Abstract
Molecular cloning of cockroach allergens and their expression as recombinant proteins have allowed a better understanding of the mechanisms of cockroach allergic disease. Recombinant cockroach allergens have been used for skin testing or in vitro methods to measure IgE antibody levels in serum. Early studies evaluating selected U.S. patients revealed that a cocktail of four cockroach allergens, Bla g 1, Bla g 2, Bla g 4, and Bla g 5, would identify 95 % of cockroach allergic patients. More recent studies pointed to an important role of sensitization to tropomyosin among certain populations, and suggested that a cocktail of five allergens Bla g 1 and/or Per a 1, Bla g 2, Bla g 4, Bla g 5, and Bla g 7, and/or Per a 7, would be expected to diagnose 50–64 % of cockroach-allergic patients worldwide. Variation in IgE reactivity profiles could be in part due to IgE responses to cross-reactive homologous allergens from different origins. The availability of purified natural or recombinant cockroach allergens provides the capacity to improve diagnosis of cockroach allergy and to develop novel forms of immunotherapy for cockroach-allergic patients.
Keywords: Cockroach, Allergy, Asthma, IgE, Skin testing, Allergy diagnosis, Recombinant allergens, Molecular cloning, Allergen structure, Tropomyosin, Allergen cross-reactivity
Introduction
Cockroach allergy has been established as an important cause of asthma for almost 50 years. In 1964, Bernton and Brown reported for the first time the presence of positive skin test to cockroach extract (44 %) in a study involving 755 patients living in New York [1]. Further studies by Kang et al. confirmed the causal relationship between cockroach allergy and asthma by demonstrating early, late-phase, and dual bronchoconstriction following inhalation of cockroach extract by cockroach-allergic asthmatic patients [2]. Although positive skin tests and specific IgE to cockroach have been reported in patients with allergic rhinitis and atopic dermatitis [3••, 4], the most consistent association of cockroach allergy with human diseases has been found with asthma.
It has been demonstrated that cockroaches may harbor bacteria, viruses, fungi, and parasites. However, transmission of diseases by cockroaches has not been precisely documented, partially because of confounding factors, including substandard hygienic conditions of some of the habitats where they live. Remarkably, asthma is the only disease consistently associated with exposure to cockroaches [5–7].
Cockroach Allergy: A Marker for Asthma Severity?
Progress in methodologies for quantification of cockroach allergens in the environment led to investigatation of the relationship between allergen exposure and sensitization and development of clinical disease. Monoclonal antibody-based ELISA for measurement of Bla g 1 and Bla g 2 were widely used, and threshold levels of exposure above which individuals would be at increased risk for sensitization or development of asthma symptoms were defined as 2 U/g and 8 U/g of dust for Bla g 1 and Bla g 2, respectively [8–10].
The National Cooperative Inner City Asthma Study (NCICAS) shed light on the role of cockroach allergy in asthma in eight inner-city areas in the United States [9]. However, cockroach-induced asthma may occur whenever substandard housing allows for cockroach infestation, including rural and semirural areas, suburbs, and small towns and cities around the world [11]. A key study by Rosenstreich et al. [9] revealed that inner-city children with asthma, who were both sensitized to cockroach and exposed to high levels of cockroach allergen in their homes, presented increased asthma morbidity, with more hospitalizations, more medical visits, and more reported symptoms [9]. These findings were confirmed in subsequent studies carried out in inner-city areas in the United States and in other countries [12–15]. Exposure to low levels of Bla g 1 and Bla g 2 has been linked to wheezing among infants in the first 3 months of life and with increased proliferative T cell responses [16]. Other reports have demonstrated an association of cockroach allergen exposure with persistent childhood wheezing [17] and severe asthma [18]. Interestingly, Bla g 2 was able to induce IgE responses at levels of exposure 10- to 100-fold lower (<1 µg/g dust) than cat and mite allergens, proving the potency of this allergen [19].
Among asthmatic inner-city children in the United States, Wang et al. demonstrated that having IgE to cockroach, and also to dust mites, was associated with a higher risk for asthma hospitalizations and corticosteroid use [20]. A recent innercity birth cohort study showed that prenatal exposure to cockroach allergen has led to a greater risk of sensitization by the age of 5–7 years. This risk was augmented by exposure to airborne nonvolatile combustion byproducts, particularly among children who were null for the Glutathione-S-transferase m 1 mutation [21••].
Another study by Wang et al. revealed an additional aspect of cockroach exposure: the possibility of driving the IgE immune response towards other cross-reactive invertebrate allergens [22••]. Analyzing 504 serum samples from the National Cooperative Inner-City Asthma Study, the authors uncovered a strong positive correlation between cockroach, shrimp and dust mite IgE levels. High exposure to cockroach in the home showed significant correlation with higher IgE levels to shrimp, in addition to higher IgE levels to cockroach. Therefore, this high cockroach exposure appeared to drive the immune response towards increasing IgE levels to shrimp. The authors hypothesized that this effect could be due to IgE responses directed to the cross-reactive allergen tropomyosin [22••] (see below). It would be interesting to investigate whether the augmented IgE response to shrimp would be clinically relevant in causing allergic reactions upon ingestion of shrimp.
The combination of sensitization and exposure to cockroach has also served as a marker for good clinical response to treatment with monoclonal anti-human IgE antibody Omalizumab among inner-city children, adolescents, and young adults with allergic asthma [23]. Strikingly, this study showed that patients who were both sensitized and exposed to cockroach (Bla g 1 levels ≥2 U/g) in their homes benefited most from Omalizumab treatment, with a 71.2 % reduction in exacerbations, greater improvement of symptoms, and greater reduction of dose of corticosteroids, as compared to the other study participants.
In conjunction, these studies indicate an important role of exposure and sensitization to cockroach in causing earlier and more severe symptoms of asthma, at a much lower dose of allergen exposure than other allergenic sources.
Cockroach Allergens Belong to Different Families of Proteins with Distinct Structures and Biological Activities
A remarkable progress in our understanding of cockroach allergy was initiated with the identification and molecular cloning of cockroach allergens [8, 24]. The first cockroach allergen to be cloned was Bla g 2 in 1995 [25]. Since then, identification of German and American cockroach allergens has progressed, and 10 allergen Groups are presently reported in the WHO/IUIS Allergen Nomenclature database (www.allergen.org). The allergens are Bla g 1, Bla g 2, Bla g 3, Bla g 4, Bla g 5, Bla g 6, Bla g 7, andBla g 8 from Blattella germanica, and Per a 1, Per a 3, Per a 6, Per a 7, Per a 9, and Per a 10 from Periplaneta americana (Table 1). Bla g 1, Bla g 2, and Bla g 4 are proteins secreted or excreted by cockroaches. Allergens with structural functions, like tropomyosins from Group 7 or allergens from Groups 6 and 8, result from degradation of dead bodies [24]. Dried secretions and remains of cockroach body parts are thought to be the origin of airborne allergens that induce sensitization in genetically predisposed individuals.
Table 1.
