Abstract
➤ Osteocytes, derived from osteoblasts, reside within bone and communicate extensively with other bone cell populations to regulate bone metabolism. The mature osteocyte expresses the protein sclerostin, a negative regulator of bone mass.
➤ In normal physiologic states, the protein sclerostin acts on osteoblasts at the surface of bone and is differentially expressed in response to mechanical loading, inflammatory molecules such as prostaglandin E2, and hormones such as parathyroid hormone and estrogen.
➤ Pathologically, sclerostin dysregulation has been observed in osteoporosis-related fractures, failure of implant osseous integration, metastatic bone disease, and select genetic diseases of bone mass.
➤ An antibody that targets sclerostin, decreasing endogenous levels of sclerostin while increasing bone mineral density, is currently in phase-III clinical trials.
➤ The osteocyte has emerged as a versatile, indispensable bone cell. Its location within bone, extensive dendritic network, and close communication with systemic circulation and other bone cells produce many opportunities to treat a variety of orthopaedic conditions.
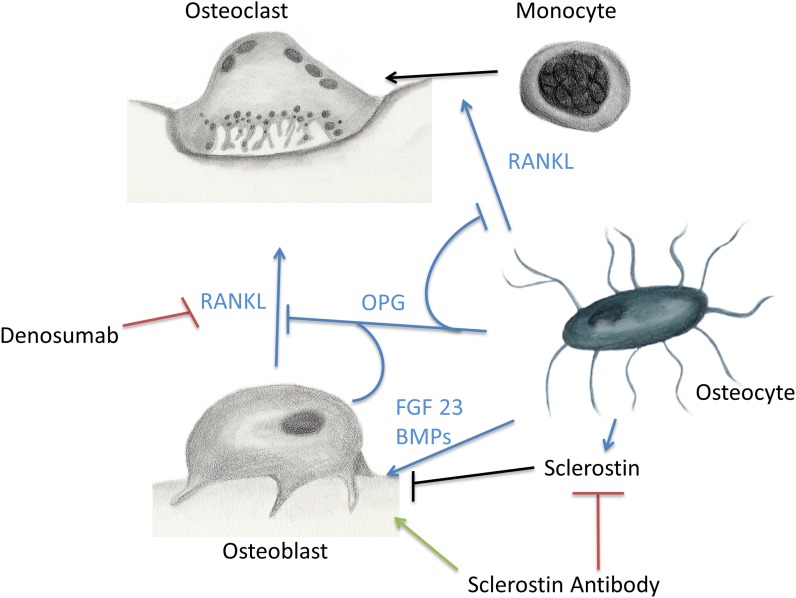
Bone is the major component of the skeletal system that provides locomotion, muscle attachment, protection of internal organs, and calcium homeostasis. In addition, bone provides a unique microenvironment in which osteoblasts, osteocytes, osteoclasts, hematopoietic cells, mesenchymal cells, and immune cells interact (Fig. 1). Monocytes differentiate and fuse to form bone-resorbing osteoclasts. Osteoblasts are derived from mesenchymal stem cells and primarily deposit new bone osteoid. Osteoblast, osteocytes, and T cells produce a key osteoclastogenic protein called receptor activator of nuclear factor kappa-β ligand (RANKL). To balance osteoclastogenesis and bone resorption, osteocytes and osteoblasts also produce osteoprotegerin (OPG) to interfere with RANKL signaling1. There is increasing evidence that bone cells act on other systems such as the central nervous system, energy metabolism, serum glucose regulation, and gonadal function2-4. One example is osteocalcin, a protein secreted by osteoblasts that modulates pancreatic insulin secretion and gonadal function5-7.
Fig. 1.
Osteocyte control of local bone environment. Osteocytes orchestrate bone resorption and bone deposition by controlling osteoclast and osteoblast activity. Osteocytes release RANKL (receptor activator of nuclear factor kappa-β ligand) to induce osteoclast differentiation, as well as OPG (osteoprotegerin) to downregulate osteoclastogenesis. Importantly, osteocytes also release FGF-23 (fibroblast growth factor-23), BMPs (bone morphogenetic proteins), and sclerostin to regulate osteoblast activity. Denosumab and sclerostin antibody are two antibodies that interact with bone cell biology to increase bone mass.
The osteocyte is the master signal sensor, integrator, and transducer of the skeleton. Osteocytes, osteoblasts, and osteoclasts are the major bone cells that orchestrate growth, maintenance, and healing of bone. Sclerostin, a glycoprotein secreted predominantly by osteocytes under physiologic conditions, is an important negative regulator of bone mass through the inhibition of bone formation by osteoblasts.
Bone cells are highly metabolically active components of bone. Mineralized bone matrix comprises hydroxyapatite, calcium, and other ions important for homeostasis. Bone matrix is a reservoir for many proteins such as collagen, osteocalcin, osteopontin, transforming growth factor, and bone morphogenetic protein (BMP). On the bone surface, osteoblasts produce new matrix while osteoclasts resorb and remodel bone. Osteocytes, which make up >95% of bone cells, are differentiated osteoblasts encased in the bone matrix8,9. The myriad functions of osteocytes have not been unraveled until recently10,11.
Osteocytes form a living network within the mineralized matrix of bone, appearing quiescent to observational researchers of the past12-14. From within a 15 to 20-μm lacunar space, the osteocyte cell body communicates via dendrites that extend through tubular canaliculi10 (Fig. 2). These dendrites contact other osteocytes, the marrow, and the osteoblast layer15,16. The distribution of osteocytes within bone is a highly organized three-dimensional matrix designed to enhance adaptation17. The vast network of osteocytes forms a large “bone membrane” and cell-matrix interface18. Osteocytes are bathed in a unique canalicular fluid that delivers nutrients, oxygen, and information from the systemic circulation. Canalicular fluid carries hormones, exchanges circulating factors, conducts mechanical signals, and provides access to potential therapeutic drugs19.
Fig. 2.
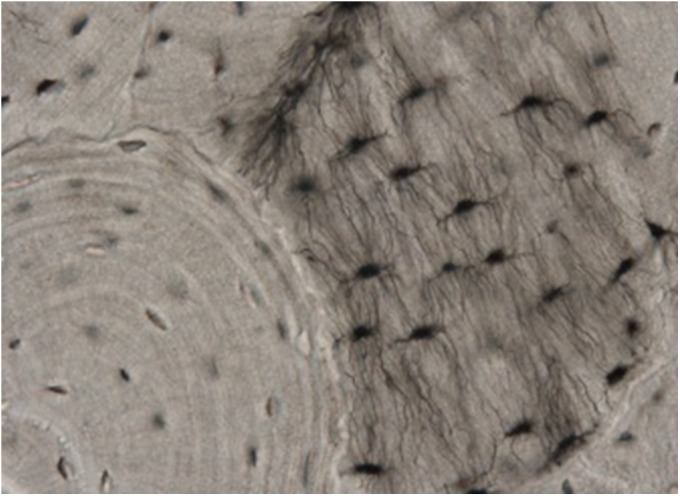
Osteocytes in native bone. Osteocytes reside within a highly organized three-dimensional matrix and communicate via dendrites to other osteocytes, the bone marrow, and osteoblasts.
