Abstract
Gamma-aminobutyric acid (GABA)-ergic disturbances are hallmark features of schizophrenia and other neuropsychiatric disorders and encompass multiple interneuronal cell types. Using bacterial artificial chromosome-driven, miRNA silencing technology we generated transgenic mouse lines that suppress glutamic acid decarboxylase 1 (GAD1) in either cholecystokinin (CCK)- or neuropeptide Y (NPY)-expressing interneurons. In situ lipidomic and proteomic analyses on brain tissue sections revealed distinct, brain region-specific profiles in each transgenic line. Behavioral analyses revealed that suppression of GAD1 in CCK+ interneurons resulted in locomotor and olfactory sensory changes, whereas suppression in NPY+ interneurons affected anxiety-related behaviors and social interaction. Both transgenic mouse lines had altered sensitivity to amphetamine albeit in opposite directions. Together, these data argue that reduced GAD1 expression leads to altered molecular and behavioral profiles in a cell type-dependent manner, and that these subpopulations of interneurons are strong and opposing modulators of dopamine system function. Furthermore, our findings also support the hypothesis that neuronal networks are differentially controlled by diverse inhibitory subnetworks.
Keywords: behavior, cholecystokinin, GABA, GAD1, MALDI-IMS, neuropeptide Y
INTRODUCTION
Gamma-aminobutyric acid (GABA) system abnormalities have been identified in a number of neuropsychiatric disorders including schizophrenia,1 bipolar disorder,2 autism,3 Rett syndrome,4 and epilepsy.5 Among these, downregulation of glutamic acid decarboxylase 1 (GAD1), the enzyme responsible for producing the majority of the GABA in the brain,6 is a robust and consistent finding in the postmortem brains of subjects with schizophrenia.7 However, what role(s) individual interneuronal cell types might have in normal or dysfunctional behavior are not well established. GABA-ergic interneurons are classified based on their morphology, physiology, receptor expression, brain distribution and molecular markers.8 This diversity allows different classes of interneurons to regulate the input, signal integration, output and population synchrony of principle cells and other interneurons in distinct and dynamic ways.9 Given the variety of interneuron types and the spectrum of behavioral abnormalities among disorders with identified GABA-ergic pathophysiology, it is possible that dysfunction of one or more interneuron classes contributes to the variety of symptoms.
Cholecystokinin (CCK) and neuropeptide Y (NPY) are expressed in nonoverlapping interneuron cell types with distinct morphology, connectivity, firing patterns and distributions in both rodents and humans.9-12 CCK+ interneurons are basket cells that primarily synapse onto cell soma or double bouquet cells that target dendrites12,13 in the amygdala, cortex, hippocampus, hypothalamus and thalamus.11 In contrast, NPY+ interneurons are neurogliaform cells that primarily signal through non-synaptic volume transmission14 and Martinotti cells that synapse onto dendrites15 in the amygdala, cortex, hippocampus, hypothalamus and striatum.10
As both of these distinct interneuron cell types appear to be among those that are affected in subjects with schizophrenia,1 we hypothesized that their dysfunction will lead to molecular and behavioral disturbances that are cell type specific (Figure 1a). To test this, we have developed two transgenic mouse lines that suppress GAD1 expression in CCK+ and NPY+ interneuron populations using novel bacterial artificial chromosome (BAC)-driven miRNA-based silencing technology.16 To uncover molecular and behavioral consequences of GAD1 deficiency in these two distinct interneuronal cell types, we performed in situ proteomic analyses on brain tissue sections and comprehensive behavioral assessments of these transgenic mice.
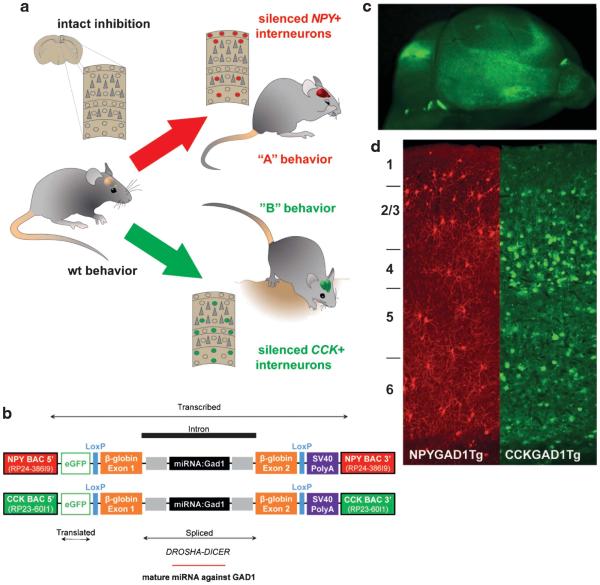
Figure 1.