Properties of cockroach allergens
| Blattella germanica | |||||
|---|---|---|---|---|---|
| Allergen | M.W.a | Function/homology | PDB ID | References | |
| Bla g 1 | ~25–90 | Midgut microvilli | |||
| Bla g 1.0101 | 46, 21 | protein homolog | [32, 34] | ||
| Bla g 1.0102 | 25–37 | [31] | |||
| Bla g 1.0201 | 56 | [32, 34] | |||
| Bla g 2 | 36 | Inactive aspartic protease | 1YG9, 2NR6b, 3LIZb | [25, 26, 43–46, 103] | |
| Bla g 3 | 79 | Hemocyanin | n.p. | ||
| Bla g 4 | 21 | Lipocalin | 3EBKc | [27, 59] | |
| Bla g 5 | 23 | Glutathione S-Transferase | [61] | ||
| Bla g 6 | Bla g 6.0101 | 17 | Troponin C | [76] | |
| Bla g 6.0201 | [76] | ||||
| Bla g 6.0301 | [76] | ||||
| Bla g 7 | 33 | Tropomyosin | [104, 105] | ||
| Bla g 8 | Myosin light chain | n.p. | |||
| Periplaneta americana | |||||
| Allergen | M.W.a | Function/homology | PDB ID | References | |
| Per a 1 | ~25–45 | Midgut microvilli | |||
| Per a 1.0101 | 26 | Protein homolog | [32, 34] | ||
| Per a 1.0102 | 26 | [33] | |||
| Per a 1.0103 | 45 | [35] | |||
| Per a 1.0104 | 31 | [33, 106] | |||
| Per a 1.0201 | 51 | [106–108] | |||
| Per a 3 | 46–79 | Arylphorin/hemocyanin | |||
| Per a 3.0101 | 79 | [48] | |||
| Per a 3.0201 | 75 | [48] | |||
| Per a 3.0202 | 56 | [50] | |||
| Per a 3.0203 | 46 | [48, 50] | |||
| Per a 6 | 17 | Troponin C | [76] | ||
| Per a 7 | 33 | Tropomyosin | |||
| Per a 7.0101 | [68] | ||||
| Per a 7.0102 | [64] | ||||
| Per a 9 | 43 | Arginine kinase | [109] | ||
| Per a 10 | 28 | Serine protease | [81–84] | ||
n.p. Not published. Available in the WHO/IUIS Allergen Nomenclature Database
Molecular weight calculated from sequence
X-ray crystal structures of Bla g 2 in complex with monoclonal antibodies that inhibit IgE binding
Structure of a P. americana homolog reported under the Protein Data Bank Accession number 3EBW
(Reprinted from Pomes and Arruda [42]; copyright 2013, with permission from Elsevier)
In addition to the allergens officially deposited in the WHO/IUIS database, other related cockroach proteins have been identified. These include a Bla g 2 homolog from P. americana (44 % identity) cloned by Dr. FT Chew [26], a Bla g 4 homolog from P. americana [27], and a troponin-T from American cockroach (with 17 % prevalence of IgE reactivity) [28].
Recently, the use of proteomic techniques combined with bioinformatic allergen database analysis led to the identification of novel IgE-binding proteins in two different populations. In Taiwan, these proteins, originated from whole-body of German cockroach, included vitellogenin, aldolase, arginine kinase, enolase, Hsp70, and triosephosphateisomerase. They have been reported as allergens in species other than B. germanica, with potential for cross-reactivity [29]. A second study revealed the presence of 12 new IgE-reactive components from German cockroach fecal extract among patients from Korea. Most of these allergens were digestive enzymes such as α-amylase, trypsin, chymotrypsin, metalloprotease, and midgut carboxy peptidase A [30•]. Further studies will be necessary to establish the importance of these allergens among larger groups of patients from different areas of the world.
Specific properties of cockroach allergens belonging to distinct groups are described in the following sections.
The Group 1 of Cross-Reactive Cockroach Allergens
Bla g 1 and Per a 1 share a 52–72 % amino acid sequence identity, and have a distinct primary structure constituted of multiple tandem repeats of ~100 amino acid residues [31–35]. A recent study showed that two repeats encapsulate a large and nearly spherical hydrophobic cavity, defining the basic structural unit of Bla g 1. This cavity has the capacity to bind various lipids, which suggests a digestive function associated with nonspecific transport of lipid molecules in cockroaches [36••]. Bla g 1 is secreted in the midgut of the cockroach digestive system, especially by females and after a food intake [37, 38]. In keeping with a function as a digestive protein, the Group 1 cockroach allergens show homology to microvillar membrane-associated proteins fromother insects [39–41], and with ANG12, a protein from Anopheles gambiae which is induced after a blood meal in the midgut of adult female mosquitoes [42]. The presence of Bla g 1 in fecal particles makes this molecule, together with Bla g 2, a good marker of cockroach allergen exposure. All environmental studies report Bla g 1 exposure in arbitrary units. Defining the basic structural unit of Bla g 1 facilitated the standardization of assays in absolute units for the assessment of environmental allergen exposure and measurement of absolute levels of Bla g 1 in extracts used for diagnosis. [36••].
The Inactive Aspartic Protease Bla g 2
Bla g 2 shares homology with the proteolytic enzymes aspartic proteases (pepsin, renin, chymosin), is secreted in the digestive system, and excreted in fecal particles together with Bla g 1. Although it was at first assumed that Bla g 2 proteolytic activity could increase allergenicity, structural and functional studies demonstrated that Bla g 2 is an inactive aspartic protease [26, 43]. X-ray crystallography and site-directed mutagenesis analysis revealed interesting mechanisms of allergen-antibody interaction and led to expression of hypoallergens as potential tools for immunotherapy [44–46].
Group 3, Homologs to Hemolymph
Per a 3 is an hexameric protein that shares homology with arylphorins or insect storage proteins (20–34 %), insect juvenile hormone-suppressible proteins (31–36 %) and arthropod hemocyanins (30–35 %) [47–49]. Per a 3 comprises different variants or isoallergens, with a wide range of skin test reactivity (26–95 %) [47, 48, 50]. Bla g 3, a B. germanica homolog, has also been reported (Table 1). Data from our group revealed that Per a 3 is a minor allergen, with only 10 %of patients with IgE antibody reactivity (unpublished observations).
Group 4, Insect Lipocalins
Cockroach allergens from Group 4 are insect lipocalins, which are small extra-cellular proteins present in secretions, with the capacity to bind small hydrophobic ligands such as pheromones, steroids, retinoids, and arachidonic acid. Most lipocalin allergens are of mammalian origin and include: Bos d 2 and Bos d 5 from cow; Can f 1, Can f 2, Can f 4, and Can f 6 from dog; Equ c 1 and Equ c 2 from horse; Fel d 4 and Fel d 7 from cat; Mus m 1 from mouse; Rat n 1 from rat; and Cav p 1, Cav p 2, and Cav p 3 from guinea pig [51–58]. The degree of homology between Bla g 4 and the mammalian allergens is approximately 15–18 %, and small cross-reactivity with mammalian allergens would be expected. Interestingly, Bla g 4 is an 18-kDa protein, expressed exclusively in the adult male reproductive system, and involved in male reproductive function [59, 60]. The X-ray crystal structures of Bla g 4 and a P. americana homolog have been published, showing a conserved typical lipocalin fold [27].
Bla g 5: A Homolog to Glutathione Transferases
Bla g 5 is a 23-kDa protein which belongs to the family of glutathione S-transferases (GST), sharing 42–51 % sequence identity with the GST-2 subfamily from insects [61]. Glutathione S-transferases are enzymes involved in detoxification of endogenous and xenobiotic toxic compounds. Allergens homologous to Bla g 5 are Der p 8 from dust mite and Alt a 13 from Alternaria alternata, and antigenic cross-reactivity has been reported between mite and cockroach GST [62]. A study by Santiago and co-workers showed crossreactive IgE responses to Bla g 5 and GST from the helminth Wuchereria bancrofti, a major lymphatic filarial pathogen of humans [63••].
