Abstract
OBJECTIVE
Liraglutide (glucagon-like peptide-1 [GLP-1] receptor agonist) and sitagliptin (dipeptidyl peptidase-4 inhibitor) are approved in Japan for treating type 2 diabetes mellitus (T2DM). We compared the efficacy and safety of adding liraglutide or sitagliptin to a sulfonylurea in Japanese T2DM patients.
METHODS
Patients aged 18 to <80 years with hemoglobin A1c (HbA1c; National Glycohemoglobin Standardization Program [NGSP]) of 6.9–9.4%, body mass index ≤35 kg/m2, and treatment with a sulfonylurea and/or one or two non-sulfonylurea oral antidiabetic drugs for greater than or equal to eight weeks before enrollment were eligible. Patients were randomized in an open-label manner to either 0.9 mg/day liraglutide (n = 50) or 50–100 mg/day sitagliptin (n = 49) and were treated for 24 weeks. Non-sulfonylureas were discontinued before randomization. Patients using other oral antidiabetic drugs started sulfonylurea treatment. The primary endpoint was the change in HbA1c from baseline to Week 24.
RESULTS
HbA1c decreased in both groups, and the reduction was significantly greater throughout in the liraglutide group except for Week 24 (0.59 ± 0.80 vs. 0.24 ± 0.94%; P = 0.0525). Fasting plasma glucose (FPG) decreased significantly in the liraglutide group compared with the sitagliptin group (−21.15 ± 31.22 vs. +0.46 ± 39.39 mg/dL; P = 0.0014). Homeostasis model assessment of β cell function and C-peptide increased significantly in the liraglutide group but not in the sitagliptin group. Hypoglycemic symptoms and adverse events occurred in four and nine patients, respectively, in the liraglutide group, and in two and five patients, respectively, in the sitagliptin group.
CONCLUSION
Treatment with liraglutide or sitagliptin together with a sulfonylurea improved HbA1c in Japanese T2DM patients in primary care. Both drugs were associated with low rates of adverse events and hypoglycemia. The improvement in β cell function probably contributed to the improvement in glycemic control in the liraglutide group.
Keywords: liraglutide, sitagliptin, sulfonylurea, RCT, Type 2 diabetes, β Cell function, C peptide
Introduction
The therapeutic efficacy of incretin-related drugs is thought to be greater in Japanese patients with type 2 diabetes mellitus (T2DM) than in Western patients, primarily because of inadequate secretion of insulin.1,2
In Japan, the maximum approved dose of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, is 0.9 mg/day. In clinical trials, this dose achieved good glycemic control when used alone3–6 or in combination with a sulfonylurea.7 In Western countries, liraglutide may be administered at higher doses of 1.2 or 1.8 mg/day,8 and substantial evidences have shown that liraglutide is effective in glycemic control alone,9 or in combination with a sulfonylurea10 and with metformin plus a sulfonylurea.11
Sitagliptin, a dipeptidyl peptidase-4 inhibitor that also targets the incretin system, has also been approved in Japan. Its approved dose is 100 mg/day, which is the same as that used in Western countries. Intriguingly, the dose of sitagliptin was not adjusted in Japanese patients, despite the possible risk of hypoglycemia when used in combination with a sulfonylurea.12
Although several studies have compared liraglutide and sitagliptin in terms of efficacy and safety, these studies were performed in Western countries and enrolled patients with inadequate glycemic control despite treatment with metformin.13,14 Therefore, from a clinical perspective, it is important to compare efficacy and safety, especially hypoglycemia, between liraglutide and sitagliptin at doses approved for Japanese patients with T2DM and inadequate glycemic control despite treatment with a sulfonylurea.
In 2001, the Japan Diabetes Clinical Data Management Study Group (JDDM) was established with the objective to conduct clinical research of diabetes in Japan.15–18 The JDDM performed this 24-week, randomized, parallel-group, open-label study in primary care settings. Japanese patients with T2DM and inadequate glycemic control on a sulfonylurea and/or other oral antidiabetic drugs were treated with 0.9 mg liraglutide or 50–100 mg sitagliptin. The efficacy of these two treatments was compared in terms of hemoglobin A1c (HbA1c) and other indices, including markers of pancreatic β cell function and cardiovascular biomarkers of glycemic/metabolic control. We also compared their safety profiles in terms of hypoglycemia and adverse events. The effects of both drugs on quality of life were evaluated using the problem areas in diabetes (PAID) questionnaire. We chose this tool because it was developed to compare diabetes-related psychosocial distress between injectable and oral therapies,19–21 and is therefore appropriate in the present study that compared an injectable drug (liraglutide) and an oral drug (sitagliptin).
Methods
This 24-week, open-label, randomized, parallel-group study was conducted at 21 primary care centers in Japan. The study consisted of a 4-week screening/run-in phase from Visit 1 and a 24-week treatment phase from Visit 2. The present study was approved by the Ethics Committee of the JDDM, as previously described.15–18 All patients provided informed consent at Visit 1 in accordance with the Guidelines for Epidemiological Studies of the Ministry of Health, Labour and Welfare of Japan. The study was registered on the University Hospital Medical Information Network (identifier: UMIN000004970).