Cell type-specific suppression of glutamic acid decarboxylase (GAD1) in vivo. In a normal brain, intact inhibitory circuits modulate the function of individual principle cells and complex networks. In this study, we hypothesized that silencing neuropeptide Y (NPY+) (red) or cholecystokinin (CCK+) (green) interneurons would lead to different patterns of behavior (a). To test this hypothesis, we created a synthetic miRNA targeted to silence GAD1, the primary enzyme responsible for gamma-aminobutyric acid synthesis in the brain, and inserted it into two BAC constructs that limited expression of the transgene to NPY+ or CCK+ neurons, respectively (b). These constructs also produced enhanced green fluorescent protein (eGFP) (pseudocolored red for NPY+ cells and green for CCK+ cells for illustration purposes) to visualize and verify construct expression patterns (c,d).
MATERIALS AND METHODS
Mice
NPYGAD1 and CCKGAD1 transgenic mice on congenic C57Bl/6 backgrounds were generated by injecting circular-modified BAC into fertilized mouse oocytes by the Vanderbilt Transgenic Mouse/ESC Shared Resource (http://www.vicc.org/research/shared/tg-mouse.php) and identified using construct-specific PCR primers. modified BACs contained an enhanced green fluorescent protein (eGFP) reporter gene and a minigene with a GAD1-targeting miRNA at the start codon of either the mNpy or mCck genes (described previously16). All experiments were conducted in accordance with Vanderbilt Animal Care and Use Committee guidelines.
Immunohistochemistry
Mice were anesthetized and perfused with ice-cold 1 × PBS followed by 4% phosphate-buffered paraformaldehyde. Brains were post fixed in 4% paraformaldehyde overnight before saturation in phosphate-buffered sucrose concentrations reaching 30%. Fifty micron sections were washed in PBS and blocked in 10% normal donkey serum in 0.1 mM PB (pH 7.4) for 1 h. Primary antibody incubations were for 72 h at 4 °C and secondary incubations were for 3h at room temperature. Secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) were diluted 1:250. For eGFP labeling, sections were incubated with either chicken anti-GFP (Abcam, Cambridge, MA, USA; 1:2000) or rabbit anti-GFP (Invitrogen, Grand Island, NY, USA; 1:2000) and donkey anti-chicken DyLight488 or donkey anti-rabbit DyLight488 secondary. GAD1-stained sections were preincubated with 70 mg ml −1 of monovalent Fab’ fragment of donkey anti-mouse immunoglobulin G (Jackson Immunoresearch) to block endogenous mouse immunoglobulins, then incubated with mouse anti-GAD1 (Millipore, Billerica, MA, USA; 1:2000) and donkey anti-mouse Cy3 secondary. CCK-stained sections were incubated with either rabbit anti-proCCK (a generous gift from Dr Andrea Varro) or rabbit anti-CCK8S (Immunostar, Hudson, WI, USA; 1:1000) and donkey anti-rabbit Cy3 secondary. Images were acquired by fluorescence microscopy (Leica Microsystems, Bannockburn, IL, USA).
Mass spectrometry
Twelve micron coronal sections from 2-month-old naive transgenic mice, NPYGAD1TG (n = 6) and CCKGAD1TG (n = 6), and wild-type (WT) littermates, NPYGAD1WT (n = 3) and CCKGAD1WT n = 3), were thaw mounted onto gold-coated steel targets. Matrix-assisted laser desorption ionization imaging mass spectrometry (MALDI-IMS) was carried out as previously described17 with modifications. Protein identification was performed using liquid chromatography-tandem mass spectrometry as previously described.18
Behavior
A separate cohort of adult male NPYGAD1TG (n = 12), NPYGAD1WT (n = 10), CCKGAD1TG (n = 12) and CCKGAD1WT (n = 12) mice were evaluated on a comprehensive behavioral testing battery. A modified Irwin Screen assessed general health, neuromuscular function and motor coordination.19 Locomotor activity and habituation were measured. Anxiety- or depression-like behavior were measured in the zero maze, forced swim and light-dark box tasks.20 Sensorimotor gating was assessed with prepulse inhibition (PPI) of acoustic startle.21 The y-maze alternation task measured working memory.22 The three-chambered social interaction task was used as described previously23 with minor modifications. A series of nonsocial (orange and almond extract, diluted 1:100 with water, McCormick, Sparks, MD, USA) and social odors (conspecific bedding) were presented by cotton swabs to measure olfactory habituation and social preference.23 Contextual and cued fear conditioning were measured as previously described24 with minor modifications. Finally, amphetamine sensitivity was measured by comparing locomotion before and after injections of 3 mg kg −1 D-amphetamine hemisulfate (Sigma-Aldrich, St Louis, MO, USA) in 0.9% saline solution. Data were collected by automated software (MEDActivity, StartleReflex and VideoFreeze; MEDAssociates, Georgia, VT, USA or ANy-maze; Stoelting, Wood Dale, IL, USA) or by blinded observers.