Groups 6, 7, and 8: Cockroach Allergens Homologous to Proteins Involved in Contraction
Group 7 comprises Bla g 7 and Per a 7, which belong to the tropomyosin family of proteins. Tropomyosin is a panallergen present in muscle of many animal species. Initially identified as a major shrimp allergen, it is present in a number of mollusks (e.g., squid), arthropods (arachnids and insects), and parasites [64–70]. IgE responses to shrimp tropomyosin have been shown to improve diagnosis of shrimp allergic patients [71, 72]. The high homology among invertebrate tropomyosins (~80 %) provides the basis for antigenic crossreactivity [73, 74]. Interestingly, tropomyosins from invertebrates share much lower homology with vertebrate homologues (~55 % identity), resulting in lack of cross-reactivity between vertebrate and invertebrate tropomyosins [75].
Additional minor cockroach allergens involved in contraction have been identified that belong to Groups 6 and 8, with homology to troponin C and myosin light chain, respectively [76]. Of importance, a myosin light chain from shrimp Litopenaeus vannamei, Lit v 3, has been identified as a major allergen recognized by 55 % (21/38) of shrimp-allergic patients [77].
Group 9: Arginine Kinases
The first arginine kinase to be described as an allergen was Pen m 2 fromthe black tiger shrimp (Penaeus monodon) [78]. Subsequently, Per a 9 was identified as a major cockroach allergen (WHO/IUIS Allergen Nomenclature Database), and a B. germanica homolog was cloned. Arginine kinases are involved in the metabolism of ATP, by catalyzing the reversible transfer of the high-energy phosphoryl group from ATP to arginine [78]. Inhibition of IgE antibody binding to dust mite, cockroach, king prawn, lobster, and mussel extracts by recombinant Plo i 1 from Indian meal moth suggested that arginine kinase is a cross-reactive invertebrate panallergen [79].
Group 10: Serine Proteases
The presence of proteases in cockroach extracts has been known for a long time [80]. Per a 10, a 28-kDa protein, was reported as a major allergen with serine protease activity in an Indian population, reacting to IgE in 82 % (37/45) of cockroach-sensitized patients [81]. The serine protease activity of Per a 10 augmented allergen-induced airway inflammation in a mouse model, and Per a 10 biased dendritic cells towards type 2 by upregulating CD86 and inducing low IL-12 secretions [82, 83]. A recombinant Per a 10 without proteolytic activity was recently produced in E. coli. The Per a 10 protein sequence exhibited 27–38% similarity to mite serine proteases, and 41–52%similarity to other insect trypsins. The allergen showed potential for immunotherapy by having reduced IgE antibody binding and histamine release [84].
Recombinant Cockroach Allergens for Diagnosis
In current clinical practice, diagnosis of cockroach allergy is performed using crude extracts by in vivo skin testing and/or in vitro measurement of specific IgE to cockroach (by ImmunoCAP). Cockroach extracts available in the United States for allergy diagnosis have up to seven-fold variation in levels of Bla g 1 and Bla g 2 [85]. Slater et al. have shown that the mean potency of three US German cockroach extracts was 3,300 BAU/mL, whereas standardized mite, cat, or grass extracts present typically 5,000–100,000 BAU/mL [86]. In a study carried out in Brazil involving children with asthma, skin prick tests were performed with B. germanica and P. americana extracts, and the results were compared to specific IgE serum levels. The concordance of skin prick test and specific IgE results was reasonable for B. germanica and weak for P americana [87]. In addition, performing skin tests with B. germanica and P. americana extracts from three different manufacturers revealed a poor correlation of the results, with sensitization rates to German and American cockroach of up to 54.1 and 59.5 %, respectively [88]. These results suggested the existence of substantial differences in the potencies or constituents of the extracts from these same species. It is clear that the use of standardized cockroach extracts of reliable potency and contents would facilitate diagnosis and treatment of cockroach allergy [89••]. A mediator release assay using human FceRI-transfected RBL cells and human serum has been used to measure cockroach extract potency. Ranking of extract potency was consistent between the mediator release assay and the ID50EAL assay, which is a functional in vivo assay of choice for standardization of extracts in the United States, suggesting that it could be a safer and more efficient method of cockroach extract standardization for diagnosis and immunotherapy [90]. Attempts to standardize cockroach extracts revealed that currently available commercial extracts tend to have low and variable potency, and that no single allergen is immunodominant in a way that it could be used to standardize extracts [89••]. Recombinant cockroach allergens could improve clinical diagnosis of cockroach allergy, since they have been successfully used for in vivo and in vitro assessment of sensitization to specific cockroach allergens [3••, 25, 29, 59, 91]. Overall, results indicate that cockroach-allergic patients present variable allergen sensitization profiles, and that no single major allergen appeared to account for most of the IgE reactivity to cockroach within a given population. In addition, the importance of individual allergens in causing sensitization varies in different areas of the world, possibly due to the influence of sensitization to cross-reactive antigens. Early studies evaluating selected U.S. cockroach allergic patients with asthma revealed that a cocktail of four cockroach allergens, Bla g 1, Bla g 2, Bla g 4, and Bla g 5, would identify 95 % of cockroach allergic patients [8, 42, 61]. More recent studies identified that sensitization to tropomyosin is important among certain populations in different areas of the world [3••, 64, 66], and suggested that a cocktail of five cockroach allergens Bla g 1 and/or Per a 1,Bla g 2,Bla g 4,Bla g 5, and Bla g 7, and/or Per a 7, would be expected to diagnose 50– 64 % of cockroach allergic patients worldwide [3••, 29, 91].
In most studies carried out in the U.S., sensitization to Bla g 2, Bla g 4, and Bla g 5 presented the highest prevalence among cockroach allergic patients with asthma [25, 59, 91]. Using streptavidin CAP and a multiplex flow cytometric assay, Satinover et al. showed that a panel of 5 recombinant allergens (rBla g 1, rBla g 2, rBla g 4, rBla g 5, and rPer a 7) could identify 64 % of cockroach-allergic US patients [91]. Prevalence of IgE antibodies was highest for rBla g 2 (54.4 %) and rBla g 5 (37.4 %) among the 118 sera analyzed. However, patterns of IgE antibody binding were unique to each subject, irrespective of their geographic location within the U.S. These results were in contrast with sensitization to indoor allergens from cat and mite, which shows a clear immunodominance of few allergens (Fel d 1 for cat and Groups 1 and 2 for mite).
In Taiwan, Chuang et al. also found heterogeneous IgE reactivity profiles, in a group of 32 cockroach-allergic patients [29]. Using recombinant cockroach allergens Bla g 1, Bla g 2, Bla g 4, Bla g 5, Bla g 7, and the newly identified B. germanica enolase, arginine kinase, and vitellogenin, the authors showed that all patients reacted to at least one allergen on an IgE dot-blot immunoassay. The prevalence of IgE recognition was highest for Bla g 2 (63 %), followed by Bla g 4 (53 %), vitellogenin (47 %), Bla g 1 and arginine kinase (34 %), Bla g 5 and Bla g 7 (31 %), and enolase (25.0 %) [29].