Patients
Patients with T2DM who satisfied the following criteria at Visit 1 (Week–4) were eligible for this study: able to attend outpatient visit every one month, age 18 to <80 years, being educated to continue diet and exercise therapy, treatment with a sulfonylurea and/or one or two other oral antidiabetic drugs for greater than or equal to eight weeks before Visit 1, HbA1c (National Glycohemoglobin Standardization Program [NGSP]) 6.9–9.4%, and body mass index ≤35 kg/m2. The sulfonylurea was administered at a stable and approved dose (2–6 mg glimepiride, 2.5–10 mg glibenclamide, or 80–160 mg gliclazide). Other oral antidiabetic drugs were discontinued from Visit 2. Patients using non-sulfonylurea oral antidiabetic drugs before enrollment started a sulfonylurea at the same time as starting the allocated study drug. The type and dose of the sulfonylurea was at the physician’s discretion. Therefore, patients only received a sulfonylurea in combination with the allocated study drug during the treatment period. Patients with any of the following criteria were excluded from the study: tendency toward repeated hypoglycemia unawareness or clinically significant hypoglycemia; maculopathy requiring urgent treatment; proliferative retinopathy; hepatic dysfunction (aspartate aminotransferase >80 IU/L or alanine aminotransferase >80 IU/L) or a past history of liver fibrosis/cirrhosis; renal impairment (estimated glomerular filtration rate <60 mL/minute/1.73 m2); known allergy to the test drugs or related products; current or history of malignant tumor with recurrence strongly suspected; women who were pregnant, breast-feeding (within one year after delivery), or intended to become pregnant; participation in another clinical trial within 12 weeks of Visit 1; treatment with liraglutide or sitagliptin within 12 weeks of Visit 1; treatment with insulin within 12 weeks of Visit 1 (patients who had used insulin for less than or equal to seven days in the last 12 weeks were eligible); current or planned systemic steroid treatment; and patients who were considered to be unsuitable for this study at the attending physician’s discretion. P-values below 5% (two-tailed) were considered to be significant. All analyses were performed with the statistical software package SPSS (SPSS Japan Inc., Tokyo, Japan).
Treatments
The subjects were randomized at Visit 2 (Week 0) when either liraglutide or sitagliptin was started. The liraglutide dose was started at 0.3 mg, and was increased to 0.6 mg at Week 1 and to 0.9 mg at Week 2. However, if necessary, the dose increase could be delayed in the event of gastrointestinal disorders or other tolerability issues. Sitagliptin was started at 50 mg/day and was increased to 100 mg/day after taking into account the occurrence of hypoglycemia and changes in glycemic control.
For patients using a sulfonylurea, the dose could be reduced at the physician’s discretion within one week of Visit 2. Glimepiride could be reduced to ≤2.0 mg, glibenclamide to ≤1.25 mg, and gliclazide to ≤40 mg according to recommendations for the use of GLP-1 receptor agonists and DPP-4 inhibitors in combination with a sulfonylurea. At Visit 4 and thereafter, the sulfonylurea dose could be increased or decreased at the physician’s discretion, taking into account the patient’s blood glucose level and risk of hypoglycemia. Otherwise, the dose used previously was continued without change.
The subjects were instructed to continue diet therapy and, if deemed necessary, exercise therapy, throughout the study. The patients were prohibited from using oral antidiabetic drugs (except sulfonylureas) and insulin.
Follow-up visits after starting treatment were scheduled every month for 24 weeks.
Endpoints
The primary efficacy endpoint was the change in HbA1c from baseline to Week 24. HbA1c values were originally determined in Japan Diabetes Society values and were converted to NGSP values using the following certified equation19: HbA1c (%, NGSP) = HbA1c (%, JDS) + 0.4%. Secondary efficacy endpoints included the following: fasting blood glucose levels, proportions of patients achieving HbA1c (NGSP) <6.9% and ≤7.4%, body weight, markers of pancreatic β cell function (homeostatic model assessment of β cell function [HOMA-β] and proinsulin/insulin ratio), cardiovascular biomarkers (N-terminal prohormone of brain natriuretic peptide [NT-proBNP], high-sensitivity C-reactive protein [hsCRP]), blood pressure, and the PAID questionnaire. Safety was evaluated in terms of hypoglycemic events and adverse events, which were evaluated by interviews at each visit.
PAID questionnaire
The PAID questionnaire was completed at baseline and at Week 24 to assess diabetes-related psychosocial distress. The PAID questionnaire consists of 20 items aimed at assessing the perspective of patients to their current emotional burden of diabetes and its treatments.20–22 The questionnaire has been extensively validated as a clinical tool and an outcome measure. The questionnaire was translated and validated in Japanese,23 and has been used in several clinical studies in Japan.24,25 In the present study, each item was scored using a five-point Likert-type scale, where 1 = not a problem and 5 = a serious problem, and the sum score was calculated as previously described.24
Statistical analysis
The sample size required to provide statistical power of 80% was calculated to be 64 patients per group based on a mean difference of −0.5% and a common standard deviation of 1.0%. Based on the assumption that the discontinuation/withdrawal rate was 15%, we intended to enroll 76 subjects per group (152 subjects in total).
