Type 2 diabetes mellitus (T2DM) is associated with macrovascular and microvascular complications as well as an increased risk for cognitive impairment (CI), ranging from mild memory impairment to fully developed dementia (1). Both vascular dementia (VD) and Alzheimer disease (AD) dementia are increased in patients with T2DM. However, it is not clear if T2DM directly causes CI and dementia or if the two disorders share a common pathophysiological process (2).
Insulin resistance, hyperglycemia, and hypoglycemia have all been implicated as risk factors for CI in T2DM (3). However, their relative importance remains uncertain. The possibility that altered brain insulin action plays a role is consistent with human data showing that AD is associated with decreased insulin receptor expression in the brain (4), impaired insulin signaling (5), and decreased insulin levels in the cerebrospinal fluid (CSF) (6). Furthermore, insulin delivery directly into the hippocampus enhances spatial memory in nondiabetic rats, whereas this response to intrahippocampal insulin is impaired in diet-induced obese rats (7). In small clinical trials, intranasal insulin delivery appears to improve memory function in patients with CI and AD (8).
Functional MRI, based on the blood oxygenation level–dependent (BOLD) contrast mechanism, has become an important tool to investigate the brain’s neurophysiologic response to specific stimuli or cognitive tasks. In contrast, resting-state functional MRI (rs-fMRI) measures spontaneous low-frequency oscillations in the BOLD signal (9). This spontaneous brain activity at rest is altered in VD (10) and AD (11) patients, and to a lesser extent in people with CI (11); however, few studies have examined the resting-state brain activity in T2DM (12,13). Musen et al. (13) compared rs-fMRI in T2DM and age-matched diabetic control subjects with no structural brain abnormalities or CI. Using a seed approach to measure brain functional connectivity during the resting state, the authors demonstrated that T2DM patients had decreased brain connectivity, which was associated with homeostasis model assessment of insulin resistance (HOMA-IR), a measure of insulin resistance. Xia et al. (12) found similar results using the rs-fMRI amplitude of low-frequency fluctuation (ALFF) approach. The ALFF approach avoids the need to select a seed region and allows assessment of the whole brain by measuring signal fluctuations over time at the voxel level (14). The hope is that such rs-fMRI signals might be a biomarker for identifying T2DM patients at risk for CI before structural brain abnormalities and/or CI can be identified. Although it is not known if CI is caused by abnormal oscillations in functional activity or if the oscillations are merely a CI biomarker, if such a tool for early CI risk detection was available, it could eventually be used for preventive dementia interventions.
In this issue, Cui et al. (15) examine the rs-fMRI spontaneous low-frequency signal oscillations and their local smoothness in T2DM patients compared with an age-, sex-, and education-matched control group. The authors used the ALFF approach defined above and also the regional homogeneity (ReHo) measure that assesses the ReHo of these oscillatory signals in a small window around each voxel (14). There were no structural brain abnormalities and both T2DM and control subjects showed higher ALFF and ReHo values in the posterior cingulated cortex and the precuneus and medial prefrontal cortex compared with global mean values. However, T2DM subjects exhibited decreased resting brain fluctuations in the occipital lobe, calcarine cortex, and postcentral gyrus, which are somatosensory and visual network systems. Performance in cognitive tests also was reduced in T2DM patients and this was associated with reduced ReHo and ALFF values in the cuneus and lingual gyrus, regions associated with visual memory and word processing (16). Interestingly, HOMA-IR was inversely correlated with neural activation in the parietal lobe, frontal lobe, middle temporal gyrus, and lingual gyrus. These results are in agreement with the two studies mentioned above (12,13), suggesting insulin resistance affects resting-state neuronal activation and may play a pathogenic role in CI in T2DM.