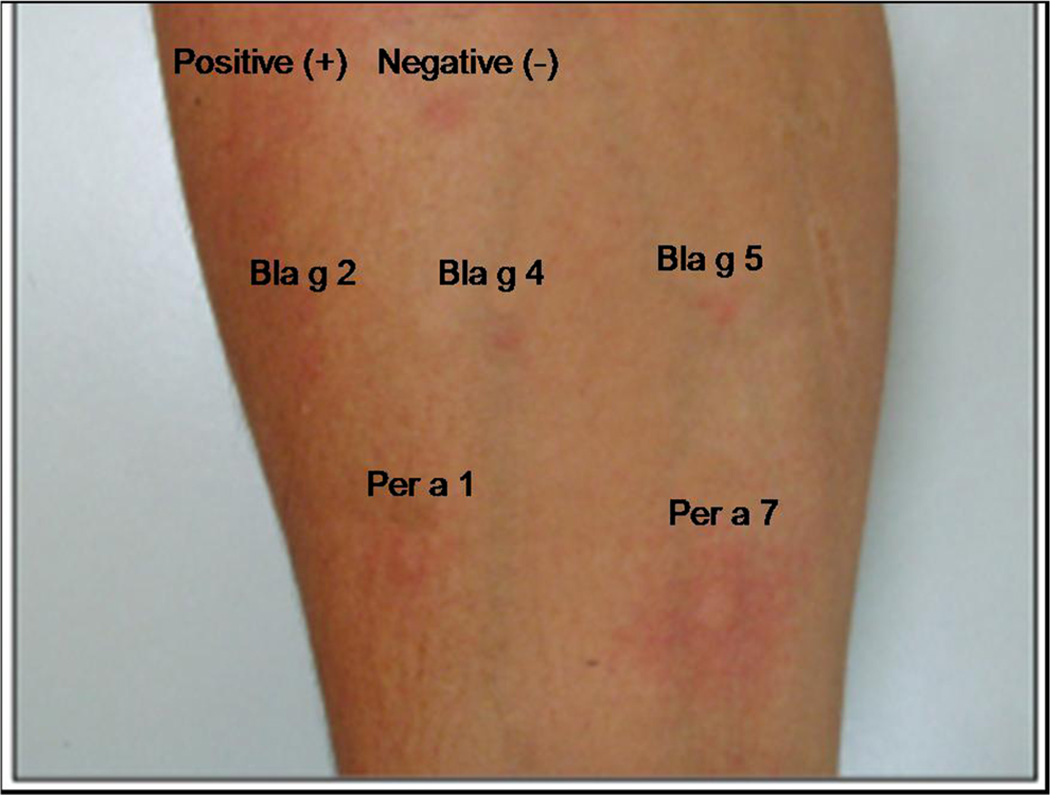
In contrast, data from Brazil revealed striking differences from the results observed in US and Taiwan. A panel of 5 recombinant allergens (rPer a 1, rPer a 7, rBla g 2, rBla g 4, and rBla g 5) was used for skin testing 57 cockroach allergic patients with asthma and/or rhinitis (Fig. 1). Twenty-four patients (42 %) had positive responses to rPer a 7 (P. americana tropomyosin), and the frequencies of sensitization to Per a 7 of 42–54 %, considering in vivo and in vitro testing, respectively, were in keeping with previous studies in Brazil [3••, 64]. On the other hand, reactivity to the other cockroach allergens tested was remarkably low: only 3 (5.3 %), 4 (7 %), 3 (5.3 %), and 4 (7 %) showed positive skin prick tests to Per a 1, Bla g 2, Bla g 4, and Bla g 5, respectively. Overall, 28/57 (49.1 %) had skin tests to at least one recombinant allergen [3••].
Fig. 1.
Skin prick testing with recombinant allergens in a cockroach-allergic patient with asthma and rhinitis. Positive skin tests were observed for rPer a 1 and rPer a 7 and negative tests were obtained for rBlag 2, rBla g 4, and rBla g 5. (Reproduced from Barbosa et al. [3••]; copyright 2013, Karger, Basel, Switzerland)
The high prevalence of IgE reactivity to tropomyosin in Brazil is in contrast to the low levels of sensitization to Per a 7 in the U.S. [90]. Interestingly, analysis carried out in various European countries also revealed a low frequency of IgE reactivity (6–18 %) to mite tropomyosin (Der p 10) among mite-allergic patients [92, 93]. Conversely, sensitization profiles in a large group of 650 allergic patients in Africa showed a 55 % prevalence of IgE to mite tropomyosin [94]. The high frequency of reactivity to cockroach tropomyosin seen in Brazil could reflect cross-reactivity to mite tropomyosin, which shares 80 % sequence identity to the cockroach homolog. Another possibility is that co-sensitization to tropomyosins from different origins occurred. In particular, the high frequency of sensitization to tropomyosins in Brazil and Africa could be due to cross-reactivity to tropomyosin from intestinal parasites, particularly Ascaris lumbricoides [66, 95, 96]. In keeping with a possible mechanism by which parasites could increase allergic reactivity through cross-reactive antibody responses, several parasite molecules have been reported to have homologs in allergen families other than tropomyosins, including lipocalins, proteins from the cupin superfamily, EF-hand proteins, profillins, and glutathione-s-transferases [97].
Sensitization to individual cockroach allergens, aiming at identifying markers for severity of disease, has been investigated among cockroach-allergic patients. Recombinant P. americana allergens (Per a 1 through 7, and Per a 9), expressed in E. coli, were used to evaluate correlations with clinical manifestations and severity of disease among patients from Taiwan [98]. IgE-binding to Per a 2 was more frequently found among the group of patients with persistent asthma, as compared to patients with rhinitis only (81 vs. 45 %, p < .05). In contrast, 80 % of rhinitis patients had IgE-binding activity to Per a 9, as compared with only 28.5 % among the patients with asthma (p < .01). The results suggested that sensitization to Per a 2 could be a marker of more severe airway disease [98].
The availability of individual recombinant and natural cockroach allergens will facilitate the diagnosis of individual profiles of IgE reactivity (highly variable for cockroach allergy) and the identification the specific allergen(s) affecting each cockroach allergic patient.
Recombinant Cockroach Allergens: Perspectives for Allergen-Specific Immunotherapy
There have been very few immunotherapy studies for cockroach allergy [99–101]. In 1988, Kang et al. reported in a small number of patients (n=11) that immunotherapy with cockroach extract resulted in improvement of immunological and clinical parameters, including symptoms and medication use, after 5 years of treatment [99]. Another study described decrease in nasal symptoms and increase in cockroach specific IgG levels, accompanied by decrease in serum levels of IL-2, IL-4, and IL-4 receptor, following 3 years of immunotherapy with cockroach extract [100]. A double-blind, placebo-controlled cockroach immunotherapy trial from India has been more recently reported [101]. Immunotherapy was performed using an in-house cockroach extract, prepared under good manufacturing practice conditions, and 50 cockroach-allergic patients with asthma and/or rhinitis were enrolled, of whom 42 (28 active treatment, 18 placebo) completed the first year of the study. At 1 year, while patients were still on buildup phase, a significant improvement in clinical scores and bronchial hyper-reactivity, accompanied by increase in cockroach specific IgG4, was observed. However, in the second year, no placebo-treated subjects were included, and only 12 patients in the active treatment group provided samples for analysis at the end of the 2-year follow-up [101]. These small studies suggested that cockroach immunotherapy may be effective.
Recently, results of four pilot studies of sublingual and subcutaneous immunotherapy (SLIT and SCIT) with German cockroach extract have been reported [102••]. The studies, aimed at assessing safety and evaluating immunological parameters, involved a total of 190 children and adults with asthma, rhinitis, or both. The administration of cockroach allergen by means of SCIT was immunologically more active than SLIT, particularly regarding the increase of IgG4 levels and blocking antibody responses. Both forms of immunotherapy posed no safety concerns to any age group. These pilot studies suggested that immunotherapy with cockroach allergen is more likely to be effective with SCIT, and provided the basis for larger-scale efficacy studies of immunotherapy for cockroach allergy [102••].