Efficacy was evaluated in the per-protocol set (PPS), which consisted of all patients who complied with the study protocol. Missing data were not substituted. Changes in clinical variables after treatment of allocated drugs from the baseline values were analyzed by paired t-test except for PAID score in which Wilcoxon rank sum test was used. For comparisons of efficacy variables between the two groups, analysis of covariance (ANCOVA) was used with the baseline value as a covariate and treatment group as a fixed effect. Proportions of patients with HbA1c <6.9% or <7.4% were compared using χ2 tests. Safety was evaluated in the intent-to-treat (ITT) set, which consisted of all randomized patients. P-values under 5% (two-tailed) were considered to be significant. All analyses were performed with the statistical software package SPSS (SPSS Japan Inc., Tokyo, Japan).
Results
Patients
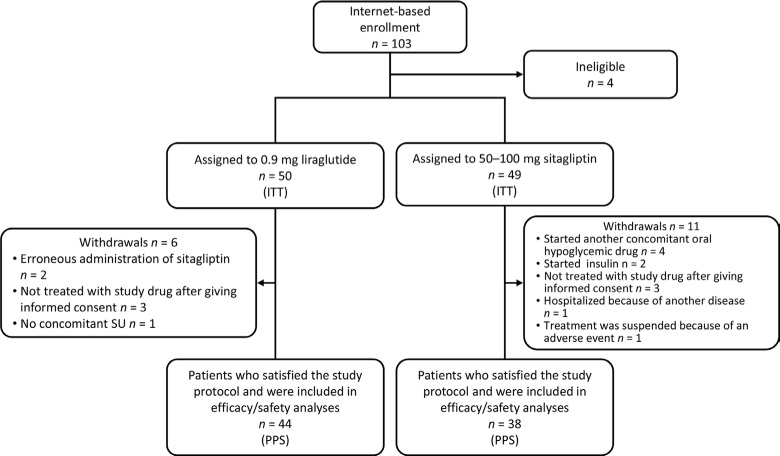
Between July 2010 and October 2012, 103 patients were registered in an internet-based case management system by the participating physicians. Of these, 99 were eligible and were randomized to either liraglutide (n = 50) or sitagliptin (n = 49) (Fig. 1). In all, 6 patients in the liraglutide group discontinued treatment and 44 patients completed the study. A total of 11 patients in the sitagliptin group discontinued treatment and 38 patients completed the study. Therefore, 82 patients were included in the PPS.
Figure 1.
Patient disposition.
Abbreviations: ITT, intent-to-treat; PPS, per-protocol set.
The baseline characteristics of the patients in both groups are presented in Table 1, in which no statistical differences were found between the two. Overall, 11 patients were using drugs other than a sulfonylurea before enrollment, with a biguanide used alone in six patients, an α-glucosidase inhibitor (α-GI) used alone in two patients, a biguanide plus an α-GI used in one patient, an α-GI plus a pioglitazone used in one patient, and a biguanide plus a pioglitazone in one patient. All of these patients switched to a sulfonylurea at randomization to the study drugs.
Table 1.
Patient characteristics at baseline.
| LIRAGLUTIDE | SITAGLIPTIN | |
|---|---|---|
| n | 50 | 49 |
| Males | 33 (66.0) | 32 (65.3) |
| Females | 17 (34.0) | 17 (34.7) |
| Age (years) | 61.1 ± 8.6 | 61.5 ± 9.7 |
| Body weight (kg) | 69.35 ± 12.68 | 67.86 ± 12.52 |
| BMI (kg/m2) | 26.00 ± 3.33 | 25.75 ± 3.42 |
| Duration of disease (years) | 11.68 ± 7.20 | 10.99 ± 6.69 |
| Prior therapy | ||
| SU alone | 25 | 21 |
| SU plus 1 other drug | 13 | 15 |
| SU plus 2 other drugs | 6 | 8 |
| Other drugs | 6 | 5 |
| SU dose | ||
| Low | 25 (53.2) | 22 (47.8) |
| High* | 22 (46.8) | 24 (52.2) |
| Hba1c (NGSP, %) | 7.69 ± 0.89 | 7.92 ± 1.02 |
| FPG (mg/dL) | 143.04 ± 35.85 | 146.09 ± 36.04 |
| F-IRI (μU/mL) | 7.46 ± 4.96 | 7.95 ± 5.89 |
| F-proinsulin (μU/mL) | 23.53 ± 14.70 | 26.44 ± 16.73 |
| HOMA-R | 2.66 ± 1.96 | 3.03 ± 2.41 |
| HOMA-β | 42.00 ± 37.14 | 38.96 ± 34.90 |
| Proinsulin/insulin ratio | 3.64 ± 1.96 | 4.69 ± 7.04 |
| CPR (ng/mL) | 2.02 ± 1.00 | 1.92 ± 0.84 |
| NT-proBNP (pg/mL) | 55.02 ± 70.98 | 47.41 ± 60.86 |
| Log-hsCRP | 6.54 ± 1.31 | 6.23 ± 1.13 |
Notes:
The high dose was defined as ≥2.0 mg for glimepiride, ≥5.0 mg for glibenclamide, and ≥80 mg for gliclazide. Values are means ± standard deviation or n (%).