Statistical analyses
Mass spectrometry P-values were calculated using pairwise two-tailed t-tests and corrected using Benjamini–Hochberg false-discovery rates.25 The magnitude of significant differences was calculated using log2-based average log ratios (ALR) where ALR = mean(log2[NPYGAD1plate 1, section a], log2[NPYGAD1plate 1 section b])-log2[NPYBACWTplate1] for each plate. For behavioral analyses, two-tailed group wise t-tests and two-way repeated measures analysis of variance (ANOVAs) were used to compare transgenic mice with WT littermate controls as appropriate for each test. Social preference was calculated as 100x(novel mouse 1 interaction time–novel object interaction time)/(novel mouse 1 interaction time–novel object interaction time); and 100x(novel mouse 2 interaction time-familiar mouse interaction time)/(novel mouse 2 interaction time-familiar mouse interaction time).
RESULTS
Transgenic mice suppress GAD1 protein expression in targeted subpopulations
Mice were generated containing BAC constructs with promoter-enhancer elements of either the NPY or CCK genes, an eGFP reporter, and a synthetic miRNA-targeting Gad1 mRNA (Figure 1b). These elements restricted eGFP expression and GAD1 suppression to either NPY+ or CCK+ interneurons and made targeted cells fluorescent (Figures 1c and d). Both transgenic lines were generated as previously described.16 Construct expression and GAD1 suppression efficacy were verified with immunohistochemistry in Tg(Npy-eGFP/miRNA:GAD1)1KM16 and Tg(Cck-eGFP/miRNA:GAD1)2KM (Supplementary Figures 1 and 2) transgenic mice, hereafter, referred to as NPYGAD1TG and CCKGAD1TG. These experiments show that the transgene was expressed specifically in NPY+ and CCK+ cells, and these subpopulations had no detectable levels of GAD1 expression.
GAD1 suppression in NPY+ or CCK+ interneurons has differential effects on the lipidome and proteome
To assess molecular changes downstream of GAD1 suppression and whether they are cell type dependent, we performed MALDI-IMS17 on brain tissue sections. Taking advantage of spatial resolution offered by this analysis, we divided sections into 10 regions of interest (Supplementary Figure 3): cortex (divided into CTXH for neocortex in hippocampal sections, CTXS for neocortex in striatal sections and MFC for the cingulate area of striatal sections), corpus callosum (divided into CORPH and CORPS for the respective sections), hippocampus (HIPP), hypothalamus (HYTH), septum (SEP), striatum (STR) and thalamus (THAL). Using this method, we reliably assessed over 400 distinct proteins, peptides and lipids (0–approximately 22 000 Da), in each brain region. GAD1 suppression in NPY+ interneurons lead to significant changes of 129 lipids, peptides or proteins (51 decreased, 65 increased and 13 had region-specific changes; Supplementary Table 1), whereas GAD1 suppression in CCK+ interneurons induced expression changes of 52 lipids, peptides or proteins (25 decreased, 23 increased and 4 had region-specific changes; Supplementary Table 2) compared with WT controls. Perhaps not surprisingly, only 15 results were common to both transgenic lines, but only three of these changed in the same direction (six changed in opposite directions and six had region-specific differences). Highlighting the utility of MALDI-IMS spatial resolution, there were only two peaks (m/z 1583.09 and 1907.27) that were significantly changed in at least three regions in NPYGAD1TG mice (Supplementary Table 1) and none in CCKGAD1TG mice (Supplementary Table 2).
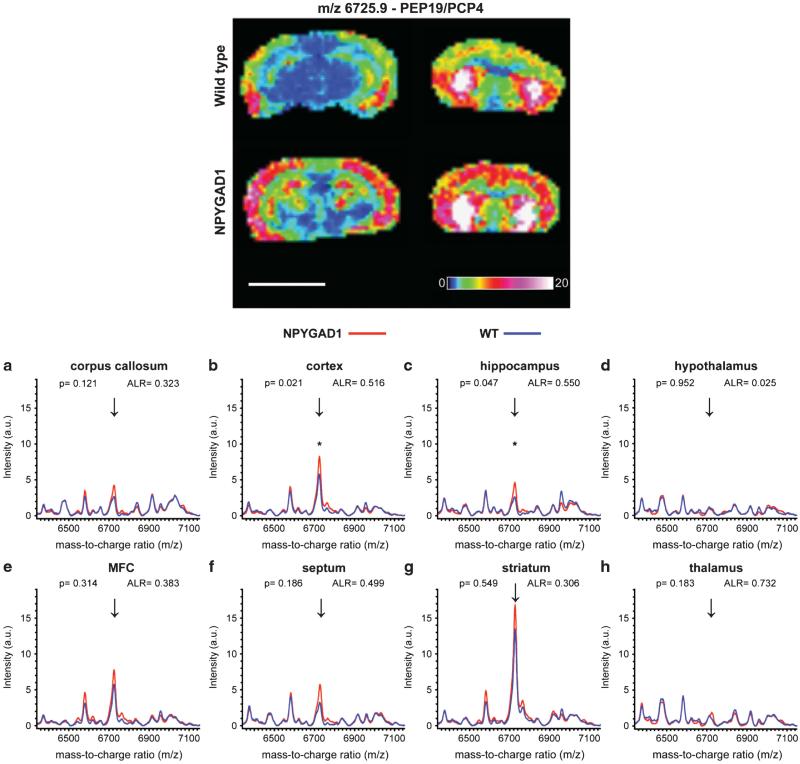
We found that m/z 6725.9 was significantly upregulated in the hippocampus (t(2) = 4.471, P = 0.047) and cortex (t(2) = 6.796, P = 0.021) of NPYGAD1TG mice (Figure 2; Supplementary Table 1). To determine its identity, protein was extracted from tissue sections and analyzed by liquid chromatography-tandem mass spectrometry similar to that described in Schey, et al.18 (data not shown). Using these methods, we conclusively identified m/z 6725.9 as PEP19, also known as PCP4.26 Interestingly, PEP19/PCP4 overexpression has been shown to disrupt neurodevelopment27 and increase neurotransmitter release including dopamine and acetylcholine.26 The peptide was also increased following chronic stress28 or amphetamine administration29 in rodents, whereas clinical gene expression studies found it to be increased in one study of patients with mental illness30 but reduced in another.31 Peaks that were similarly upregulated in the hippocampus and cortex (m/z 1201.9, 1450.2, 1583.1 and 1907.3) were putatively identified as a phosphosphingolipid, a glycerophosphoglycerophosphoglycerol, an acidic glycosphingolipid and a neutral glycosphingolipid, respectively, using the Nature Lipidomics Gateway informatics platform (see Supplementary Methods for details). Further studies are needed to fully identify and evaluate these and other results in the data set.