Conclusions
Cockroach allergy is associated with increased morbidity and high risk for emergency room visits and admissions due to asthma exacerbations among asthmatic patients. Continued exposure to low levels of cockroach allergens in the indoor environment leads to sensitization in susceptible individuals, and subsequent exacerbation of symptoms. Progress in molecular cloning and expression of recombinant allergens has led to an improvement in knowledge of the structure and function of cockroach allergens, which may be useful for developing novel strategies for diagnosis and therapy of cockroach-allergic patients. At present, ten Groups of well-characterized cockroach allergens from both B. germanica and P. americana, the most common intra-domiciliary cockroach species, have been accepted by the WHO/IUIS Allergen Nomenclature database. The use of recombinant allergens for diagnostic purposes has been investigated in clinical studies by performing either skin testing or measurements of serum-specific IgE in groups of cockroach allergic patients. Overall, the results have revealed heterogeneous IgE reactivity profiles among cockroach-allergic patients. However, further studies are needed to better characterize the relative importance of different cockroach allergens in larger number of patients, which will provide insights into cockroach allergen sensitization depending on allergen exposure, geographic location, and genetic background. These studies would help in defining the most appropriate selection of cockroach allergens to be used for diagnosis and therapy in a given area. Ultimately, recombinant allergens could be used in clinical trials for immunotherapy of cockroach-allergic patients, particularly those at a higher risk for more severe disease including children and young adults living in inner-city environments.
Acknowledgments
Research carried out in Brazil by L. Karla Arruda has been supported by São Paulo State Research Funding Agency (FAPESP) and Brazilian National Research Council – National Institutes of Science and Technology, Institute of Investigation in Immunology (CNPq–INCT–iii). Part of the research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI077653 (PI: AP andMDC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Martin D. Chapman has received NIH grant support (no. AI077653, through Indoor Biotechnologies, Inc.), has served on boards for Virginia Bio and the Charlottesville Business Innovation Council, is employed as the president/CEO of Indoor Biotechnologies, Inc., holds US patent 5,869,288 (through Indoor Biotechnologies, Inc.), holds stock/stock options in Indoor Biotechnologies, Inc., and has received royalties from the University of Virginia.
Anna Pomés has received NIAID/NIH grant support (no. R01AI077653) and is employed by Indoor Biotechnologies, Inc.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Conflict of Interest L. Karla Arruda, Michelle C.R. Barbosa, Ana Beatriz R. Santos, and Adriana S. Moreno declare that they have no conflict of interest.
Contributor Information
L. Karla Arruda, Email: karla@fmrp.usp.br, Department of Medicine, Ribeirao Preto Medical School, University of Sao Paulo, Av. Bandeirantes 3900, Ribeirao Preto, SP 14049-900, Brazil.
Michelle C. R. Barbosa, Email: mchllch@hotmail.com, Department of Medicine, Ribeirao Preto Medical School, University of Sao Paulo, Av. Bandeirantes 3900, Ribeirao Preto, SP 14049-900, Brazil.
Ana Beatriz R. Santos, Email: biaro7@hotmail.com, Institute of Health Sciences, Paulista University Campus Campinas, Av. Comendador Enzo Ferrari, 280, Swift, Campinas, SP 13043-900, Brazil.
Adriana S. Moreno, Email: drismoreno@yahoo.com, Department of Medicine, Ribeirao Preto Medical School, University of Sao Paulo, Av. Bandeirantes 3900, Ribeirao Preto, SP 14049-900, Brazil.
Martin D. Chapman, Email: mdc@inbio.com, Indoor Biotechnologies, Incorporated, 1216 Harris Street, Charlottesville, VA 22903, USA.
Anna Pomés, Email: apomes@inbio.com, Indoor Biotechnologies, Incorporated, 1216 Harris Street, Charlottesville, VA 22903, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bernton H, Brown H. Insect allergy—Preliminary studies of the cockroach. J Allergy. 1964;35:506–513. doi: 10.1016/0021-8707(64)90082-6. [DOI] [PubMed] [Google Scholar]
- 2.Kang B, Vellody D, Homburger H, Younginger JW. Cockroach as a cause of allergic asthma. Its specificity and immunologic profile. J Allergy Clin Immunol. 1979;63:80–86. doi: 10.1016/0091-6749(79)90196-9. [DOI] [PubMed] [Google Scholar]
- 3. Barbosa MC, Santos AB, Ferriani VP, Pomés A, Chapman MD, Arruda LK. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. 2013;161:213–219. doi: 10.1159/000346318. This is the first study using recombinant cockroach allergens for skin testing in patients with asthma and/or rhinitis living in Brazil. The results revealed that skin testing with recombinant allergens was safe and that cockroach tropomyosin (Per a 7 allergen) was the major sensitizing allergen. By contrast, studies carried out in the United States revealed low levels of sensitization to Per a 7, and more frequent reactions to Bla g 2 and Bla g 5 among cockroach-allergic patients.
- 4.Michel S, Yawalkar N, Schnyder B, Fischer B, Helbling A. Eczematous skin reaction to atopy patch testing with cockroach in patients with atopic dermatitis. J Investig Allergol Clin Immunol. 2009;19:173–179. [PubMed] [Google Scholar]
- 5.Arruda LK, Pomés A. Every Cockroach Is Beautiful to Its Mother. Int Arch Allergy Immunol. 2013;161:289–292. doi: 10.1159/000350207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornwell PB. The Cockroach. Vol. 1. London: Hutchinson; 1968. [Google Scholar]
- 7.Sookrung N, Chaicumpa W. A revisit to cockroach allergens. Asian Pac J Allergy Immunol. 2010;28:95–106. [PubMed] [Google Scholar]
- 8.Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107:419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 10.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 11.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, et al. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan WJ, Rangsithienchai PA, Wood RA, Rivard D, Chinratanapisit S, Perzanowski MS, et al. Pest and allergen exposure and abatement in inner-city asthma: a work group report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee. J Allergy Clin Immunol. 2010;125:575–578. doi: 10.1016/j.jaci.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perzanowsky MS, Platts-Mills TAE. Further confirmation of the relevance of cockroach and dust mite sensitization to inner-city asthma morbidity. Clin Exp Allergy. 2009;39:1291–1293. doi: 10.1111/j.1365-2222.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 14.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–684. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarinho E, Schor D, Veloso MA, Rizzo JA. There are more asthmatics in homes with high cockroach infestation. Braz J Med Biol Res. 2004;37:503–510. doi: 10.1590/s0100-879x2004000400007. [DOI] [PubMed] [Google Scholar]
- 16.Finn PW, Boudreau JO, He H, Wang Y, Chapman MD, Vincent C, et al. Children at risk for asthma: home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105:933–942. doi: 10.1067/mai.2000.106546. [DOI] [PubMed] [Google Scholar]
- 17.Silva JM, Camara AA, Tobias KR, Macedo IS, Cardoso MR, Arruda E, et al. A prospective study of wheezing in young children: the independent effects of cockroach exposure, breast-feeding and allergic sensitization. Pediatr Allergy Immunol. 2005;16:393–401. doi: 10.1111/j.1399-3038.2005.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey CD, Celedon JC, Sredl DL, Weiss ST, Cloutier MM. Predictors of disease severity in children with asthma in Hartford, Connecticut. Pediatr Pulmonol. 2005;39:268–275. doi: 10.1002/ppul.20177. [DOI] [PubMed] [Google Scholar]
- 19.Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TA. Mite cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case-control study of three schools. Thorax. 1999;54:675–680. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy. 2009;39:1381–1389. doi: 10.1111/j.1365-2222.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131:886–893. doi: 10.1016/j.jaci.2012.12.666. This is a very interesting study which showed that prenatal exposure to cockroach allergen among Dominican and African-American pregnant women in New York was associated with a greater risk of allergic sensitization at the ages of 5–7 years. This risk was increased only among children who were also exposed to nonvolatile polycyclic aromatic hydrocarbons (PAHs), with children null for the Glutathione-S-transferase μ 1 (GSTM1) mutation being particularly vulnerable. The study highlights the fact that combustion byproducts can act as adjuvants in the development of cockroach sensitization in urban environments.