Abbreviations: BMI, body mass index; SU, sulfonylurea; HbA1c, hemoglobin A1c; NGSP, National Glycohemoglobin Standardization Program; FPG, fasting plasma glucose; F-IRI, fasting immunoreactive insulin; F-proinsulin, fasting proinsulin; HOMA-R, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of β cell function; CPR, C-peptide immunoreactivity; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; hsCRP, high-sensitivity C-reactive protein.
The mean doses of liraglutide and sitagliptin at Week 24 were 0.81 ± 0.22 mg/day and 56.1 ± 16.6 mg/day, respectively. The liraglutide dose at the last visit was 0.3 mg in 5 patients, 0.6 mg in 2 patients, and 0.9 mg in 37 patients. The sitagliptin dose at the last visit was 25 mg in 1 patient, 50 mg in 29 patients, and 100 mg in 8 patients. The sulfonylurea dose was similar in both groups.
HbA1c
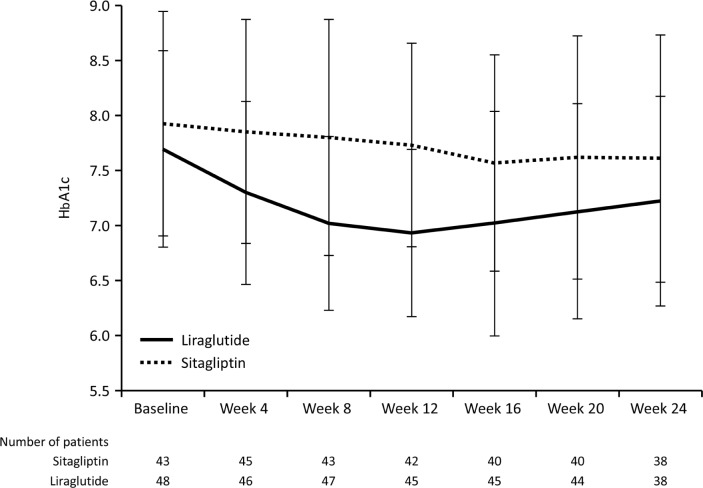
Change in HbA1c from baseline to each visit and the time-course of HbA1c are shown in Table 2 and Figure 2. In the liraglutide group, decreases in HbA1c from baseline were apparent at Week 4 onward, with a decrease of >0.5% at Week 8 onward (P < 0.001 vs. baseline at Weeks 4–24). The reduction was greatest at Week 12 (−0.78 ± 0.78%). The decrease in HbA1c was maintained through to Week 24, at which time the change from baseline was −0.59 ± 0.80%. HbA1c also decreased over time in the sitagliptin group, although the decrease from baseline was only statistically significant at Week 16 (−0.34 ± 0.91%). The change in HbA1c from baseline to Week 24 was −0.24 ± 0.94%, which was not significant. The change in HbA1c from baseline to Week 24 was therefore greater in the liraglutide group than in the sitagliptin group, although the difference was not statistically significant (P = 0.0525). When we repeated the analysis in the per-protocol population, the change in HbA1c from baseline to Week 24 was −0.63 ± 0.85% in the liraglutide group versus −0.19 ± 0.85% in the sitagliptin group, which was statistically significant (P = 0.0491).
Table 2.
Change in HbA1c from baseline to each visit and proportion of patients with HbA1c <6.9%.
| BASELINE | WEEK 4 | WEEK 8 | WEEK 12 | WEEK 16 | WEEK 20 | WEEK 24 | |
|---|---|---|---|---|---|---|---|
| Change from baseline (mean ± SD) | |||||||
| Liraglutide | 7.69 ± 0.89 | −0.39 ± 0.40* | −0.69 ± 0.64* | −0.78 ± 0.78* | −0.73 ± 1.00* | −0.65 ± 0.89* | −0.59 ± 0.80* |
| Sitagliptin | 7.92 ± 1.02 | −0.06 ± 0.51 | −0.17 ± 0.74 | −0.24 ± 0.78 | −0.34 ± 0.91† | −0.27 ± 0.90 | −0.24 ± 0.94 |
| P-value (ANCOVSA) | – | 0.0002 | <0.0001 | <0.0001 | 0.0161 | 0.0275 | 0.0525 |
| Patients with HbA1c <6.9%, n/N (%) | |||||||
| Liraglutide | 6/48 (12.5) | 15/46 (32.6) | 22/47 (46.8) | 21/45 (46.7) | 19/45 (42.2) | 18/44 (40.9) | 15/38 (39.5) |
| Sitagliptin | 5/43 (11.6) | 6/45 (13.3) | 7/43 (16.3) | 6/42 (14.3) | 11/40 (27.5) | 12/40 (30.0) | 10/38 (26.3) |
| P-value (χ2 test) | 0.8986 | 0.0291 | 0.002 | 0.0011 | 0.1563 | 0.2973 | 0.2222 |
Notes:
P < 0.0001 and
P = 0.0319 versus baseline (paired t-test). Because missing data were not substituted, the numbers of patients (N) at each time-point varied according to the number of patients analyzed.
Figure 2.
Time-course of changes in HbA1c (NGSP).
Notes: Values are means ± standard deviation.