Figure 2.
Glutamic acid decarboxylase 1 (GAD1) suppression in neuropeptide Y (NPY+) interneurons leads to an increase in the expression of a Da peptide, identified as PEP19/PCP4. Representative matrix-associated laser desorption ionization mass spectrometry (MALDI-IMS) expression intensity image of m/z 6725.9 from wild-type (top row) and NPYGAD1 (bottom row) brain sections on the same MALDI target plate. m/z 6725.9 (arrows) was detected in the corpus callosum (a), cortex (b), hippocampus (c), medial frontal cortex (e), septum (f) and striatum (g). It was not detected in hypothalamus (d) or thalamus (h). m/z 6725.9 was significantly increased in the cortex (b) and hippocampus (c). The peptide was identified as PEP19, also known as PCP4, in a separate experiment. Data points represent mean intensity. ALR, average log ratio. *P < 0.05; a.u., arbitrary units. Scale bar, 5 mm.
GAD1 suppression in NPY+ or CCK+ interneurons has differential effects on behavior
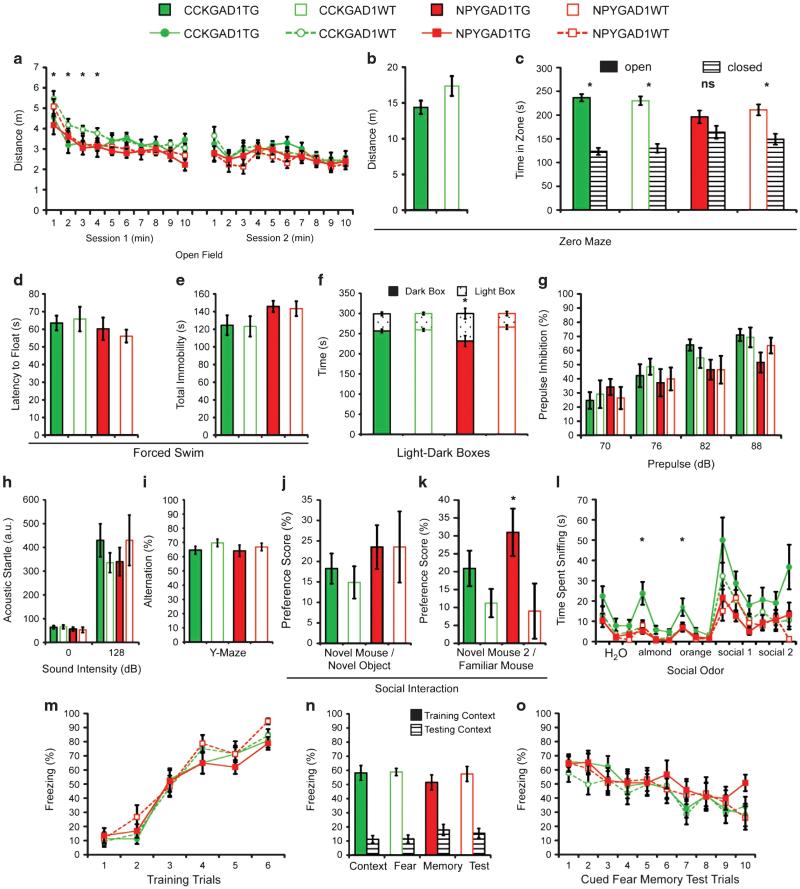
Adult male mice were evaluated using a battery of tasks chosen to assess a broad range of behavior (see Supplementary Methods for full details). NPYGAD1 and CCKGAD1 lines were tested independently using identical testing parameters and compared against WT littermates. Mice were visually indistinguishable and did not display any general health or neuromuscular problems (Supplementary Figure 4a,b). CCKGAD1TG mice exhibited decreased locomotor activity during the initial portion of the open-field test (two-way repeated measures ANOVA time × genotype interaction, F(9,198) = 2.0938, P = 0.032; Figure 3a). All groups displayed normal habituation to the open-field arena (two-way repeated measures ANOVA main effect of time: NPYGAD1 F (9,180) = 12.2300, P = 0.000; CCKGAD1 F(9,198) = 10.5489, P = 0.000). CCKGAD1TG mice also trended toward hypoactivity on the elevated zero maze (two-tailed independent samples t-test: t(22) = −1.7822, P = 0.089; Figure 3b).