- 22. Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011;128:834–837. doi: 10.1016/j.jaci.2011.07.045. In this study, the authors report an interesting observation, that increasing exposure to cockroach allergen in the home is associated not only with increasing in IgE to cockroach but also with increasing in IgE to shrimp. This could be due to cross-reactive IgE responses to shared allergens, including tropomyosin.
- 23.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomés A, Wünschmann S, Hindley J, Vailes LD, Chapman MD. Cockroach allergens: function, structure and allergenicity. Protein Pept Lett. 2007;14:960–969. doi: 10.2174/092986607782541178. [DOI] [PubMed] [Google Scholar]
- 25.Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, Vedvick TS, et al. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2. Sequence homology to the aspartic proteases. J Biol Chem. 1995;270:19563–19568. doi: 10.1074/jbc.270.33.19563. [DOI] [PubMed] [Google Scholar]
- 26.Gustchina A, Li M, Wünschmann S, Chapman MD, Pomés A, Wlodawer A. Crystal structure of cockroach allergen Bla g 2, an unusual zinc binding aspartic protease with a novel mode of self-inhibition. J Mol Biol. 2005;348:433–444. doi: 10.1016/j.jmb.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 27.Tan YW, Chan SL, Ong TC, Yit le Y, Tiong YS, Chew FT, et al. Structures of two major allergens, Bla g 4 and Per a 4, from cockroaches and their IgE binding epitopes. J Biol Chem. 2009;284:3148–3157. doi: 10.1074/jbc.M807209200. [DOI] [PubMed] [Google Scholar]
- 28.Khantisitthiporn O, Sookrung N, Tungtrongchitr A, Tongtawe P, Bunnag C, Srimanote P, et al. Native troponin-T of the American cockroach (CR), Periplaneta americana, binds to IgE in sera of CR allergic Thais. Asian Pac J Allergy Immunol. 2007;25:189–197. [PubMed] [Google Scholar]
- 29.Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. 2010;10:3854–3867. doi: 10.1002/pmic.201000348. [DOI] [PubMed] [Google Scholar]
- 30. Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol. 2013;161:315–324. doi: 10.1159/000347034. In this study from Korea, Jeong et al. have have found that some of 12 IgE-binding proteins identified by proteomics strategy were digestive enzymes, including α-amylase, midgut carboxypeptidase A, chymotrypsin, astacin-like metalloprotease, and trypsin. α-Amylase was found to be an important allergen with a 41 % prevalence of IgE reactivity. Amylase activity was previously reported from various cockroach gut compartments and salivary glands. The results raise the interesting possibility that α-amylase could be another invertebrate cross-reactive allergen in the cockroach, in addition to tropomyosin, glutathione S-transferase, arginine kinase. and myosin light chain, which share homologs in mites, shrimp. and parasites.
- 31.Helm R, Cockrell G, Stanley JS, Brenner RJ, Burks W, Bannon GA. Isolation and characterization of a clone encoding a major allergen (Bla g Bd90K) involved in IgE-mediated cockroach hypersensitivity. J Allergy Clin Immunol. 1996;98:172–180. doi: 10.1016/s0091-6749(96)70240-3. [DOI] [PubMed] [Google Scholar]
- 32.Pomés A, Melén E, Vailes LD, Retief JD, Arruda LK, Chapman MD. Novel allergen structures with tandem amino acid repeats derived from German and American cockroach. J Biol Chem. 1998;273:30801–30807. doi: 10.1074/jbc.273.46.30801. [DOI] [PubMed] [Google Scholar]
- 33.Wu CH, Wang NM, Lee MF, Kao CY, Luo SF. Cloning of the American cockroach Cr-PII allergens: evidence for the existence of cross-reactive allergens between species. J Allergy Clin Immunol. 1998;101:832–840. doi: 10.1016/S0091-6749(98)70312-4. [DOI] [PubMed] [Google Scholar]
- 34.Melén E, Pomés A, Vailes LD, Arruda LK, Chapman MD. Molecular cloning of Per a 1 and definition of the cross-reactive Group 1 cockroach allergens. J Allergy Clin Immunol. 1999;103:859–864. doi: 10.1016/s0091-6749(99)70430-6. [DOI] [PubMed] [Google Scholar]
- 35.Yang CY, Wu JD, Wu CH. Sequence analysis of the first complete cDNA clone encoding an American cockroach Per a 1 allergen. Biochim Biophys Acta. 2000;1517:153–158. doi: 10.1016/s0167-4781(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 36. Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD, et al. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.06.014. This very recent study revealed that the Bla g 1 allergen has a novel fold with a capacity to bind various lipids, which suggests a digestive function associated with nonspecific transport of lipid molecules in cockroaches. Characterizing the basic structural unit of Bla g 1 allowed for absolute standardization of measurements of environmental allergen exposure.