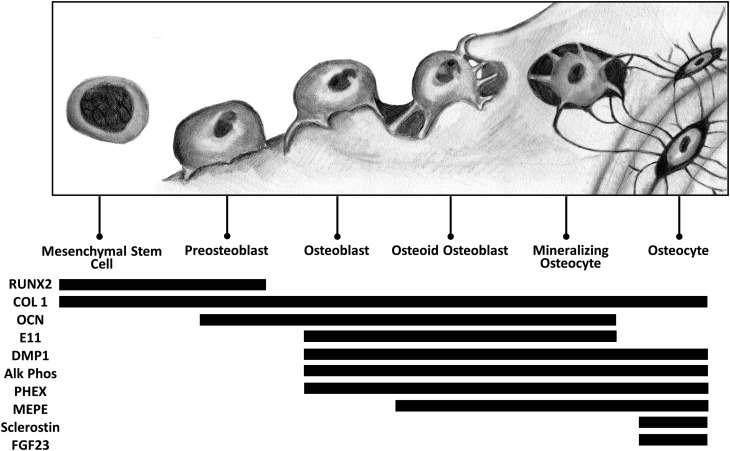
The signaling pathways underlying the osteoblastic differentiation into osteocytes are under investigation12,20-24. Several key proteins that identify the maturing osteocyte, such as E11, alkaline phosphatase, Pi-regulating endopeptidase on chromosome X (PHEX), matrix extracellular phosphoglycoprotein (MEPE), sclerostin, and fibroblast growth factor-23 (FGF-23), are differentially expressed (Figs. 1 and 3). Only after completion of differentiation does the osteocyte produce sclerostin and FGF-23, which are important factors in osteocyte-osteoblast communication. FGF-23 acts locally within the bone and also acts on the kidneys to influence phosphate homeostasis; however, discussion of osteocyte expression of this intriguing protein is beyond the scope of this review.
Fig. 3.
Osteocyte differentiation. Osteocytes differentiate from osteoblasts. The precise mechanism of differentiation is unknown; however, several distinct markers are expressed during osteocyte maturation. RUNX2 = runt-related transcription factor-2, COL 1 = type-I collagen, OCN = osteocalcin, DMP1 = dentin matrix acidic phosphoprotein 1, Alk Phos = alkaline phosphatase, PHEX = Pi-regulating endopeptidase on chromosome X, MEPE = matrix extracellular phosphoglycoprotein, and FGF23 = fibroblast growth factor-23.
Since its initial description in 1958, sclerostin has emerged as an important osteocytic secretion with implications for a variety of bone density diseases25. Although osteocytes are the best-researched producers of sclerostin, mRNA (messenger RNA) has also been detected in chondrocytes, kidney, lung, vasculature, and heart26-28. Sclerostin is a SOST gene product under nuanced regulatory control. It is integral to osteocyte function as a signal to damp the action of osteoblast bone deposition and to control bone metabolism. Its anti-anabolic effect on bone has rendered it an important molecule in fracture-healing, osteoporosis, metastatic disease, and a variety of other disorders. Development of sclerostin antibodies has shown promising results and wide applicability to a multitude of orthopaedic conditions29-32.
Physiologic Roles of Sclerostin
Sclerostin expression is regulated by a wide variety of factors, including mechanosensation, local cytokines, and endocrine factors.
Osteocytes are integral mechanosensors of bone, and osteocytes regulate bone mass in response to mechanical loading33. The lacunae and the canalicular network among osteocytes may act as mechanical strain amplifiers, in order to increase osteocyte sensitivity to mechanical loading34-36. The exact location of signal detection is not known, and may reside within the osteocyte cell body or dendrites, or both. Sclerostin is differentially regulated depending on skeletal mechanical loading37. Areas of concentrated strain in the skeleton show decreased levels of sclerostin38. In microgravity and decreased mechanical loading experiments, both upregulation of sclerostin and decreased bone mineral density occur39. Clinically, the serum sclerostin level is increased in healthy adult males during bed rest40. In cases of chronic unloading, such as spinal cord injury, levels of sclerostin also increased and correlated with osteoporosis progression41.
Downregulation of sclerostin is associated with increased osteogenesis and bone mass42. Sclerostin is thought to act by inhibition of the Wnt/β-catenin pathway in osteoblasts, binding at the LRP (low-density lipoprotein receptor-related protein) 5/6 receptor on the osteoblast cell membrane43. Therapeutically, antibodies against sclerostin protein (AMG 785; Amgen, Thousand Oaks, California) reduce levels of sclerostin and restore bone mineral density44. In this pathway, it has been hypothesized that sclerostin travels through the canalicular network to the bone surface to reach target osteoblasts at the bone surface and activate the Wnt pathway45,46. Subsequently, sclerostin promotes osteoblast apoptosis to ultimately decrease osteoid deposition.
In response to mechanical loading, osteocytes release prostaglandins47. In vitro studies have shown that prostaglandin E2 rapidly inhibits sclerostin through the EP4 receptor48. This release serves to regulate osteoblast proliferation and can also interact with the Wnt/β-catenin pathway. It has also been suggested by in vitro data that hypoxia inhibits sclerostin expression in osteocytes through the Wnt/β-catenin pathway49. Many other inflammatory molecules, such as tumor necrosis factor, oncostatin M, cardiotrophin-1, and leukemia inhibitory factor, rapidly downregulate sclerostin expression in osteocyte cell lines50-53.
Osteocytes are sensitive to a variety of systemic hormones that change bone mass by altering the expression of sclerostin. Studies have shown rapid, efficient communication between osteocytes and systemic circulation19.
