Abstract
Astrocytes protect neurons but also evoke a proinflammatory response to injury and viral infections including HIV. We investigated the mechanism of HIV-1 infection in primary astrocytes, which showed minimal but productive viral infection independent of CXCR4. As with ectopic-CD4-expressing astrocytes, lysosomotropic agents led to increased HIV-1 infection in wild-type but not Rab 5, 7, and 11-ablated astrocytes. Instead, HIV-1 infection was decreased in Rab-depleted astrocytes, corroborating viral entry by endocytosis. HIV-1 produced persistent infection in astrocytes (160 days); no evidence of latent infection was seen. Notably, one caveat is that endosomal modifiers enhanced wild-type HIV-1 infection (M- and T-tropic) in astrocytes, suggesting endocytic entry of the virus. Impeding endocytosis by inhibition of Rab5, 7 or 11 will inhibit HIV infection in astrocytes. Although the contribution of such low-level infection in astrocytes to neurological complications is unclear, it may serve as an elusive viral reservoir in the central nervous system.
Keywords: lysosomotropic agents, proteasome-inhibitors, HIV-1 persistence, Rab, HIV-LTR, TNPO3, LSP1
Introduction
The central nervous system (CNS) is a target of HIV-1 infection (Epstein et al., 1984; Fox and Cottler-Fox, 1986; Gabuzda et al., 1986; Levy et al., 1985; Navia et al., 1986; Petito et al., 1985; Wiley et al., 1986) and may serve as an important viral reservoir (Churchill et al., 2009). HIV-1 enters the CNS early after transmission (Churchill et al., 2006; Ragin et al., 2012; Resnick et al., 1988) and persists for the entire life of the individual. HIV-1 infection in microglia and macrophages is often productive (Gartner et al., 1986; Koenig et al., 1986; Pumarola-Sune et al., 1987; Strizki et al., 1996; Vazeux et al., 1987; Wiley et al., 1986), while infection in astrocytes ranges from unproductive to productive (Boutet et al., 2001; Chiodi et al., 1987; Churchill et al., 2009; Gorry et al., 1999; Koyanagi et al., 1987; Trillo-Pazos et al., 2003; Wiley and Achim, 1997). The importance of such an infection in astrocytes is unclear.
Astrocytes are the predominant neuro-glial cells (10:1) involved in brain plasticity and neuroprotection by detoxifying glutamate (Giulian et al., 1993; Piani and Fontana, 1994). Astrocytes are also important in HIV-1-mediated neuropathology, serving as inflammatory cells in response to viral products and as possible viral reservoir. Earlier reports have shown that in infected brain tissues, up to 25% of astrocytes carry HIV-1 DNA (Churchill et al., 2006; Dewhurst et al., 1987; Trillo-Pazos et al., 2003). In particular, primary HIV-1 isolates are efficient in infecting primary human fetal astrocytes (Churchill et al., 2009; Thompson et al., 2004). However, some studies have also suggested intracellular restrictions, with the presence of efficient early viral transcripts but low levels of late transcripts responsible for structural proteins (Neumann et al., 1995).
Restrictions on viral replication in astrocytes have been specifically attributed to the malfunction of HIV-1 Rev protein (Neumann et al., 1995). Rev is involved in transporting unspliced and partially spliced viral-mRNA transcripts from the nucleus to cytoplasm (Fischer et al., 1994; Malim et al., 1989; Noguchi et al., 2012). A few studies have also suggested restrictions at the viral entry level owing to the absence of CD4-receptor in astrocytes (Boutet et al., 2001; Canki et al., 2001; Schweighardt and Atwood, 2001) and the presence of a compensatory viral entry mechanism. A large body of data has shown HIV-1 entry into astrocytes to be CD4-, CXCR4-, and CCR5-independent (Sabri et al., 1996; Schweighardt et al., 2001). Thus, HIV-1 may use alternate receptors such as mannose receptor or DC-SIGN (Deiva et al., 2006) to enter astrocytes. Using VSV-pseudotyped virus or transient transfection of HIV-1 infectious molecular clone in astrocytes showed high levels of HIV replication and release of infectious virus particles (Bencheikh et al., 1999; Tornatore et al., 1991). Thus, suggesting that the intracellular environment in astrocytes is conducive to HIV-1 replication.