Overall, 39.5 and 26.3% of patients in the liraglutide and sitagliptin groups had HbA1c <6.9% at Week 24. The responder rate was greatest at Week 8 and 12 in the liraglutide group (46.8 and 46.7%, respectively) and at Week 20 in the sitagliptin group (30.0%).
Secondary endpoints
The changes in secondary endpoint from baseline to Week 24 are summarized in Table 3. Body weight decreased by 0.60 kg in the liraglutide group and increased by 0.29 kg in the sitagliptin group, although these changes were not significant. Fasting plasma glucose (FPG) decreased significantly from baseline to Week 24 in the liraglutide group (−21.15 ± 31.22 mg/dL, P < 0.0001) but not in the sitagliptin group (+0.46 ± 39.39 mg/dL, P = 0.9403), corresponding to a significant between-group difference (P = 0.0014). In terms of other secondary endpoints, although HOMA-β and CPR increased significantly in the liraglutide group but not in the sitagliptin group, the between-group differences were not statistically significant.
Table 3.
Changes in secondary endpoints from baseline to week 24.
| PARAMETER | LIRAGLUTIDE | SITAGLIPTIN | P-VALUE‡ | ||||
|---|---|---|---|---|---|---|---|
| n* | CHANGE FROM BASELINE | P-VALUE† | n* | CHANGE FROM BASELINE | P-VALUE† | ||
| Body weight (kg) | 36 | −0.60 ± 2.44 | 0.1513 | 38 | 0.29 ± 2.05 | 0.3848 | 0.0969 |
| BMI (kg/m2) | 36 | −0.23 ± 0.98 | 0.167 | 38 | 0.11 ± 0.78 | 0.3784 | 0.1027 |
| FPG (mg/dL) | 46 | −21.15 ± 31.22 | <0.0001 | 41 | 0.46 ± 39.39 | 0.9403 | 0.0014 |
| F-IRI (μU/mL) | 46 | 0.68 ± 3.39 | 0.183 | 42 | 1.46 ± 9.35 | 0.3178 | 0.5771 |
| F-proinsulin (μU/mL) | 46 | 1.06 ± 16.79 | 0.671 | 42 | 2.75 ± 20.64 | 0.393 | 0.683 |
| HOMA-R | 46 | −0.17 ± 1.54 | 0.4619 | 41 | 1.12 ± 7.07 | 0.3166 | 0.2108 |
| HOMA-β | 46 | 26.18 ± 73.64 | 0.02 | 41 | 6.01 ± 33.75 | 0.2612 | 0.0944 |
| Proinsulin/insulin ratio | 46 | −0.26 ± 2.28 | 0.4377 | 42 | −1.07 ± 6.64 | 0.3008 | 0.7114 |
| CPR (ng/mL) | 46 | 0.21 ± 0.50 | 0.0073 | 42 | 0.09 ± 0.93 | 0.5277 | 0.4162 |
| NT-proBNP (pg/mL) | 46 | −6.91 ± 42.04 | 0.2707 | 42 | 1.05 ± 60.71 | 0.9115 | 0.6202 |
| Log-hsCRP | 46 | 0.01 ± 1.40 | 0.9631 | 42 | 0.26 ± 1.18 | 0.1603 | 0.7435 |
| PAID total score | 39 | −2.41 ± 7.97 | 0.1026 | 35 | −2.97 ± 15.67 | 0.2829 | 0.7286 |
Notes:
The number of patients differs between each parameter because data are shown only for patients with data for the individual parameter at Week 24 and the last observation was not carried forward to impute missing data.
Versus baseline (paired t-test, except PAID total score [Wilcoxon signed-rank test]).
For liraglutide versus sitagliptin (ANCOVA, except PAID total score [Wilcoxon rank-sum test]). Values are means ± standard deviation.
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; F-IRI, fasting immunoreactive insulin; F-proinsulin, fasting proinsulin; HOMA-R, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of β cell function; CPR, C-peptide immunoreactivity; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; hsCRP, high-sensitivity C-reactive protein; PAID, problem areas in diabetes survey.
The PAID total score decreased slightly and to a similar extent in the liraglutide (−2.41 ± 7.97) and sitagliptin (−2.97 ± 15.67) groups. However, the changes from baseline to Week 24 were not significant in either group.
There were no significant changes in the other secondary endpoints in either group.
Safety
Adverse events were reported in four patients in the liraglutide group, and included injection site erythema in two patients, common cold in one patient, diarrhea in one patient, and unruptured cerebral aneurysm in one patient. No one discontinued the liraglutide group because they were transient or considered to have no definite causal relationship. Adverse events were also reported by two patients in the sitagliptin group; both events were hand stiffness. One of these patients discontinued administration of sitagliptin because of the adverse event.
Hypoglycemic symptoms were reported in nine patients in the liraglutide group (18.0% of patients in the ITT set). The symptoms included hand tremor in three patients, a floating feeling in three patients, cold sweat in one patient, floating feeling/cold sweat/hand tremor in one patient, and hand tremor/cold sweat/weakness in one patient. Hypoglycemic symptoms were also reported in five patients in the sitagliptin group (10.2% of patients in the ITT set). The symptoms included a floating feeling in one patient, lassitude in one patient, hand tremor in one patient, dizziness in one patient, and weakness/cold sweat in one patient. None of the patients in either group discontinued treatment because of hypoglycemic symptoms. None of the patients experienced hypoglycemia requiring assistance from another person. The patients did not self-monitor blood glucose at the time of hypoglycemic symptoms.