For example, parathyroid hormone (PTH) is a resorptive signal released into the bloodstream by the parathyroid glands to act on bone in response to low levels of circulating calcium. PTH acts on osteocytes by altering sclerostin expression. Constitutively active PTH receptor-1 (PTHR1) in osteocytes is sufficient to increase bone modeling and remodeling due to the inhibition of sclerostin expression54,55. In a study of twenty-seven postmenopausal women treated with intermittent PTH, levels of sclerostin in the peripheral blood and bone-marrow plasma were decreased significantly56. Evidence has shown that PTH causes increased proteasomal degradation of runt-related transcription factor-2 (RUNX2) protein, which is a direct upregulator of SOST expression57-59. One possible pathway for the anabolic effect of intermittent PTH is inhibition of sclerostin expression in osteocytes.
Sex steroids are important for bone growth and maintenance. Estrogen signaling affects osteocyte regulation of bone density. Deletion of the estrogen receptor-α results in decreased sensitivity to mechanical loading, and estrogen withdrawal induces osteocyte apoptosis59,60. In men with idiopathic osteoporosis, circulating sclerostin levels correlate with estrogen exposure, possibly reflecting lower osteocyte cell mass or number61,62. In elderly men treated with gonadotropin-releasing hormone (GnRH) and 17β-estradiol, serum sclerostin levels were significantly reduced, while men treated with GnRH agonist and testosterone showed increased circulating sclerostin61. Studies on circulating sclerostin levels and sex hormones have not, however, clearly demonstrated that sclerostin levels are due to osteocyte expression. At this stage, much research is based primarily on circulating levels of sclerostin and is largely preliminary.
It is well known that patients with type-II diabetes mellitus have associated low bone mass and poor fracture-healing. In experimental rats with type-II diabetes mellitus, treatment with sclerostin antibody increases bone mass and bone strength and improves bone defect regeneration63. It has been proposed that bone defect regeneration may be dysregulated in patients with type-II diabetes mellitus because of premature callus resorption before adequate ossification occurs64,65.
The Role of Sclerostin and Osteocytes in Orthopaedic Disorders
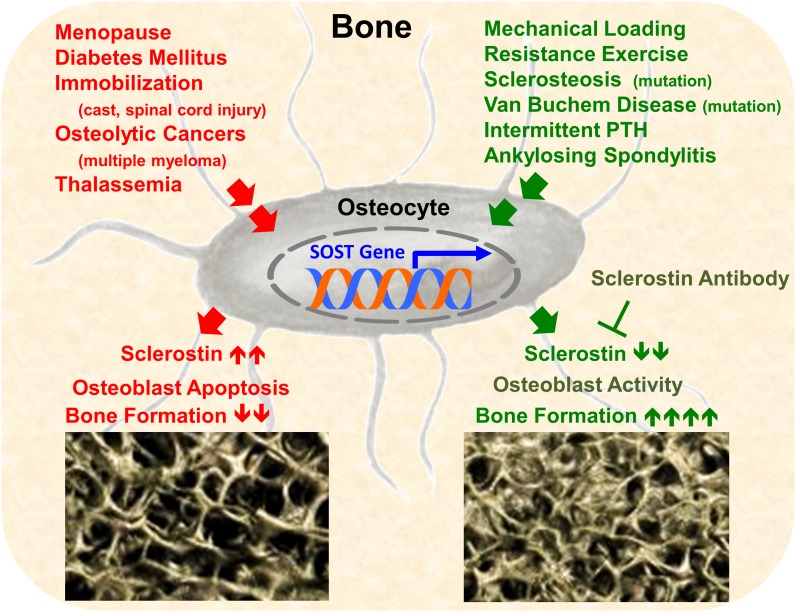
Osteocytes and their secretory products are under investigation in a variety of known bone diseases. Osteoporosis, fracture-healing, implant osseous integration, metastatic bone disease, and genetic bone disease each involve and affect osteocyte biology (Fig. 4). Sclerostin, secreted by mature osteocytes, is a subject of much interest as it pertains to bone disorders.
Fig. 4.
Sclerostin and disease. Many orthopaedic conditions act on the osteocyte to increase sclerostin expression (red) or decrease expression (green) and, subsequently, downregulate or upregulate bone formation, respectively. PTH = parathyroid hormone.
Fracture-Healing
Understanding and improving fracture-healing is a major area of research. Optimization of this system would decrease morbidity and mortality due to traumatic and pathologic fractures. Initial disruption of the vasculature at a fracture site induces cell necrosis; however, it has been suggested that surviving osteocytes are important for robust cellular recruitment and OPG release in initial fracture-healing stages66. Sclerostin expression is important during later callus remodeling by downregulating callus formation and maturation67,68.
Rodent models suggest downregulation of sclerostin is beneficial in fracture-healing. In mice lacking the sclerostin gene, healing was significantly hastened as evidenced by increased bone area, decreased cartilage area, and osseous bridging in the callus as early as fourteen days (no wild-type mice displayed osseous bridging at this time)69. Sclerostin antibody, when administered twice weekly after fracture, showed increased bone formation, bone mineral density, and bone strength as early as two weeks after fracture. At six weeks, the maximum failure load was increased by 68%70. Sclerostin antibody administered to rats with a critical-sized femoral defect and mice with a non-critical-sized femoral defect showed earlier healing, complete union, and physiologic maturation of the defect71,72. Similar conclusions can be drawn from the rat model of experimental periodontitis, in which sclerostin antibody treatment increased alveolar bone regeneration and filled substantial oral bone defects73.
Contrastingly, in a study of seventy-five human patients with long-bone fractures, fracture hematoma and serum sclerostin levels were elevated, evidencing a local and systemic role for sclerostin in fracture-healing74. Modulation of the Wnt pathway may be necessary for proper fracture-healing. While sclerostin activates the Wnt pathway, dickkopf-related protein 1 (Dkk-1), a protein that also acts at LRP6 to inhibit Wnt signaling, inhibits fracture-healing75. In a head-to-head clinical trial comparing zoledronic acid and denosumab treatment with Dkk-1 and sclerostin serum levels in postmenopausal women, it appeared that zoledronic acid and denosumab exert opposite effects on the Wnt pathway to ultimately decrease bone resorption76.
In postmenopausal women with osteoporosis, sclerostin levels may predict the risk of fracture: an increased level of serum sclerostin, independent of bone mineral density, age, and other confounding factors, strongly correlates with fracture risk77. Fragility fractures are a major complication of osteoporosis and are difficult to surgically stabilize because of inadequate bone strength to hold metallic implants used for internal fixation, with a high rate of delayed fracture union and nonunion. In the first few days after fracture, inflammatory molecules such as interleukin-6 are expressed at the fracture site, which may induce bone-remodeling gene expression and sclerostin downregulation after the fourth day78. Ovariectomized rats treated with sclerostin antibody showed enhanced, accelerated bone repair in proximal tibial defects after one week79. Sclerostin antibody not only improves bone-healing but also has anabolic effects on the entire skeleton. Sclerostin antibody may reduce the risk of nonunion after surgical stabilization due to fixation failure80.