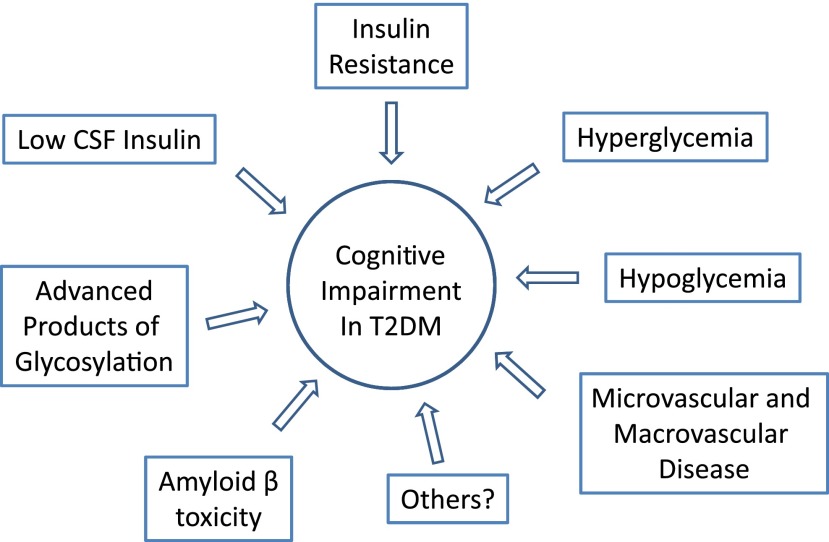
The study by Cui et al. (15) has limitations that could potentially affect data interpretation. Differential exclusion criteria were used for CI. The Montreal Cognitive Assessment, a superior test for detection of mild CI, was used only to exclude control subjects. Cognitive function thus was superior in control subjects (vs. T2DM, P < 0.01) due to selection bias, which could have accounted for some changes seen in T2DM. Another limitation is discrepancies in waist circumference (a marker of abdominal fat and insulin resistance), which was greater in the T2DM subjects. Body weight and BMI were not reported. Obesity has been associated with alterations in rs-fMRI (17) and therefore could influence data independent of T2DM. The T2DM group was also very heterogeneous in terms of duration of disease, glycemic control, and type of diabetes treatment. Furthermore, technical factors could contribute to some differences observed: 1) whether motion was balanced across groups (an influence on ALFF) (18), 2) the high sensitivity of the ALFF approach to vessel flow, and 3) the dependence upon an arbitrary threshold for the number of voxels used in calculating ReHo. Thus, many factors could be affecting the reported changes observed in the resting-state brain activity. There is great need to define the pathophysiology of CI in T2DM. rs-fMRI data may provide a noninvasive approach to detect relatively early changes in neural networks, but little is known about how potential biological mediators, such as insulin resistance, low insulin levels in the CSF, amyloid deposition, advanced products of glycosylation, and vascular disease, affect brain oscillations and/or brain networks and relate to CI (Fig. 1). Moreover, we need to determine if treatment of T2DM or insulin resistance ameliorates rs-fMRI abnormalities and if they directly affect CI. While the Action to Control Cardiovascular Risk in Diabetes–Memory in Diabetes (ACCORD–MIND) study did not show improved cognition after intensive diabetes management (19), hypoglycemia may have limited detection of beneficial outcomes (20). The ongoing studies with intranasal insulin may help clarify some of these issues (8). Undoubtedly, longitudinal studies will be needed to establish whether rs-fMRI changes will correctly identify T2DM patients at risk for CI.
Figure 1.
Schematic representation of the pathophysiological factors associated with the development of CI in patients with T2DM.
The growing aging population and prevalence of obesity and diabetes will likely increase CI and dementia cases in the next few decades. Looking to the future, we need to better understand the mechanisms linking T2DM to CI so that new interventions can be developed to prevent or reverse early stages of CI.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 749.
References
- 1.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger JA. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimers Dis 2012;30(Suppl. 2):S185–S198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan MWR. R D Lawrence Lecture 2010. The brain as a target organ in type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med 2011;28:141–147 [DOI] [PubMed] [Google Scholar]
- 4.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 2005;7:63–80 [DOI] [PubMed] [Google Scholar]
- 5.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 1998;50:164–168 [DOI] [PubMed] [Google Scholar]
- 7.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem 2010;93:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiherr J, Hallschmid M, Frey WH, 2nd, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 2013;27:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–541. [DOI] [PubMed]
- 10.Sun YW, Qin LD, Zhou Y, et al. Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav Brain Res 2011;223:388–394 [DOI] [PubMed] [Google Scholar]
- 11.Binnewijzend MA, Schoonheim MM, Sanz-Arigita E, et al. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2012;33:2018–2028 [DOI] [PubMed] [Google Scholar]
- 12.Xia W, Wang S, Sun Z, et al. Altered baseline brain activity in type 2 diabetes: A resting-state fMRI study. Psychoneuroendocrinology 2013;38:2493–2501 [DOI] [PubMed] [Google Scholar]
- 13.Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 2012;61:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22:394–400 [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Jiao Y, Chen Y-C, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 2014;63:749–760 [DOI] [PubMed] [Google Scholar]
- 16.Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci 2000;267:1909–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tregellas JR, Wylie KP, Rojas DC, et al. Altered default network activity in obesity. Obesity (Silver Spring) 2011;19:2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog RI, Sherwin RS. Diabetes. Can tight glycemic control in diabetes benefit cognition? Nat Rev Neurol 2011;8:124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]