Discussion
This 24-week, multicenter, open-label, randomized, parallel-group study was conducted to compare the efficacy and safety of two incretin therapies, namely liraglutide and sitagliptin, in Japanese patients with T2DM and inadequate glycemic control despite using a sulfonylurea or one/two non-sulfonylurea drugs. We found that treatment with either liraglutide or sitagliptin in combination with a sulfonylurea reduced HbA1c levels, suggesting that either drug should be considered for use in combination with a sulfonylurea in patients with inadequate glycemic control despite using one or more oral antidiabetic drugs. Notably, the reduction in HbA1c was significantly greater in the liraglutide group than in the sitagliptin group up to Week 20. Unfortunately, the significance of the reduction was borderline at Week 24, probably because of the small numbers. We suggest that GLP-1 receptor agonists may be more effective than DPP-4 inhibitors in Japanese patients.
It is thought that pancreatic β cells have reached a state of exhaustion and are unable to secrete sufficient insulin in patients with inadequate glycemic responses to a sulfonylurea.26 GLP-1 receptor agonists and DPP-4 inhibitors augment glucose-dependent insulin secretion from β cells and suppress glucagon secretion from pancreatic α cells.27 Although the former effect is dependent on functional β cells, we confirmed that liraglutide, in particular, was still capable of improving glycemic control. One explanation is that liraglutide has greater suppressive effects on glucagon secretion,6 which might be reflected by the greater reduction in FPG in the liraglutide group than in the sitagliptin group. Alternatively, the β cell exhaustion might represent desensitization to glucose, and this might be restored by liraglutide via the GLP-1 receptor through changes in signal transduction and increased availability of releasable insulin.28 This latter possibility is supported by the significant increases in HOMA-β and C-peptide levels observed in this study in the liraglutide group. Similar findings were reported in a prior study in Western patients treated with 1.2 or 1.8 mg liraglutide or 100 mg sitagliptin in combination with metformin. In this study, fasting C-peptide, fasting proinsulin-to-insulin ratio, and HOMA-β improved significantly in the liraglutide group over 26 weeks29 and 1 year.14 Thus, the results of studies in Japanese and non-Japanese patients suggest that treatment with a GLP-1 analog, such as liraglutide, might have greater blood glucose-lowering effects than sitagliptin in patients with inadequate glycemic responses to a sulfonylurea or other oral drugs.
In the liraglutide group, the magnitude of the decrease in HbA1c was greatest at Week 12, at which time the proportion of responders with HbA1c <6.9% was also greatest. The magnitude of the decrease in HbA1c from baseline was smaller at subsequent visits. Consistent with our study, other Japanese studies revealed that HbA1c decreased over the first 8–12 weeks and was maintained thereafter in patients treated with liraglutide alone4 or in combination with a sulfonylurea.7 However, because the baseline HbA1c levels were greater in these earlier studies, the reductions in HbA1c were greater in these studies (−1.88 and −1.56%) than in our study (−0.59%). Nevertheless, these reductions in HbA1c were achieved in patients treated with 0.9 mg/day liraglutide, which is half the maximum dose used in Western studies (1.8 mg/day).9,10 In our study, 39.5% of liraglutide-treated patients achieved HbA1c <6.9%. Therefore, the results of our study and of prior studies in Japan4,7 indicate that 0.9 mg/day is an appropriate dose for Japanese patients.
Prior studies have shown that GLP-1 receptor agonists are associated with body weight reductions. For example, in a meta-analysis30 of three clinical studies in non-Japanese patients, liraglutide was associated with a reduction in body weight of −2.12 kg (95% CI: −2.60, −1.63 kg) at 1.2 mg/day and of −2.70 kg (95% CI: −3.22, −2.18 kg) relative to active controls (sitagliptin,29 glimepiride,31 and insulin glargine).1 In Japanese studies, however, the effects of liraglutide on body weight are less clear. For example, in a 24-week monotherapy study, body weight was reduced by 0.92 kg in the liraglutide group but increased by 0.99 kg in the glibenclamide group (P < 0.0001).4 By contrast, in another study in which Japanese patients were treated with liraglutide in combination with a sulfonylurea, there was a smaller reduction in body weight in the liraglutide group (−0.37 kg) than in the placebo group (−1.12 kg; P = 0.0071) over 24 weeks.7 In the present study, body weight decreased by 0.60 kg in the liraglutide group and increased by 0.29 kg in the sitagliptin group, although these changes were not significant. Taken together, the results of these studies suggest that liraglutide has weaker body weight-reducing effects in Japanese patients than in non-Japanese patients when used in combination with a sulfonylurea. A larger reduction in body weight was observed when liraglutide was administered as monotherapy, with a mean weight change of -0.92 kg compared with +0.99 for glibenclamide-treated patients (difference: −1.91 kg; P < 0.0001).4 A likely explanation for the smaller weight change in Japanese patients is that body weight and body mass index are lower in Japanese patients than in non-Japanese patients, which might limit the capacity for body weight reductions. Indeed, in a prior study, it was noted that body mass index at baseline was correlated with weight loss and was the only independent determinant of weight loss,32 although it remains to be seen whether this is also true in Japanese patients. Alternatively, the dose of liraglutide used in Japanese studies is half of that used in other countries, and this might have an impact on the magnitude of the body weight reduction.