These data taken together paint an interesting potential role for sclerostin in fracture-healing. Although neutralization of sclerostin in rodent models enhances bone formation, human patients demonstrate increased levels of sclerostin during physiologic fracture-healing. Furthermore, levels of sclerostin are altered in an osteoporotic state. These data suggest the regulation of bone mineralization, callus ossification, and remodeling are closely related to sclerostin expression. Osteocytic secretion of sclerostin at specific time points after skeletal injury most likely plays a critical regulatory role in bone-healing.
Implant Osseous Integration
Orthopaedic implants in joint arthroplasty are most likely to fail because of late aseptic loosening81. After surgery, all prostheses inevitably generate wear particles that embed in adjacent tissues. Implant failure often is accompanied by peri-implant bone resorption and osteocyte death82.
Studies have shown that wear particles generated from implants significantly altered osteocyte function in vitro resulting in Akt inactivation and cell apoptosis, providing a link between macroscopic findings and molecular biology83. To increase bone formation around implants, Liu et al. and Virdi et al. treated experimental rat implant models with sclerostin antibodies84,85. Sclerostin antibody in those studies prevented the negative effect of wear particles and increased bone volume. Osteocyte viability and maintenance of bone mass are critical to reduce the complication rate of joint arthroplasty; revision rates for total hip and total knee replacements are projected to increase to 137% and 601%, respectively, by 203086.
Metastatic Bone Cancer and Cancer-Induced Bone Loss
Metastatic and primary cancers that involve osseous lesions may interact closely with osteocyte protein products. Sclerostin may be a key mediator of cancer-induced bone disease and represents a promising therapeutic avenue for bone metastasis in breast cancer and myeloma-related bone disease87. Other targets include OPG, RANK/RANKL, cathepsin K, and other molecular pathways as potential emerging targets for metastatic bone pain88,89.
In multiple myeloma, myeloma cells override osteocyte regulation of bone cells. Myeloma cells release RANKL and inhibit production of as well as induce degradation of OPG90. Additional studies have revealed that myeloma cells also suppress osteoblasts by secreting sclerostin91. A positive correlation between severity of bone disease and circulating levels of serum sclerostin suggests multiple myeloma cells utilize sclerostin in the formation of osteolytic lesions92.
In some disease states, such as Paget disease of bone and prostate cancer, there is a substantially increased number of osteoblasts and osteoblastic activity93-96. In addition to increased bone mineralization and osteoblastic lesions, respectively, these patients demonstrate levels of sclerostin that correlate with the rate of bone turnover97. Perhaps these data illustrate compensatory sclerostin release from osteocytes to downregulate an overwhelming level of osteoblast activity. Alternatively, sclerostin may induce osteocytic RANKL production, upregulating osteoclast activity and bone resorption98.
Breast cancer metastatic cells also modulate bone cell activity. In osteolytic metastatic breast cancers, RUNX2 and coactivator core-binding factor beta (CBFB) modulate both osteoclast and osteoblast function. Specifically, osteopontin secretion by breast cancer metastatic cells activates osteoclasts99, while osteoblasts may be inhibited by RUNX2/CBFB-dependent sclerostin expression in osteoblasts100. Sclerostin domain containing 1 (SOSTDC1), a gene from the sclerostin family, is active in the BMP and Wnt signaling pathways and may be downregulated in breast cancer101. Higher levels of SOSTDC1 mRNA in patients correlate with metastatic-free survival rates, while primary breast tumors exhibit decreased levels of SOSTDC1 signaling. These data, taken together, suggest a complex role for sclerostin in breast cancer metastatic disease.
Genetic Bone Diseases
A number of bone diseases are due to genetic aberrations that specifically implicate osteocytes. Whether osteocyte dysfunction or dysfunction of osteocyte gene products is culpable in each disease is often uncertain. However, specific dysregulation of sclerostin results in characteristic bone abnormalities.
Sclerostin dysregulation is most specifically seen in sclerosteosis and van Buchem disease. In sclerosteosis, patients have increased bone mineral density and syndactyly due to sclerostin loss-of-function mutations. Only six mutations have been identified in sclerosteosis, and they involve both the sclerostin gene and LRP526,27,102-104. van Buchem disease is a similar, less severe disease of generalized osteosclerosis. Increased osteoblast activity is due to a regulatory domain deletion downstream of the SOST gene105,106. In human teeth and growth plates from patients affected by van Buchem disease, osteocytes did not produce appreciable levels of sclerostin107. Clinically, levels of serum sclerostin in van Buchem disease show a gene-dose effect of the deletion (as measured by serum sclerostin levels) with relation to the phenotypic severity of disease108.
Although sclerostin is clearly implicated in van Buchem disease and sclerosteosis, it may also play a relevant role in osteogenesis imperfecta. Osteogenesis imperfecta, classically due to a defect in type-I collagen, is characterized by osteopenia and brittle bones prone to fracture. In a mouse model of osteogenesis imperfecta, short-term sclerostin-neutralizing antibody improved bone mass and reduced fractures109.
Other genetic diseases have shown extensive bone involvement and may be related to osteocytic dysfunction. For example, ankylosing spondylitis is a disease of ectopic bone formation that causes a bamboo-like appearance of the spine with limited mobility and lower back pain. Altered OPG levels in ankylosing spondylitis favor osteoblastic activity in active disease states and ectopic bone formation110. Osteoporosis due to thalassemia also shows high bone turnover and increased Dkk-1 serum concentrations. High sclerostin in these patients was not reduced after zoledronic acid administration, suggesting high osteocyte activity111.
Emerging Osteocyte and Sclerostin-Targeted Therapeutic Options
Sclerostin is emerging as a key molecule in governing bone health. As the source of sclerostin, osteocytes are master integrators of chemical and mechanical signals that affect profound changes within the human body. The intimate access of the vascular system through the canalicular fluid and osteocytic network provides an ideal delivery system for a variety of small molecules51. Understanding the systems that regulate osteocyte function and expression of sclerostin are important facets of ongoing research in bone health. Additional applications of sclerostin action are also emerging, as in chondrocyte expression of sclerostin in osteoarthritis112. To manage sclerostin levels is to protect the delicate balance among the osteocyte, the osteoblast, and the osteoclast.