In our earlier study (Vijaykumar et al., 2008), HIV infection in astrocytes has been shown to occur via endocytosis. However, this study did not investigate the mechanism of viral endocytosis. Endocytosis is a complex process wherein virus particles move along the intracellular endocytic pathway involving early, late, and recycling endosomes. Endosome functioning involves Rabs and associated proteins (Martinez and Goud, 1998). Rabs are gaunosine triphosphatases (GTPases) of the Ras superfamily. Rab proteins are important factors in discriminating between pathways leading to different intracellular locations (Macovei et al., 2013; Mainou and Dermody, 2012). Rab proteins regulate specific steps of endocytosed vesicles from the plasma membrane to early endosomes (Rabs 4 and 5) (Gorvel et al., 1991), late endosomes and lysosomes (Rab7) (Bucci et al., 2000), and vesicle-recycling endosomes (Rabs 4 and 11) (Ullrich et al., 1996; Urbe et al., 1993).
To investigate the regulation of HIV-1 infection in astrocytes, we used sensitive detection methods to examine endocytosis- mediated viral infection. HIV-1 infection was minimally productive owing to viral entry restrictions and degradation of endocytosed viral particles in endosomes. The productive HIV-1 infection in astrocytes persisted for several months without cell death and with no transinfection to bystander astrocytes.
Results
Minimum productive HIV-1 infection independent of CD4 and CXCR4 in astrocytes
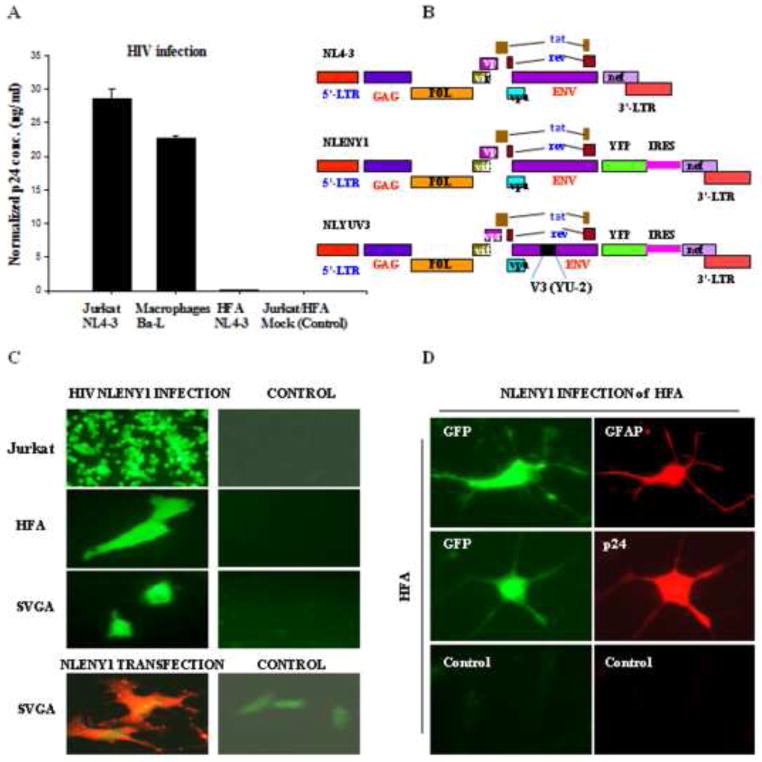
To elucidate the mechanism of HIV-1 infection in astrocytes, we initially infected HFA and SVGA or SVGA reporter cells (Table S1), using either recombinant (NLENY1) YFP-expressing NL4-3 virus (Fig. 1A, B) (Kutsch et al., 2002; Mehla et al., 2010; Vijaykumar et al., 2008) or wild-type virus (Fig. 1A, B). Experiments were also done with HIV-1 (wild-type) on SVGA reporter cells containing either HIV-LTR-GFP/RFP (Tat-dependent) or HIV-LTR-gag-GFP-RRE (Rev-dependent) DNA cassettes (Table S1) (Zhang et al., 2010). Following infection of astrocytes with HIV Ba-L, HIV-IIIB, NL4-3, or NLENY1 viruses, we observed a weak infection (Fig. 1A). Productive infection at the single-cell level (Fig. 1C) was corroborated by p24 immunostaining in YFP-positive HIV-infected GFAP+-astrocytes (Fig 1C, D), although, viral infection was undetectable by p24 ELISA in culture supernatants (Fig. 1A). Live fluorescence microscopy, however, showed infection in HFA on Day 5, which peaked around Day 15 after infection. In subsequent studies, we used T-tropic HIV-1 NL-4-3 genetically engineered (NLENY1, X4) and M-tropic NLYUV3 (R5) as reporter viruses to infect HFA. The overall natural HIV-1 infection in primary astrocytes was less than 0.025%, but profound infection occurred in primary macrophages and lymphocytes (Fig. 1A). Further validating HIV-1 infection, ectopic introduction of HIV-1 infectious molecular clone into SVGA cells showed abundant p24 expression on immunostaining (Fig. 1C bottom panel).