In the present study, we also assessed diabetes-related distress using the PAID questionnaire, in which lower scores indicate less distress.16,18 There were small reductions in PAID total scores in both groups, although the changes were not statistically significant in either group. These results suggest that the administration of liraglutide via subcutaneous injection does not negatively affect the daily life of patients with relatively advanced diabetes mellitus relative to an oral tablet, and that improvements in other aspects of the PAID questionnaire might outweigh possible negative perceptions of injections. A similar finding was observed in a previous paper in which liraglutide improved treatment satisfaction compared with sitagliptin owing to the greater reductions in HbA1c and body weight.33
Limitations
Some limitations of this study warrant mention. First, although the sample size required to achieve statistical power of 80% was calculated to be 64 patients per group, only 50 and 49 patients were enrolled into the liraglutide and sitagliptin groups, respectively, of whom 44 and 38 completed the study, respectively. Thus, the study was potentially underpowered to detect significant differences in HbA1c at Week 24. This might also have affected the ability to detect significant differences in secondary efficacy variables. Possible reasons for the lower than planned enrollment might include the fact that relatively few patients at the participating institutes were being treated with a sulfonylurea and that patients were more frequently prescribed other drugs, such as metformin and α-GI inhibitors. Second, in the circumstances that prevailing physician’s prescription in 2011 in Japan was an inclusion of DPP-4 inhibitors with metformin and other oral drugs, together with minimization of sulfonylurea dosing that had been performed to most of the patients in each clinic, physician’s discretion somehow did not fit to the study protocol resulting in the small numbers of participants and the occurrence of cases outside of the protocol.
Third, some patients in the liraglutide group had not been increased to the maximum permitted dose of 0.9 mg/day, with a mean dose of 0.81 mg/day at Week 24. The mean dose of sitagliptin at Week 24 was 56 mg/day, which indicates that the majority of patients were using 50 mg/day throughout the study. It is possible that a greater reduction in HbA1c would have been observed in the sitagliptin group if the dose had been increased to 100 mg/day.
Conclusion
In conclusion, this study in primary care settings showed that treatment with liraglutide or sitagliptin in combination with a sulfonylurea improved HbA1c in Japanese patients with T2DM and inadequate glycemic control at baseline. Considering that the reduction in HbA1c was greater in the liraglutide group, our results suggest that GLP-1 receptor agonists may be more effective than DPP-4 inhibitors in patients with advanced diabetes characterized by inadequate glycemic control despite oral treatment. The present results also suggest the improvement in glycemic control in the liraglutide group was possibly mediated by improvements in β cell function. Further studies are needed to evaluate the therapeutic efficacy of these treatment regimens and to identify patients who might best benefit from the addition of incretin therapy to ongoing treatments.
Acknowledgments
We wish to thank Dr Koichi Iwasaki, Dr Morifumi Yanagisawa, Dr Daigaku Uchida, Dr Nobuichi Kuribayashi, Dr Kazuhiro Miyazawa, Dr Hideki Wakamatsu, Dr Fuminobu Okuguchi, Dr Yoshio Kurihara, Dr Masahiko Takai, Dr Michiko Chousa, Dr Katsuhide Sugimoto, Dr Koichi Kawai, Dr Katsuya Yamazaki, Dr Kokichi Tanaka, Dr Masato Takagi, Dr Hajime Maeda, and Dr Shinichiro Shirabe for technical support and scientific advice. Analysis of data was conducted by Mebix Co. Ltd. We also wish to thank Nicholas D. Smith, PhD from Elsevier Japan KK., for English proofreading and editorial support.
Footnotes
ACADEMIC EDITOR: Yasuo Ito, Editor in Chief
FUNDING: This study was supported by a grant from the Japan Diabetes Foundation, which had no influence on the content of the paper.