An antibody against sclerostin, as discussed above, has been developed specifically for the treatment of osteoporosis113-115. The sclerostin antibody (AMG 785; Amgen) decreases the endogenous levels of sclerostin, allowing for osteogenesis by improved osteoblast survival. Many exciting new applications for sclerostin antibody have been discussed in this article, including sclerosteosis, van Buchem disease, fracture-healing, and osteoporosis. Phase-I and II clinical trials of AMG 785 are ongoing to assess the effectiveness of antisclerostin antibody for the treatment of osteoporosis. A phase-I trial in 2011 showed single doses of AMG 785 were generally well tolerated116. Phase-II clinical trials enrolled women with femoral neck T-scores between −2.0 and −3.5 treated with five subcutaneous dosing regimens. The results showed increased bone mineral density at the lumbar spine and proximal part of the femur at twelve months after treatment with the five dosing regimens compared with other available therapies117. Additionally, Amgen performed phase-II trials to assess AMG 785 fracture-healing efficacy in patients who were fifty-five to ninety-five years old after hip fracture and surgical fixation118. In February 2013, Amgen and collaborator UCB SA halted patient testing mid-stage because AMG 785 treatment did not improve the time of healing of the fractures, as assessed on radiographs, compared with a placebo. Phase-III trials are currently under way for the treatment of osteoporosis in postmenopausal women. Despite an unremarkable toxicity profile in these trials, it appears sclerostin has other important functions in the bone-marrow environment on immune cell support; studies of sclerostin dysregulation have shown B-cell apoptosis and decreases in B-cell populations119.
Many studies have identified diseases in which osteocytes have been abolished or decreased in number. In multiple myeloma, osteocyte apoptosis is markedly increased compared with that in normal patients and patients with monoclonal gammopathy of undetermined significance (MGUS)120. Osteocytes lose control of sclerostin secretion in this clinical scenario. Patients with Crohn disease with major mineral deficiencies due to gastrointestinal damage have also demonstrated deleterious effects on osteocytes and decreased bone remodeling121. However, animal models of Crohn disease treated with sclerostin antibody have dramatic improvements in bone density122. Additionally, bisphosphonates, calcitonin, and QVDOPh, a pan-caspase inhibitor, each show promise in maintaining osteocytes in disease states123.
The network formed by osteocyte dendrites has also been implicated in disease and may represent aberrant communication between osteocytes and the osteoblasts on the bone surface. For example, in osteoporosis, osteocyte dendrites demonstrate a decreased number of connections, and the carefully laid three-dimensional network is disturbed124. Conversely, in osteomalacia, there is an increased number of dendrites and connectivity within the network8. In osteomalacia, the extracellular osseous matrix is hypomineralized; recent studies have shown that hypermineralization of osteocyte lacunae is associated with other disease processes, such as osteoarthritis125. Changes in the morphology of osteocytes and their extracellular environment is intimately affected by and related to disease process and bone density.
Systemic diseases are also related to osteocyte and bone cell activity. Hyperparathyroidism, in which PTH is increased, promotes pathologic osteoclast activity and, in turn, results in destruction of the bone. However, intermittent PTH administration decreases sclerostin levels and increases bone mineral density56. Thus, PTH function as related to bone mass is directly related to temporal administration. This novel effect of intermittent PTH administration yielded the development of teriparatide (Eli Lilly, Indianapolis, Indiana), which has efficacious therapeutic effects in the treatment of osteoporosis and has been shown to decrease sclerostin expression and increase osteocyte density126.
Overview and Future Directions
Treatments to increase bone mass are undergoing rapid changes. In addition to AMG 785, bisphosphonates denosumab (Amgen) and teriparatide (Eli Lilly) became available for clinical applications with a high level of enthusiasm and expanding indications. (Denosumab was approved by the U.S. Food and Drug Administration [FDA] for use in the treatment of osteoporosis and skeleton-related events in bone metastases from solid tumors; bisphosphonates were approved by the FDA for use in the prevention and treatment of osteoporosis and other bone diseases, such as Paget disease; and teriparatide was FDA approved for osteoporosis treatment in men and postmenopausal women at risk of fracture.) However, early studies have indicated several adverse side effects associated with denosumab, alendronate, and teriparatide127,128. Therefore, there is a constant need for advancement of clinical bone biology and for the development of new therapeutics.
Currently available data regarding osteocyte function have already given rise to a variety of therapeutic measures to alter osteocyte function. Osteocytes, the predominant resident cells of bone, and osteocyte-derived proteins such as sclerostin provide ample opportunities to develop novel strategies to treat many orthopaedic conditions.
Footnotes
Investigation performed at the Center for Orthopaedic Research at Columbia University Medical Center, New York, NY
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011October;17(10):1231-4 Epub 2011 Sep 11 [DOI] [PubMed] [Google Scholar]
- 2.Elefteriou F Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008May15;473(2):231-6 Epub 2008 Mar 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein LS, Xie T, Zhang QH, Chen M. Studies of the regulation and function of the Gs alpha gene Gnas using gene targeting technology. Pharmacol Ther. 2007August;115(2):271-91 Epub 2007 Apr 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon R, Meays D, Kardos N, Frangos J. Regulation of energy metabolism by osteocytes. J Bone Miner Res. 2010; 25(Suppl 1). [Google Scholar]
- 5.Fernández-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gómez-Ambrosi J, Moreno-Navarrete JM, Frühbeck G, Martínez C, Idoate F, Salvador J, Forga L, Ricart W, Ibañez J. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009January;94(1):237-45 Epub 2008 Oct 14 [DOI] [PubMed] [Google Scholar]
- 6.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011April;26(4):677-80 [DOI] [PubMed] [Google Scholar]
- 7.Breuil V, Euller-Ziegler L. Gonadal dysgenesis and bone metabolism. Joint Bone Spine. 2001February;68(1):26-33 [DOI] [PubMed] [Google Scholar]
- 8.Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004January;36(1):1-8 [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Cooper RR. Bone Biology. J Bone Joint Surg Am. 1995August1;77(8):1276-1289 [Google Scholar]