- 37.Gore JC, Schal C. Gene expression and tissue distribution of the major human allergen Bla g 1 in the German cockroach, Blattella germanica L. (Dictyoptera: Blattellidae) J Med Entomol. 2004;41:953–960. doi: 10.1603/0022-2585-41.5.953. [DOI] [PubMed] [Google Scholar]
- 38.Gore JC, Schal C. Expression, production and excretion of Bla g 1, amajor human allergen, in relation to food intake in the German cockroach, Blattella germanica. Med Vet Entomol. 2005;19:127–134. doi: 10.1111/j.0269-283X.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira AH, Cristofoletti PT, Lorenzini DM, Guerra LO, Paiva PB, Briones MR, et al. Identification of midgut microvillar proteins from Tenebrio molitor and Spodoptera frugiperda by cDNA library screenings with antibodies. J Insect Physiol. 2007;53:1112–1124. doi: 10.1016/j.jinsphys.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Morlais I, Mori A, Schneider JR, Severson DW. A targeted approach to the identification of candidate genes determining susceptibility to Plasmodium gallinaceum in Aedes aegypti. Mol Genet Genomics. 2003;269:753–764. doi: 10.1007/s00438-003-0882-7. [DOI] [PubMed] [Google Scholar]
- 41.Shao L, Devenport M, Fujioka H, Ghosh A, Jacobs-Lorena M. Identification and characterization of a novel peritrophic matrix protein, Ae-Aper50, and the microvillar membrane protein, AEG12, from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:947–959. doi: 10.1016/j.ibmb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Pomés A A, Arruda LK. Investigating cockroach allergens: Aiming to improve diagnosis and treatment of cockroach allergic patients. Methods. 2013 Aug 2; doi: 10.1016/j.ymeth.2013.07.036. doi:pii: S1046-2023(13)00285-5. 10.1016/j.ymeth.2013.07.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wünschmann S, Gustchina A, Chapman MD, Pomés A. Cockroach allergen Bla g 2: an unusual aspartic proteinase. J Allergy Clin Immunol. 2005;116:140–145. doi: 10.1016/j.jaci.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Gustchina A, Alexandratos J, Wlodawer A, Wünschmann S, Kepley CL, et al. Crystal structure of a dimerized cockroach allergen Bla g 2 complexed with a monoclonal antibody. J Biol Chem. 2008;283:22806–22814. doi: 10.1074/jbc.M800937200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Gustchina A, Glesner J, Wünschmann S, Vailes LD, Chapman MD, et al. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186:333–340. doi: 10.4049/jimmunol.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glesner J, Wünschmann S, Li M, Gustchina A, Wlodawer A, Himly M, et al. Mechanisms of allergen-antibody interaction of cockroach allergen Bla g 2 with monoclonal antibodies that inhibit IgE antibody binding. PLoS One. 2011;6:e22223. doi: 10.1371/journal.pone.0022223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu CH, Lan JL. Cockroach hypersensitivity: isolation and partial characterization of major allergens. J Allergy Clin Immunol. 1988;82:727–735. doi: 10.1016/0091-6749(88)90071-1. [DOI] [PubMed] [Google Scholar]
- 48.Wu CH, Lee MF, Liao SC, Luo SF. Sequencing analysis of cDNA clones encoding the American cockroach Cr-PI allergens. Homology with insect hemolymph proteins. J Biol Chem. 1996;271:17937–17943. doi: 10.1074/jbc.271.30.17937. [DOI] [PubMed] [Google Scholar]
- 49.Mindykowski B, Jaenicke E, Tenzer S, Cirak S, Schweikardt T, Schild H, et al. Cockroach allergens Per a 3 are oligomers. Dev Comp Immunol. 2010;34:722–733. doi: 10.1016/j.dci.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Wu CH, Lee MF, Wang NM, Luo SF. Sequencing and immunochemical characterization of the American cockroach Per a 3 (Cr-PI) isoallergenic variants. Mol Immunol. 1997;34:1–8. doi: 10.1016/s0161-5890(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 51.Smith W, Butler AJ, Hazell LA, Chapman MD, Pomés A, Nickels DG, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–1738. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- 52.Konieczny A, Morgenstern JP, Bizinkauskas CB, Lilley CH, Brauer AW, Bond JF, et al. The major dog allergens, Can f 1 and Can f 2, are salivary lipocalin proteins: cloning and immunological characterization of the recombinant forms. Immunology. 1997;92:577–586. doi: 10.1046/j.1365-2567.1997.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Böcskei Z, Groom CR, Flower DR, Wright CE, Phillips SE, Cavaggioni A, et al. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature. 1992;360:186–188. doi: 10.1038/360186a0. [DOI] [PubMed] [Google Scholar]
- 54.Virtanen T, Kinnunen T. Mammalian allergens. Clin Allergy Immunol. 2008;21:201–218. [PubMed] [Google Scholar]
- 55.Virtanen T, Kinnunen T, Rytkönen-Nissinen M. Mammalian lipocalin allergens-insights into their enigmatic allergenicity. Clin Exp Allergy. 2012;42:494–504. doi: 10.1111/j.1365-2222.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 56.Nordlund B, Konradsen JR, Kull I, Borres MP, Önell A, Hedlin G, et al. IgE antibodies to animal-derived lipocalin, kallikrein and secretoglobin are markers of bronchial inflammation in severe childhood asthma. Allergy. 2012;67:661–669. doi: 10.1111/j.1398-9995.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 57.Fahlbusch B, Rudeschko O, Szilagyi U, Schlott B, Henzgen M, Schlenvoigt G, et al. Purification and partial characterization of the major allergen, Cav p 1, from guinea pig Cavia porcellus. Allergy. 2002;57:417–422. doi: 10.1034/j.1398-9995.2002.13540.x. [DOI] [PubMed] [Google Scholar]
- 58.Hilger C, Swiontek K, Kler S, Diederich C, Lehners C, Vogel L, et al. Evaluation of two new recombinant guinea-pig lipocalins, Cav p 2 and Cav p 3, in the diagnosis of guinea-pig allergy. Clin Exp Allergy. 2011;41:899–908. doi: 10.1111/j.1365-2222.2011.03726.x. [DOI] [PubMed] [Google Scholar]
- 59.Arruda LK, Vailes LD, Hayden ML, Benjamin DC, Chapman MD. Cloning of cockroach allergen, Bla g 4, identifies ligand binding proteins (or calycins) as a cause of IgE antibody responses. J Biol Chem. 1995;270:31196–31201. doi: 10.1074/jbc.270.52.31196. [DOI] [PubMed] [Google Scholar]
- 60.Fan Y, Gore JC, Redding KO, Vailes LD, Chapman MD, Schal C. Tissue localization and regulation by juvenile hormone of human allergen Bla g 4 from the German cockroach, Blattella germanica (L.) Insect Mol Biol. 2005;14:45–53. doi: 10.1111/j.1365-2583.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 61.Arruda LK, Vailes LD, Platts-Mills TA, Hayden ML, Chapman MD. Induction of IgE antibody responses by glutathione S-transferase from the German cockroach (Blattella germanica) J Biol Chem. 1997;272:20907–20912. doi: 10.1074/jbc.272.33.20907. [DOI] [PubMed] [Google Scholar]
- 62.Huang CH, Liew LM, Mah KW, Kuo IC, Lee BW, Chua KY. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin Exp Allergy. 2006;36:369–376. doi: 10.1111/j.1365-2222.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 63. Santiago HC, Leevan E, Bennuru S, Ribeiro-Gomes F, Mueller E, Wilson M, et al. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin Immunol. 2012;130:248–256. doi: 10.1016/j.jaci.2012.02.045. This is an intereseting study which showed that filarial infection in humans was associated with increased prevalence of cross-reactive IgE responses to Bla g 5 (cockroach GST), with possible clinical implications. Experimentally, mice infected with filaria developed anti-filaria GST IgE and showed immediate skin test reactivity to Bla g 5. It was shown that cockroach and helminth GST cross-react because of remarkable molecular and structural similarities.