Figure 3.
Glutamic acid decarboxylase 1 (GAD1) suppression has a cell type-specific impact on behavior. Adult male mice were evaluated in an extensive behavioral testing battery. Mice with GAD1-suppressing bacterial artificial chromosome (BAC) constructs in cholecystokinin (CCK +) (CCKGAD1TG, n = 12) or neuropeptide Y (NPY+) (NPYGAD1TG, n = 12) interneurons displayed different patterns of behavior compared with wild-type (WT) littermate controls (CCKGAD1WT, n = 12; NPYGAD1WT, n = 10). CCKGAD1 mice were hypoactive in the open field (a) and on the zero maze (b). NPYGAD1TG mice did not spend more time in the closed zone of the zero maze (c). There were no difference in latency to float (d) or total immobility (e) in the forced swim test. NPYGAD1TG mice had a significantly reduced preference for the dark box in the light-dark box anxiety test (f). There were no differences in prepulse inhibition (g) or acoustic startle response (h) (a.u., arbitrary units). All groups displayed normal alternation in the y-maze (i). In the three-chambered social task, both mouse lines displayed normal sociability (j); however, NPYGAD1TG mice had a significantly increased preference for social novelty (k). CCKGAD1TG mice spent significantly more time investigating a nonsocial olfactory stimulus but all groups investigated social olfactory stimuli similarly (l). Finally, there were no differences in learning (m) or memory of contextual (n) or auditory cued (o) fear conditioning or cued fear extinction (o). *P o 0.05; NS, nonsignificant; a.u., arbitrary units.
We assessed anxiety-like behavior using the elevated zero maze and light-dark box paradigms.20 NPYGAD1TG mice displayed reduced anxiety-like behavior in both paradigms. Mice typically spend significantly more time in the closed zone than the open zone of the zero maze;20 however, NPYGAD1TG mice failed to do so (paired t-test: t(11) = 1.207, P = 0.253; Figure 3c). Similarly, they had a significantly reduced aversion to the light box compared with littermate controls (two-tailed independent samples t-test: t(20) = − 2.247, P = 0.036; Figure 3f).
The Porsolt forced swim task20 measured depression-like behaviors by the duration each animal attempted to escape after being placed in water (latency to float) and the total immobility time during the session. There were no differences between groups in either latency to float (independent samples t-test: NPYGAD1 t(20) = −0.538, P = 0.596; CCKGAD1 t(22) = 0.279, P = 0.783; Figure 3d) or total immobility (independent samples t-test: NPYGAD1 t(20) = −0.291, P = 0.774; CCKGAD1 t(22) = −0.078, P = 0.939; Figure 3e).
PPI tested the animals’ sensorimotor-gating capabilities21. All groups showed appropriate levels of inhibition to each of four prepulse levels and there were no significant differences in percent PPI (two-way repeated measures ANOVA: NPYGAD1 F (19) = 0.036, P = 0.852; CCKGAD1 F(22) = 0.000, P = 0.998; Figure 3g) or in baseline acoustic startle without the prepulse (independent samples t-test: NPYGAD1 t(19) = − 0.788, P = 0.440; CCKGAD1 t(22) = 1.166, P = 0.256; Figure 3h). Data from one NPYGAD1TG mouse was removed due to equipment malfunction.
We tested hippocampal function using y-maze spontaneous alternation.22 All groups alternated at the expected level and there were no differences between groups (independent samples t-test: NPYGAD1 t(20) = − 0.551, P = 0.587; CCKGAD1 t(22) = − 1.370, P = 0.184; Figure 3i).
Social behavior was evaluated using the three-chamber social task.23 After an acclimation phase, the first interaction phase tested sociability by comparing preference for investigating a social stimulus mouse (first novel mouse) placed in a pencil cup in one chamber instead of an empty cup in the opposite chamber (novel object). The second interaction phase measured preference for social novelty by introducing a second stimulus mouse (second novel mouse) and comparing interaction time with the second novel mouse versus the first novel mouse (now the ‘familiar’ mouse). All groups showed preferences for the first novel mouse over the novel object (Figure 3j). However, during the second interaction phase, NPYGAD1TG mice showed significantly stronger preferences for social novelty (two-tailed independent samples t-test; t(20) = 2.178, P = 0.042; Figure 3k).