- 10.Palumbo C, Palazzini S, Marotti G. Morphological study of intercellular junctions during osteocyte differentiation. Bone. 1990;11(6):401-6 [DOI] [PubMed] [Google Scholar]
- 11.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more [Epub ahead of print]. Endocr Rev. 2013October;34(5):658-90 Epub 2013 Apr 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonewald LF The amazing osteocyte. J Bone Miner Res. 2011February;26(2):229-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtrop ME Light and electron microscopical structure of bone forming cells. In: Hall BK, editor. Bone: a treatise, Vol 1: the osteoblast and osteocyte. Caldwell, NJ: The Telford Press; 1990. p 1-40 [Google Scholar]
- 14.Palumbo C A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell Tissue Res. 1986;246(1):125-31 [DOI] [PubMed] [Google Scholar]
- 15.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001February;28(2):145-9 [DOI] [PubMed] [Google Scholar]
- 16.Palumbo C, Palazzini S, Zaffe D, Marotti G. Osteocyte differentiation in the tibia of newborn rabbit: an ultrastructural study of the formation of cytoplasmic processes. Acta Anat (Basel). 1990;137(4):350-8 [DOI] [PubMed] [Google Scholar]
- 17.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994July;55(3):287-99 [DOI] [PubMed] [Google Scholar]
- 18.Ramp WK Cellular control of calcium movements in bone. Interrelationships of the bone membrane, parathyroid hormone and alkaline phosphatase. Clin Orthop Relat Res. 1975Jan-Feb;(106):311-22 [PubMed] [Google Scholar]
- 19.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998February;22(2):107-17 [DOI] [PubMed] [Google Scholar]
- 20.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006January;235(1):176-90 [DOI] [PubMed] [Google Scholar]
- 21.Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010March;1192(1):437-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W, Byrne MH, Wang Y, Krane SM. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J Clin Invest. 2000October;106(8):941-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005January1;118(Pt 1):147-56 Epub 2004 Dec 15 [DOI] [PubMed] [Google Scholar]
- 24.van der Plas A, Aarden EM, Feijen JH, de Boer AH, Wiltink A, Alblas MJ, de Leij L, Nijweide PJ. Characteristics and properties of osteocytes in culture. J Bone Miner Res. 1994November;9(11):1697-704 [DOI] [PubMed] [Google Scholar]
- 25.Truswell AS Osteopetrosis with syndactyly; a morphological variant of Albers-Schönberg’s disease. J Bone Joint Surg Br. 1958May;40(2):209-18 [PubMed] [Google Scholar]
- 26.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001March1;10(5):537-43 [DOI] [PubMed] [Google Scholar]
- 27.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001March;68(3):577-89 Epub 2001 Feb 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008June;23(6):860-9 [DOI] [PubMed] [Google Scholar]
- 29.Papapoulos SE Targeting sclerostin as potential treatment of osteoporosis. Ann Rheum Dis. 2011March;70Suppl 1:i119-22 [DOI] [PubMed] [Google Scholar]
- 30.Moester MJ, Papapoulos SE, Löwik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010August;87(2):99-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki R [Anti-sclerostin antibodies]. Clin Calcium. 2011January;21(1):94-8 [Article in Japanese] [PubMed] [Google Scholar]
- 32.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009April;24(4):578-88 [DOI] [PubMed] [Google Scholar]
- 33.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011July;26(7):1425-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marotti G, Farneti D, Remaggi F, Tartari F. Morphometric investigation on osteocytes in human auditory ossicles. Ann Anat. 1998October;180(5):449-53 [DOI] [PubMed] [Google Scholar]
- 35.Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735-43 Epub 2005 Jul 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech. 2007;40(10):2199-206 Epub 2007 Jan 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003December1;22(23):6267-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006Oct-Dec;6(4):354. [PubMed] [Google Scholar]
- 39.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012September;97(9):E1736-40 Epub 2012 Jul 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012February;27(2):352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009October;24(10):1651-61 [DOI] [PubMed] [Google Scholar]
- 42.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009October;24(10):1651-61 [DOI] [PubMed] [Google Scholar]
- 43.Ott SM Sclerostin and Wnt signaling—the pathway to bone strength. J Clin Endocrinol Metab. 2005December;90(12):6741-3 [DOI] [PubMed] [Google Scholar]
- 44.Niziolek PJ, Farmer TL, Cui Y, Turner CH, Warman ML, Robling AG. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011November;49(5):1010-9 Epub 2011 Aug 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005May20;280(20):19883-7 Epub 2005 Mar 18 [DOI] [PubMed] [Google Scholar]
- 46.Lewiecki EM Monoclonal antibodies for the treatment of osteoporosis. Expert Opin Biol Ther. 2013February;13(2):183-96 Epub 2012 Dec 19 [DOI] [PubMed] [Google Scholar]
- 47.Tian X, Jee WS, Li X, Paszty C, Ke HZ. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone. 2011February;48(2):197-201 Epub 2010 Sep 17 [DOI] [PubMed] [Google Scholar]
- 48.Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One. 2011;6(3):e17772 Epub 2011 Mar 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genetos DC, Toupadakis CA, Raheja LF, Wong A, Papanicolaou SE, Fyhrie DP, Loots GG, Yellowley CE. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010May15;110(2):457-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG, Nicola NA, Gillespie MT, Martin TJ, Sims NA. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest. 2010February;120(2):582-92 Epub 2010 Jan 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, Gillespie MT, Martin TJ, Sims NA. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008December;23(12):2025-32 [DOI] [PubMed] [Google Scholar]
- 52.Cornish J, Callon K, King A, Edgar S, Reid IR. The effect of leukemia inhibitory factor on bone in vivo. Endocrinology. 1993March;132(3):1359-66 [DOI] [PubMed] [Google Scholar]
- 53.Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, Fazzalari NL, Evdokiou A, Atkins GJ. Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res. 2009August;24(8):1434-49 [DOI] [PubMed] [Google Scholar]
- 54.Schipani E, Kruse K, Jüppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995April7;268(5207):98-100 [DOI] [PubMed] [Google Scholar]
- 55.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001February;107(3):277-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010November;95(11):5056-62 Epub 2010 Jul 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003December12;278(50):50259-72 Epub 2003 Oct 1 [DOI] [PubMed] [Google Scholar]
- 58.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005November;146(11):4577-83 Epub 2005 Aug 4 [DOI] [PubMed] [Google Scholar]