COMPETING INTERESTS: KY discloses personal fees from MSD, Astellas, Taisho-Toyama and Novo Nordisk. NY discloses grants and personal fees from Sanofi, and personal fees from MSD, Takeda, Sanwa Kagaku Kenkyusho, Eli Lilly Japan and AstraZeneca, outside the submitted work. AK discloses personal fees from Astellas, MSD, and Sunstar, outside the submitted work. Other authors disclose no competing interests.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
Author Contributions
HY, MO, and HT designed the experiments. HY and AK mainly reviewed and edited the manuscript, and all authors contributed to the writing of the manuscript. HY, KH, KY, MO, GL, NY, and HT participated in recruitment and clinical work and conducted the research study. HY, KH, KY, MO, and GL were primarily responsible for data collection, integrity, and analysis. NY, HT, and AK contributed to the discussion of the data and performed critical review of the manuscript. HY is the guarantor of this work and full access to all the data in the study and takes responsibility of the data and accuracy of the data analysis. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46:1453–1469. doi: 10.1345/aph.1R041. [DOI] [PubMed] [Google Scholar]
- 2.Yabe D, Seino Y. Liraglutide in adults with type 2 diabetes: global perspective on safety, efficacy and patient preference. Clin Med Insights Endocrinol Diabetes. 2011;4:47–62. doi: 10.4137/CMED.S5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaku K, Rasmussen MF, Nishida T, Seino Y. Fifty-two-week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon-like peptide-1 analog liraglutide vs glibenclamide in patients with type 2 diabetes. J Diabetes Invest. 2011;2:441–447. doi: 10.1111/j.2040-1124.2011.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013–1022. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 5.Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;81:161–168. doi: 10.1016/j.diabres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Seino S, Rasmussen MF, Nishida T, Kaku K. Glucagon-like peptide-1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs sulfonylurea monotherapy in Japanese patients with type 2 diabetes: results of a 52-week, randomized, multicenter trial. J Diabetes Invest. 2011;2:280–286. doi: 10.1111/j.2040-1124.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaku K, Rasmussen MF, Clauson P, Seino Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:341–347. doi: 10.1111/j.1463-1326.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- 8.Gough SC. Liraglutide: from clinical trials to clinical practice. Diabetes Obes Metab. 2012;14(suppl 2):33–40. doi: 10.1111/j.1463-1326.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- 9.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 10.Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukai E, Fujimoto S, Inagaki N. Severe hypoglycemia by combination therapy with dipeptidyl peptidase-4 (DPP-4) inhibitors and sulfonylureas. Nihon Rinsho. 2011;69:907–911. [PubMed] [Google Scholar]
- 13.Charbonnel B, Steinberg H, Eymard E, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56:1503–1511. doi: 10.1007/s00125-013-2905-1. [DOI] [PubMed] [Google Scholar]
- 14.Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamazaki K, Hirao K, et al. The status of diabetes control and antidiabetic drug therapy in Japan—a cross-sectional survey of 17,000 patients with diabetes mellitus (JDDM 1) Diabetes Res Clin Pract. 2006;73:198–204. doi: 10.1016/j.diabres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Hirao K, Arai K, Yamauchi M, Takagi H, Kobayashi M. Six-month multicentric, open-label, randomized trial of twice-daily injections of biphasic insulin aspart 30 versus multiple daily injections of insulin aspart in Japanese type 2 diabetic patients (JDDM 11) Diabetes Res Clin Pract. 2008;79:171–176. doi: 10.1016/j.diabres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama H, Araki S, Haneda M, et al. Chronic kidney disease categories and renal-cardiovascular outcomes in type 2 diabetes without prevalent cardiovascular disease: a prospective cohort study (JDDM25) Diabetologia. 2012;55:1911–1918. doi: 10.1007/s00125-012-2536-y. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama H, Matsushima M, Kawai K, et al. Low incidence of cardiovascular events in Japanese patients with type 2 diabetes in primary care settings: a prospective cohort study (JDDM 20) Diabet Med. 2011;28:1221–1228. doi: 10.1111/j.1464-5491.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- 19.The Committee of Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc. 2010;53:450–456. [Google Scholar]
- 20.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 21.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the problem areas in diabetes (PAID) questionnaire. Diabet Med. 2003;20:69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 22.Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 23.Ishii H. Psycho-behavioral problems in diabetes treatment. J Jpn Diabetes Soc. 2000;43:13–16. [In Japanese] [Google Scholar]
- 24.Hayashino Y, Okamura S, Matsunaga S, Tsujii S, Ishii H. The association between problem areas in diabetes scale scores and glycemic control is modified by types of diabetes therapy: diabetes distress and care registry in Tenri (DDCRT 2) Diabetes Res Clin Pract. 2012;97:405–410. doi: 10.1016/j.diabres.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii S, Hayashino Y, Ishii H. Diabetes distress, but not depressive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: diabetes distress and care registry at tenri (DDCRT 1) Diabet Med. 2012;29:1451–1455. doi: 10.1111/j.1464-5491.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- 26.Del Prato S Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006;55:S20–S27. doi: 10.1016/j.metabol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A. Second line therapy: type 2 diabetic subjects failing on metformin GLP-1/DPP-IV inhibitors versus sulphonylurea/insulin: for GLP-1/DPP-IV inhibitors. Diabetes Metab Res Rev. 2012;28(suppl 2):21–25. doi: 10.1002/dmrr.2350. [DOI] [PubMed] [Google Scholar]
- 28.Rustenbeck I. Desensitization of insulin secretion. Biochem Pharmacol. 2002;63:1921–1935. doi: 10.1016/s0006-2952(02)00996-6. [DOI] [PubMed] [Google Scholar]
- 29.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 30.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;1986;3(1):ii:e00. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Chen L, Ji Q, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*) Diabetes Obes Metab. 2011;13:81–88. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 32.Fadini GP, Simioni N, Frison V, et al. Independent glucose and weight-reducing effects of Liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol. 2013;50(6):943–949. doi: 10.1007/s00592-013-0489-3. [DOI] [PubMed] [Google Scholar]
- 33.Davies M, Pratley R, Hammer M, Thomsen AB, Cuddihy R. Liraglutide improves treatment satisfaction in people with type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med. 2011;28:333–337. doi: 10.1111/j.1464-5491.2010.03074.x. [DOI] [PubMed] [Google Scholar]