In addition to social interaction, we tested olfactory sensory capability and sensitivity to social cues.23 Mice were presented with consecutive social and nonsocial odors. CCKGAD1TG mice spent significantly more time sniffing almond (two-way repeated measures ANOVA, F(1,22) = 7.231, P < 0.05) and orange (two-way repeated measures ANOVA, F(1,22) = 5.831, P < 0.05) nonsocial odors than littermate controls (Figure 3l) and showed a trend toward increased investigation of social odors (two-way repeated measures ANOVA, F(1,22) = 3.035, P = 0.095).
Contextual and cued fear conditioning assessed hippocampus- and amygdala-dependent learning and memory in a single task.24 All groups rapidly learned to associate the tone with the shock and reached high levels of freezing (Figure 3m). All groups displayed appropriate contextual fear memory, defined by elevated freezing during the initial 3 min in the training context compared with the testing context (Figure 3n). All groups displayed appropriate cued fear memory, defined by increased freezing behavior during the tone relative to the novel context baseline (Figure 3o). In addition, a significant decrease in freezing with repeated cue exposures in the absence of the shock indicated appropriate fear extinction (Figure 3o).
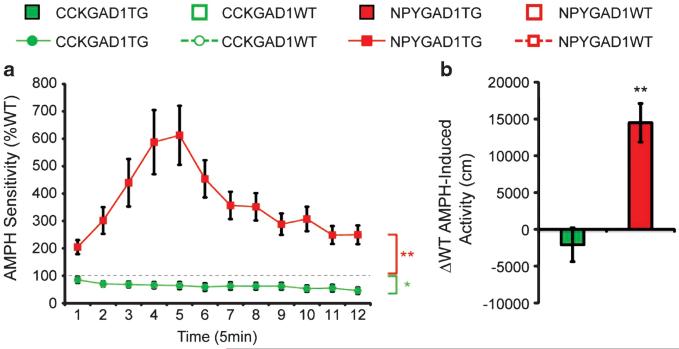
To assess the response of dopaminergic circuitry to psychostimulants, we measured locomotor responses following an amphetamine injection (3 mg kg −1). Amphetamine sensitivity was defined as the change in locomotor activity following amphetamine injection. NPYGAD1TG mice displayed ~600% greater peak responses to amphetamine than littermate controls (two-way repeated measures ANOVA, time × genotype interaction F(11) = 6.3646, P = 0.000), whereas, in a separate experiment, CCKGAD1TG mice showed an ~50% reduced peak response (two-way repeated measures ANOVA, time × genotype interaction F(11) = 2.015, P = 0.028; Figure 4a). NPYGAD1TG mice displayed significantly greater amphetamine-induced locomotor activity compared with WT controls over the entire session (t(20) = 4.477, P = 0.000; Figure 4b). Stereotypies were not different (data not shown).
Figure 4.
Glutamic acid decarboxylase 1 (GAD1) suppression has cell type-specific augmentation or attenuation of amphetamine (AMPH)-induced locomotion. All mice displayed increased locomotor activity in response to a 3 mg kg −1 injection of amphetamine. AMPH sensitivity was defined as the magnitude of the locomotor response after AMPH injection relative to baseline locomotor activity. NPYGAD1TG mice were ~600% more sensitive to AMPH compared with wild-type (WT) littermate controls, whereas CCKGAD1TG mice were ~50% less sensitive (a). Total locomotor activity across the entire post-injection session was decreased in CCKGAD1TG mice and increased in NPYGAD1TG mice compared with WT littermate controls (b). **P < 0.01; *P < 0.05.
DISCUSSION
GAD1 downregulation has been shown to effectively silence individual interneurons32 and has been described as a primary mechanism of behavioral dysfunction in other animal models.33,34 However, the molecular and behavioral consequences of restricted GAD1 suppression in distinct interneuron populations have not been well understood to date. Our results are very informative in this regard and several interesting conclusions can be drawn from these experiments. First, suppressing GAD1 in as few as 8–17% of GABA-ergic interneurons is sufficient to induce molecular and behavioral changes that are dependent on interneuron cell types affected. Second, as a result of interneuron suppression, large numbers of lipids, peptides, and proteins undergo adaptive molecular changes in multiple, unaltered cell types. Third, given the small percentage of overlapping proteomic changes across multiple brain regions, the effects of interneuron dysfunction are region specific. Fourth, altered expression of other proteins linked to psychiatric disorders suggests that the observed changes might be directly related to human disease. Fifth, GAD1 suppression in NPY+ and CCK+ interneurons leads to opposing effects on dopamine-dependent behavior highlighting the complex interplay between GABA-ergic and dopaminergic systems. Finally, the pattern of behavioral results raises the possibility that dysfunction of distinct interneuron classes is related to different psychiatric spectrum domains. It is also noteworthy that our study highlights the power of coupling the MALDI-IMS high-throughput approach with brain histology for better understanding region-specific molecular events across various animal models.