- 59.Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006August;21(8):1297-306 [DOI] [PubMed] [Google Scholar]
- 60.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997September;82(9):3128-35 [DOI] [PubMed] [Google Scholar]
- 61.Mödder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011January;26(1):27-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lapauw B, Vandewalle S, Taes Y, Goemaere S, Zmierczak H, Collette J, Kaufman JM. Serum sclerostin levels in men with idiopathic osteoporosis. Eur J Endocrinol. 2013April;168(4):615-20 Epub 2013 Mar 15 [DOI] [PubMed] [Google Scholar]
- 63.Jähn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, Bonewald LF. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur Cell Mater. 2012;24:197-209; discussion 209-10. Epub 2012 Sep 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007April;22(4):560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kayal RA, Alblowi J, McKenzie E, Krothapalli N, Silkman L, Gerstenfeld L, Einhorn TA, Graves DT. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009February;44(2):357-63 Epub 2008 Oct 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirakawa K, Hirota S, Ikeda T, Yamaguchi A, Takemura T, Nagoshi J, Yoshiki S, Suda T, Kitamura Y, Nomura S. Localization of the mRNA for bone matrix proteins during fracture healing as determined by in situ hybridization. J Bone Miner Res. 1994October;9(10):1551-7 [DOI] [PubMed] [Google Scholar]
- 67.Power J, Poole KE, van Bezooijen R, Doube M, Caballero-Alías AM, Lowik C, Papapoulos S, Reeve J, Loveridge N. Sclerostin and the regulation of bone formation: Effects in hip osteoarthritis and femoral neck fracture. J Bone Miner Res. 2010August;25(8):1867-76 [DOI] [PubMed] [Google Scholar]
- 68.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008October;19(5):459-66 Epub 2008 Jul 25 [DOI] [PubMed] [Google Scholar]
- 69.Li C, Ominsky MS, Tan HL, Barrero M, Niu QT, Asuncion FJ, Lee E, Liu M, Simonet WS, Paszty C, Ke HZ. Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone. 2011December;49(6):1178-85 Epub 2011 Aug 26 [DOI] [PubMed] [Google Scholar]
- 70.Cui L, Cheng H, Song C, Li C, Simonet WS, Ke HZ, Li G. Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact. 2013June;13(2):178-84 [PubMed] [Google Scholar]
- 71.Virk MS, Alaee F, Tang H, Ominsky MS, Ke HZ, Lieberman JR. Systemic administration of sclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J Bone Joint Surg Am. 2013April17;95(8):694-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jawad MU, Fritton KE, Ma T, Ren PG, Goodman SB, Ke HZ, Babij P, Genovese MC. Effects of sclerostin antibody on healing of a non-critical size femoral bone defect. J Orthop Res. 2013January;31(1):155-63 Epub 2012 Aug 8 [DOI] [PubMed] [Google Scholar]
- 73.Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV, Ke HZ, Liu M, Giannobile WV. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res. 2013November;28(11):2347-56 [DOI] [PubMed] [Google Scholar]
- 74.Sarahrudi K, Thomas A, Albrecht C, Aharinejad S. Strongly enhanced levels of sclerostin during human fracture healing. J Orthop Res. 2012October;30(10):1549-55 Epub 2012 Apr 16 [DOI] [PubMed] [Google Scholar]
- 75.Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009July;7(2):64-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P. Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naive postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab. 2013August;98(8):3206-12 Epub 2013 Jun 20 [DOI] [PubMed] [Google Scholar]
- 77.Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res. 2012December;27(12):2592-602 [DOI] [PubMed] [Google Scholar]
- 78.Caetano-Lopes J, Lopes A, Rodrigues A, Fernandes D, Perpétuo IP, Monjardino T, Lucas R, Monteiro J, Konttinen YT, Canhão H, Fonseca JE. Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing. PLoS One. 2011;6(2):e16947 Epub 2011 Feb 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDonald MM, Morse A, Mikulec K, Peacock L, Yu N, Baldock PA, Birke O, Liu M, Ke HZ, Little DG. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res. 2012October;30(10):1541-8 Epub 2012 Mar 27 [DOI] [PubMed] [Google Scholar]
- 80.Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011May;26(5):1012-21 [DOI] [PubMed] [Google Scholar]
- 81.Grandjean-Laquerriere A, Laquerriere P, Guenounou M, Laurent-Maquin D, Phillips TM. Importance of the surface area ratio on cytokines production by human monocytes in vitro induced by various hydroxyapatite particles. Biomaterials. 2005May;26(15):2361-9 [DOI] [PubMed] [Google Scholar]
- 82.Lange T, Schilling AF, Peters F, Haag F, Morlock MM, Rueger JM, Amling M. Proinflammatory and osteoclastogenic effects of beta-tricalciumphosphate and hydroxyapatite particles on human mononuclear cells in vitro. Biomaterials. 2009October;30(29):5312-8 Epub 2009 Jul 3 [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Yan M, Yu A, Mao H, Zhang J. Inhibitory effects of β-tricalciumphosphate wear particles on osteocytes via apoptotic response and Akt inactivation. Toxicology. 2012July16;297(1-3):57-67 Epub 2012 Apr 12 [DOI] [PubMed] [Google Scholar]
- 84.Liu S, Virdi AS, Sena K, Sumner DR. Sclerostin antibody prevents particle-induced implant loosening by stimulating bone formation and inhibiting bone resorption in a rat model. Arthritis Rheum. 2012December;64(12):4012-20 [DOI] [PubMed] [Google Scholar]
- 85.Virdi AS, Liu M, Sena K, Maletich J, McNulty M, Ke HZ, Sumner DR. Sclerostin antibody increases bone volume and enhances implant fixation in a rat model. J Bone Joint Surg Am. 2012September19;94(18):1670-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007April;89(4):780-5 [DOI] [PubMed] [Google Scholar]
- 87.Gkotzamanidou M, Dimopoulos MA, Kastritis E, Christoulas D, Moulopoulos LA, Terpos E. Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin Ther Targets. 2012August;16(8):761-9 Epub 2012 Jul 5 [DOI] [PubMed] [Google Scholar]
- 88.Coluzzi F, Di Bussolo E, Mandatori I, Mattia C. Bone metastatic disease: taking aim at new therapeutic targets. Curr Med Chem. 2011;18(20):3093-115 [DOI] [PubMed] [Google Scholar]
- 89.Coluzzi F, Mandatori I, Mattia C. Emerging therapies in metastatic bone pain. Expert Opin Emerg Drugs. 2011September;16(3):441-58 Epub 2011 May 5 [DOI] [PubMed] [Google Scholar]
- 90.Sezer O, Heider U, Zavrski I, Kühne CA, Hofbauer LC. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003March15;101(6):2094-8 Epub 2002 Nov 7 [DOI] [PubMed] [Google Scholar]
- 91.Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, Rinaldi E, Curci P, Liso V, Passeri G, Zallone A, Rizzi R, Grano M. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011June;1(6):e27 Epub 2011 Jun 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012September15;131(6):1466-71 Epub 2011 Dec 21 [DOI] [PubMed] [Google Scholar]