- 64.Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, et al. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329–337. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 65.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 66.Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121:1040–1046. doi: 10.1016/j.jaci.2007.12.1147. [DOI] [PubMed] [Google Scholar]
- 67.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol. 1993;151:5354–5363. [PubMed] [Google Scholar]
- 68.Asturias JA, Gómez-Bayón N, Arilla MC, Martínez A, Palacios R, Sánchez-Gascón F, et al. Molecular characterization of American cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J Immunol. 1999;162:4342–4348. [PubMed] [Google Scholar]
- 69.Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, et al. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994;94:882–890. doi: 10.1016/0091-6749(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 70.Lopata AL, O'Hehir RE, Lehrer SB. Shellfish allergy. Clin Exp Allergy. 2010;40:850–858. doi: 10.1111/j.1365-2222.2010.03513.x. [DOI] [PubMed] [Google Scholar]
- 71.Yang AC, Arruda LK, Santos AB, Barbosa MC, Chapman MD, Galvão CE, et al. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol. 2010;125:872–878. doi: 10.1016/j.jaci.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Gámez C, Sánchez-García S, Ibáñez MD, López R, Aguado E, López E, et al. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy. 2011;66:1375–1383. doi: 10.1111/j.1398-9995.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 73.Reese G, Schicktanz S, Lauer I, Randow S, Lüttkopf D, Vogel L, et al. Structural, immunological and functional properties of natural recombinant Pen a 1, the major allergen of Brown Shrimp, Penaeus aztecus. Clin Exp Allergy. 2006;36:517–524. doi: 10.1111/j.1365-2222.2006.02454.x. [DOI] [PubMed] [Google Scholar]
- 74.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 75.Jenkins JA, Breiteneder H, Mills EN. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J Allergy Clin Immunol. 2007;120:1399–1405. doi: 10.1016/j.jaci.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Hindley J, Wünschmann S, Satinover SM, Woodfolk JA, Chew FT, Chapman MD, et al. Bla g 6: a troponin C allergen from Blattella germanica with IgE binding calcium dependence. J Allergy Clin Immunol. 2006;117:1389–1395. doi: 10.1016/j.jaci.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibáñez MD, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008;122:795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 78.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170:445–453. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 79.Binder M, Mahler V, Hayek B, Sperr WR, Scholler M, Prozell S, et al. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001;167:5470–5477. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 80.Wongtim S, Lehrer SB, Salvaggio JE, Horner WE. Protease activity in cockroach and basidiomycete allergen extracts. Allergy Proc. 1993;14:263–268. doi: 10.2500/108854193778811946. [DOI] [PubMed] [Google Scholar]
- 81.Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. 2008;63:768–776. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 82.Sudha VT, Arora N, Singh BP. Serine protease activity of Per a 10 augments allergen-induced airway inflammation in a mouse model. Eur J Clin Invest. 2009;39:507–516. doi: 10.1111/j.1365-2362.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 83.Goel C, Govindaraj D, Singh BP, Farooque A, Kalra N, Arora N. Serine protease Per a 10 from Periplaneta americana bias dendritic cells towards type 2 by upregulating CD86 and low IL-12 secretions. Clin Exp Allergy. 2012;42:412–422. doi: 10.1111/j.1365-2222.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- 84.Govindaraj D, Gaur SN, Arora N. Characterization of recombinant Per a 10 from Periplaneta americana. Clin Vaccine Immunol. 2013;20:262–268. doi: 10.1128/CVI.00461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patterson ML, Slater JE. Characterization and comparison of commercially available German and American cockroach allergen extracts. Clin Exp Allergy. 2002;32:721–727. doi: 10.1046/j.1365-2222.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- 86.Slater JE, James R, Pongracic JA, Liu AH, Sarpong S, Sampson HA, et al. Biological potency of German cockroach allergen extracts determined in an inner city population. Clin Exp Allergy. 2007;37:1033–1039. doi: 10.1111/j.1365-2222.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 87.Lopes MI, Miranda PJ, Sarinho E. Use of the skin prick test and specific immunoglobulin E for the diagnosis of cockroach allergy. J Pediatr (Rio J) 2006;82(3):204–209. doi: 10.2223/JPED.1487. [DOI] [PubMed] [Google Scholar]
- 88.Londres MI, Sarinho FW, Miranda PJ, Solé D, Sarinho E. Allergy to cockroaches: challenges in diagnosis. Clin Lab. 2011;57:969–974. [PubMed] [Google Scholar]
- 89. Portnoy J, Chew GL, Phipatanakul W, Williams PB, Grimes C, Kennedy K, et al. Environmental assessment and exposure reduction of cockroaches: A practice parameter. J Allergy Clin Immunol. 2013;132:802–808. doi: 10.1016/j.jaci.2013.04.061. An excellent review developed by the Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma & Immunology (AAAAI), the American College of Allergy, Asthma & Immunology (ACAAI), and the Joint Council of Allergy, Asthma & Immunology on assessment of cockroach exposure and evidence-based recommendations to decrease levels of cockroach allergens in the environment, which could be beneficial to patients with cockroach-induced asthma.
- 90.Nowak-Wegrzyn AH, Bencharitiwong R, Schwarz J, David G, Eggleston P, Gergen PJ, et al. Mediator release assay for assessment of biological potency of German cockroach allergen extracts. J Allergy Clin Immunol. 2009;123:949–955. doi: 10.1016/j.jaci.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Satinover SM, Reefer AJ, Pomés A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115:803–809. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 92.Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004;34:597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- 93.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, et al. Variability of IgE reactivity profiles among Europeanmite allergic patients. Eur J Clin Invest. 2008;38:959–965. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 94.Westritschnig K, Sibanda E, Thomas W, Auer H, Aspock H, Pittner G, et al. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. 2003;33:22–27. doi: 10.1046/j.1365-2222.2003.01540.x. [DOI] [PubMed] [Google Scholar]
- 95.Sereda MJ, Hartmann S, Lucius R. Helminths and allergy: the example of tropomyosin. Trends Parasitol. 2008;24:272–278. doi: 10.1016/j.pt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fitzsimmons CM, Dunne DW. Survival of the fittest: allergology or parasitology? Trends Parasitol. 2009;25:447–451. doi: 10.1016/j.pt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Lee MF, Song PP, Hwang GY, Lin SJ, Chen YH. Sensitization to Per a 2 of the American cockroach correlates with more clinical severity among airway allergic patients in Taiwan. Ann Allergy Asthma Immunol. 2012;108:243–248. doi: 10.1016/j.anai.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Kang BC, Johnson J, Morgan C, Chang JL. The role of immunotherapy in cockroach asthma. J Asthma. 1988;25:205–218. doi: 10.3109/02770908809071367. [DOI] [PubMed] [Google Scholar]
- 100.Alonso A, Albonico JF, Mouchian K, Scavini LM, Iraneta SG, Pionetti CH. Imunological changes during cockroach immunotherapy. J Investig Allergol Clin Immunol. 1999;9:299–304. [PubMed] [Google Scholar]
- 101.Srivastava D, Gaur SN, Arora N, Singh BP. Clinicoimmunological changes post-immunotherapy with Periplaneta americana. Eur J Clin Invest. 2011;41:879–888. doi: 10.1111/j.1365-2362.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 102. Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.08.047. This very recent study reports the outcomes of four pilot studies on SLIT and SCIT using B.germanica extract. The results showed that IT with cockroach is safe, and that SCIT appears to be more effective in terms of inducing immunological responses than SLIT. The studies reported in this publication provide the basis for continuing investigation of the efficacy of IT in treatment of cockroach-allergic patients with asthma, rhinitis, or both.
- 103.Pomés A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165:391–397. doi: 10.1164/ajrccm.165.3.2104027. [DOI] [PubMed] [Google Scholar]
- 104.Jeong KY, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Allergenicity of recombinant Bla g 7, German cockroach tropomyosin. Allergy. 2003;58:1059–1063. doi: 10.1034/j.1398-9995.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 105.Jeong KY, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Analysis of amino acid sequence variations and immunoglobulin E-binding epitopes of German cockroach tropomyosin. Clin Diagn Lab Immunol. 2004;11:874–878. doi: 10.1128/CDLI.11.5.874-878.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu CH, Hsieh MJ, Huang JH, Luo SF. Identification of low molecular weight allergens of American cockroach and production of monoclonal antibodies. Ann Allergy Asthma Immunol. 1996;76:195–203. doi: 10.1016/S1081-1206(10)63422-9. [DOI] [PubMed] [Google Scholar]
- 107.Wu CH, Wang NM, Lee MF. Sequencing analysis of a cDNA encoding the American cockroach (Periplaneta americana) Cr-PII allergen. Allergy Clin Immunol Int Suppl. 1997;4:11. [Google Scholar]
- 108.Wang NM, Lee MF, Wu CH. Immunologic characterization of a recombinant American cockroach (Periplaneta americana) Per a 1 (Cr-PII) allergen. Allergy. 1999;54:119–127. doi: 10.1034/j.1398-9995.1999.00902.x. [DOI] [PubMed] [Google Scholar]
- 109.Sookrung N, Chaicumpa W, Tungtrongchitr A, Vichyanond P, Bunnag C, Ramasoota P, et al. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ Health Perspect. 2006;114:875–880. doi: 10.1289/ehp.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]