Previous studies have detailed interneuron cell type diversity to the extent of their synaptic contacts onto principle cells and other interneurons.8,9 CCK+ and NPY+ interneurons represent only 8–17% of interneurons35,36 and an even smaller percentage of all neurons. Yet, altering their GABA-ergic phenotype is sufficient to induce changes in considerable numbers of lipids, peptides and proteins. As these two nonoverlapping interneuronal subpopulations have vastly different morphology, physiology, receptor expression, brain distribution and molecular content, it is perhaps not surprising that their inactivation results in fundamentally different molecular changes. However, it was somewhat unexpected that a single-molecular manipulation (GAD1 suppression) led to region-specific molecular changes in both lines. Of these, we found increased PEP19/PCP4 in the hippocampus and cortex of NPYGAD1TG mice as a result of GAD1 suppression (1.55-fold, P = 0.047 and 1.516-fold, P = 0.021, respectively), but not in CCKGAD1TG animals. Discovering altered expression of a peptide previously linked to neurodevelopment,27,28 dopamine system function26,29 and neuropsychiatric disorders28,30,31 as a result of empirically modifying another7 supports the concept that diverse genetic factors can converge onto common molecular and behavioral dysfunction.37-39
Recent findings suggest that GAD1 expression and behavioral dysfunction are tightly correlated and subtle decreases in GABA signaling give rise to behavioral changes.33,40 Our data suggest that this modulation is dependent on interneuron cell types and may be region specific. Some of our findings could have relatively straightforward interpretations. For example, increased olfactory investigation in CCKGAD1TG mice may be explained by disrupting the inhibition of primary olfactory cortex that has high densities of CCK+ interneurons.11,41 Yet our most intriguing finding, the opposing effects of amphetamine on locomotor behavior, is likely to be far more complex. We propose that the opposite response of NPYGAD1TG and CCKGAD1TG mice to amphetamine is due to alterations in dopaminergic tone as a result of hippocampal dysfunction. NPY+ and CCK+ interneurons are both distributed in the hippocampus,10,11 but serve very different functions. NPY+ neurogliaform cells maintain tonic inhibition of entire regions through volume transmission.14,42 Disinhibiting hippocampal circuits by suppressing GAD1 in these cells can drive the activity of dopamine neurons in the ventral–tegmental area,43 resulting in increased sensitivity to amphetamine and reduced anxiety-like behaviors.44 In contrast, CCK+ basket cells regulate parvalbumin+ basket cells through synaptic contacts.45 By disinhibiting PV+ cells, GAD1 suppression in CCK+ interneurons could result in a net increase of inhibitory tone in the hippocampus that would diminish hippocampal-ventral–tegmental area loop signaling and lead to locomotor hypoactivity and reduced sensitivity to amphetamine. Alternatively, the divergent behavioral results could be explained by disrupting amygdalar46 or striatal circuits;47 however, GAD1 suppression in the amygdala alone does not change anxiety-like behavior,40 and tonically disinhibiting the striatum would likely augment the hippocampal-ventral–tegmental area loop activity mentioned above. Clearly, these hypotheses will have to be tested in further experiments. Regardless of the exact mechanism at work, it is fascinating to consider that specific GABA-ergic circuits can modulate and potentially unbalance the dopamine system in opposite directions and what that could mean for behavioral spectrum disorders.
Our data also have broader, conceptual implications. The idea of the anatomical substrate defining specific brain function has been around for almost a century: in 1929, Herrick proposed that fundamental anatomical building blocks govern complex behavioral responses.48 More recently, a hypothesis emerged that large networks can be controlled by diverse subnetworks,49 and further evidence suggests that different network dynamics of interneuron classes underlie behavioral function.50 We believe that our findings provide strong behavioral and molecular support for this view while expanding the scope beyond a single brain region and elaborating upon the behavioral impact of cell type-specific interneuron dysfunction. In this context, we can consider the interneurons as anatomically encoded, critical modular building blocks that are directly responsible for various behavioral domains. Thus, silencing different inhibitory subnetworks, driven by various inhibitory interneuronal subclasses, will lead to different behavioral phenotypes. Furthermore, modulating the inhibitory subnetworks will not only alter the behavior, but also result in brain and lipid composition disruption in the homogenous excitatory network. We also hypothesize that in pathological conditions, such as schizophrenia, the modular building blocks of the inhibitory subnetworks might be related to various symptom domains, either by direct effect on inhibitory subnetworks, or indirectly, through secondary effects on the homogenous excitatory network.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Vanderbilt Transgenic Mouse/Embryonic Stem Cell Shared resource for generating the transgenic animals, the Vanderbilt Murine Neurobehavioral Laboratory, especially Gregg Stanwood and John Allison, for consultation on behavioral tasks and equipment use, the Proteomics Core of the Mass Spectrometry Research Center at Vanderbilt University, especially David Anderson and Kristie Rose, for assistance with the identification of PEP19, and Andrea Varro from the University of Liverpool for her generosity in sharing the proCCK antibodies. This work was supported by National Institutes of Health R01 MH067234 and by the Vanderbilt Kennedy Center. MJS was supported by a Vanderbilt Brain Institute Scholar Award.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Detailed methods are described in Supplementary Methods.