- 93.Masrour Roudsari J, Mahjoub S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget's specimens. Caspian J Intern Med. 2012Summer;3(3):478-83 [PMC free article] [PubMed] [Google Scholar]
- 94.Pabinger C1, Heu C, Frohner A, Dimai HP. Pregnancy- and lactation-associated transient osteoporosis of both hips in a 32 year old patient with osteogenesis imperfecta. Bone. 2012July;51(1):142-4 [DOI] [PubMed] [Google Scholar]
- 95.Larson SR1, Chin J, Zhang X, Brown LG, Coleman IM, Lakely B, Tenniswood M, Corey E, Nelson PS, Vessella RL, Morrissey C. Prostate cancer derived prostatic acid phosphatase promotes an osteoblastic response in the bone microenvironment. Clin Exp Metastasis. 2014February;31(2):247-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005September15;65(18):8274-85 [DOI] [PubMed] [Google Scholar]
- 97.Yavropoulou MP, van Lierop AH, Hamdy NA, Rizzoli R, Papapoulos SE. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012July;51(1):153-7 Epub 2012 May 2 [DOI] [PubMed] [Google Scholar]
- 98.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900 Epub 2011 Oct 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inman CK, Shore P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem. 2003December5;278(49):48684-9 Epub 2003 Sep 23 [DOI] [PubMed] [Google Scholar]
- 100.Mendoza-Villanueva D, Zeef L, Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13(5):R106 Epub 2011 Oct 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clausen KA, Blish KR, Birse CE, Triplette MA, Kute TE, Russell GB, D’Agostino RB Jr, Miller LD, Torti FM, Torti SV. SOSTDC1 differentially modulates Smad and beta-catenin activation and is down-regulated in breast cancer. Breast Cancer Res Treat. 2011October;129(3):737-46 Epub 2010 Nov 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhadada SK, Rastogi A, Steenackers E, Boudin E, Arya A, Dhiman V, Bhansali A, Van Hul W. Novel SOST gene mutation in a sclerosteosis patient and her parents. Bone. 2013February;52(2):707-10 Epub 2012 Oct 16 [DOI] [PubMed] [Google Scholar]
- 103.Balemans W, Cleiren E, Siebers U, Horst J, Van Hul W. A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone. 2005June;36(6):943-7 [DOI] [PubMed] [Google Scholar]
- 104.Kim CA, Honjo R, Bertola D, Albano L, Oliveira L, Jales S, Siqueira J, Castilho A, Balemans W, Piters E, Jennes K, Van Hul W. A known SOST gene mutation causes sclerosteosis in a familial and an isolated case from Brazilian origin. Genet Test. 2008December;12(4):475-9 [DOI] [PubMed] [Google Scholar]
- 105.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002February;39(2):91-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, Papapoulos S, Hamersma H, Brunkow ME. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002June15;110(2):144-52 [DOI] [PubMed] [Google Scholar]
- 107.van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ, Van Hul W, Hamersma H, Dikkers FG, Hamdy NA, Papapoulos SE, Löwik CW. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009June;88(6):569-74 [DOI] [PubMed] [Google Scholar]
- 108.van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013April;28(4):848-54 [DOI] [PubMed] [Google Scholar]
- 109.Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013January;28(1):73-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylan A, Sari I, Akinci B, Bilge S, Kozaci D, Akar S, Colak A, Yalcin H, Gunay N, Akkoc N. Biomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitis. BMC Musculoskelet Disord. 2012;13:191 Epub 2012 Oct 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voskaridou E, Christoulas D, Plata E, Bratengeier C, Anastasilakis AD, Komninaka V, Kaliontzi D, Gkotzamanidou M, Polyzos SA, Dimopoulou M, Terpos E. High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res. 2012November;44(12):909-13 Epub 2012 May 11 [DOI] [PubMed] [Google Scholar]
- 112.Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, Cake MA, Read RA, Bateman JF, Sambrook PN, Little CB. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011July;19(7):874-85 Epub 2011 May 12 [DOI] [PubMed] [Google Scholar]
- 113.Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, Shimada T. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009November;24(11):1879-88 [DOI] [PubMed] [Google Scholar]
- 114.Tian X, Setterberg RB, Li X, Paszty C, Ke HZ, Jee WS. Treatment with a sclerostin antibody increases cancellous bone formation and bone mass regardless of marrow composition in adult female rats. Bone. 2010September;47(3):529-33 Epub 2010 Jun 1 [DOI] [PubMed] [Google Scholar]
- 115.Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, Dwyer D, Stouch B, Thway TM, Stolina M, Ominsky MS, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010December;25(12):2647-56 Epub 2010 Jul 16 [DOI] [PubMed] [Google Scholar]
- 116.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011January;26(1):19-26 [DOI] [PubMed] [Google Scholar]
- 117.Reid IR Osteoporosis treatment (ECTS 2013). In: IBMS BoneKEy. Meeting Report from the European Calcified Tiessue Society Annual Congress; Lisbon, Portugal; 2013 May 18-21. Article no. 392
- 118.Amgen MD Study to assess fracture healing with sclerostin antibody. U.S. National Institutes of Health; 2000. http://clinicaltrials.gov/ct2/show/NCT01081678. Accessed 2013 Nov 18 [Google Scholar]
- 119.Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res. 2012July;27(7):1451-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012June;26(6):1391-401 Epub 2012 Jan 6 [DOI] [PubMed] [Google Scholar]
- 121.Oostlander AE, Bravenboer N, Sohl E, Holzmann PJ, van der Woude CJ, Dijkstra G, Stokkers PC, Oldenburg B, Netelenbos JC, Hommes DW, van Bodegraven AA, Lips P; Dutch Initiative on Crohn and Colitis (ICC). Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn’s disease. Gastroenterology. 2011January;140(1):116-23 Epub 2010 Sep 18 [DOI] [PubMed] [Google Scholar]
- 122.Eddleston A, Marenzana M, Moore AR, Stephens P, Muzylak M, Marshall D, Robinson MK. A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J Bone Miner Res. 2009October;24(10):1662-71 [DOI] [PubMed] [Google Scholar]
- 123.Bonewald LF Osteocytes. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3rd ed Vol 1 Elsevier; 2007. p 169-90 [Google Scholar]
- 124.Bonewald LF Osteocyte biology: its implications for osteoporosis. J Musculoskelet Neuronal Interact. 2004March;4(1):101-4 [PubMed] [Google Scholar]
- 125.Carpentier VT, Wong J, Yeap Y, Gan C, Sutton-Smith P, Badiei A, Fazzalari NL, Kuliwaba JS. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: implications for bone remodeling. Bone. 2012March;50(3):688-94 Epub 2011 Dec 7 [DOI] [PubMed] [Google Scholar]
- 126.Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010June;151(6):2641-9 Epub 2010 Apr 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009January;24(1):153-61 [DOI] [PubMed] [Google Scholar]
- 128.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC, Hodsman AB. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002October;87(10):4528-35 [DOI] [PubMed] [Google Scholar]