REFERENCES
- 1.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 3.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 4.Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol. 1999;156:345–352. doi: 10.1006/exnr.1999.7030. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd KG, Bossi L, Morselli PL, Munari C, Rougier M, Loiseau H. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- 6.Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 8.Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 10.Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 11.Meziane H, Devigne C, Tramu G, Soumireu-Mourat B. Distribution of cholecystokinin immunoreactivity in the BALB/c mouse forebrain: an immunocytochemical study. J Chem Neuroanat. 1997;12:191–209. doi: 10.1016/s0891-0618(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 12.Hornung JP, De Tribolet N, Tork I. Morphology and distribution of neuropeptide-containing neurons in human cerebral cortex. Neuroscience. 1992;51:363–375. doi: 10.1016/0306-4522(92)90321-r. [DOI] [PubMed] [Google Scholar]
- 13.Freund TF, Magloczky Z, Soltesz I, Somogyi P. Synaptic connections, axonal and dendritic patterns of neurons immunoreactive for cholecystokinin in the visual cortex of the cat. Neuroscience. 1986;19:1133–1159. doi: 10.1016/0306-4522(86)90129-6. [DOI] [PubMed] [Google Scholar]
- 14.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, et al. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbett KA, Horvath S, Ebert PJ, Schmidt MJ, Lwin K, Mitchell A, et al. Novel animal models for studying complex brain disorders: BAC-driven miRNA-mediated in vivo silencing of gene expression. Mol Psychiatry. 2010;15:987–995. doi: 10.1038/mp.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods. 2007;4:828–833. doi: 10.1038/nmeth1094. [DOI] [PubMed] [Google Scholar]
- 18.Schey KL, Anderson DM, Rose KL. Spatially-directed protein identification from tissue sections by top-down LC-MS/MS with electron transfer dissociation. Anal Chem. 2013;85:6767–6774. doi: 10.1021/ac400832w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 20.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 21.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 22.Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 23.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DR, Gallagher M, Stanton ME. Genetic background differences and non-associative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 26.Harashima S, Wang Y, Horiuchi T, Seino Y, Inagaki N. Purkinje cell protein 4 positively regulates neurite outgrowth and neurotransmitter release. J Neurosci Res. 2011;89:1519–1530. doi: 10.1002/jnr.22688. [DOI] [PubMed] [Google Scholar]
- 27.Mouton-Liger F, Thomas S, Rattenbach R, Magnol L, Larigaldie V, Ledru A, et al. PCP4 (PEP19) overexpression induces premature neuronal differentiation associated with Ca(2-) /calmodulin-dependent kinase II-delta activation in mouse models of Down syndrome. J Comp Neurol. 2011;519:2779–2802. doi: 10.1002/cne.22651. [DOI] [PubMed] [Google Scholar]
- 28.Daniels WM, Marais L, Stein DJ, Russell VA. Exercise normalizes altered expression of proteins in the ventral hippocampus of rats subjected to maternal separation. Exp Physiol. 2012;97:239–247. doi: 10.1113/expphysiol.2011.061176. [DOI] [PubMed] [Google Scholar]
- 29.Romanova EV, Lee JE, Kelleher NL, Sweedler JV, Gulley JM. Comparative peptidomics analysis of neural adaptations in rats repeatedly exposed to amphetamine. J Neurochem. 2012;123:276–287. doi: 10.1111/j.1471-4159.2012.07912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teyssier JR, Ragot S, Chauvet-Gelinier JC, Trojak B, Bonin B. Activation of a DeltaFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011;133:174–178. doi: 10.1016/j.jad.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Guillozet-Bongaarts AL, Hyde TM, Dalley RA, Hawrylycz MJ, Henry A, Hof PR, et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry advance online publication. 2013 Mar 26; doi: 10.1038/mp.2013.30. doi:10.1038/mp.2013.30 (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2013 doi: 10.1093/schbul/sbs195. doi:10.1093/schbul/sbs195 (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirnics K, Levitt P, Lewis DA. Critical appraisal of DNA microarrays in psychiatric genomics. Biol Psychiatry. 2006;60:163–176. doi: 10.1016/j.biopsych.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 39.Horvath S, Mirnics K. Breaking the gene barrier in schizophrenia. Nat Med. 2009;15:488–490. doi: 10.1038/nm0509-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry. 2012;2:e181. doi: 10.1038/tp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekstrand JJ, Domroese ME, Feig SL, Illig KR, Haberly LB. Immunocytochemical analysis of basket cells in rat piriform cortex. J Comp Neurol. 2001;434:308–328. [PubMed] [Google Scholar]
- 42.Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, et al. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci. 2010;30:9898–9909. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neuro. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrick CJ. Anatomical patterns and behavior patterns. Physiol Zool. 1929;II:439–448. [Google Scholar]
- 49.Liu YY, Slotine JJ, Barabasi AL. Controllability of complex networks. Nature. 2011;473:167–173. doi: 10.1038/nature10011. [DOI] [PubMed] [Google Scholar]
- 50.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.