Figure 1. HIV-1 infection in astrocytes.
(A) HIV-1 infection in Jurkat (NLENY1), primary macrophages (Ba-L), and primary human fetal (HFA) astrocytes (NLENY1). Cells were infected with HIV-1, then the culture supernatants from infected or uninfected cells were collected, and analyzed for viral p24 (ELISA). p24 levels in ng/ml, Jurkat (7dpi), macrophages (12 dpi), and HFA (15 dpi) are shown. (B) Diagrammatic view of HIV-1 infectious molecular clones, NL4-3 (wild-type), genetically engineered NL4-3 showing YFP (NLENY1) insertion and NLYUV3 (R-tropic) wherein V3 region of NL4-3 is replaced with V3 of HIV-1 YU-2. (C) HIV-1 infection in HFA: Upper panel shows productive NLENY1 infection after 7 days in Jurkat cells (positive control). Top second panel: HFA infection with NLENY1 virus (1.0 μg/mL p24) showing YFP fluorescence at 10 days post infection (dpi). Top third panel: SVGA were infected with NLENY1 virus (1.0 μg/mL p24) showing infected (YFP positive) cells (5dpi). Bottom panel: SVGA cells transfected with NLENY1 infectious molecular clone or pEGFP vector were immunostained after 48 h for p24 protein. (D) NLENY1 infected HFA: upper panel shows HIV-1 infection (YFP-positive) in HFA (GFAP- positive); middle panel: NLENY1 infection in HFA showing YFP and p24 expression. Bottom panel: uninfected HFA as controls for p24 antibody and isotype IgG (negative control)
A weak HIV-1 infection was observed in astrocytes, possibly because of a viral entry restriction or a post-entry block in viral replication, as suggested in earlier studies (Canki et al., 2001; Gorry et al., 1999; Neumann et al., 1995; Schweighardt and Atwood, 2001; Schweighardt et al., 2001). To resolve the issue of low HIV-1 infection, we first investigated astrocytes for expression of viral entry receptor CD4 and chemokine receptors CXCR4 and CCR5. RT-PCR showed a positive message for CXCR4 and a weak message for CD4 amplicons (Fig. S1), but flow cytometric analysis confirmed only CXCR4 expression (~94%) in SVGA and HFA (Table S2). To corroborate the general expression pattern of these receptors, astrocytes from different human fetuses were analyzed by flow cytometry, which consistently showed expression of CXCR4, but not CD4 and CCR5 (data not shown). The intracellular adhesion molecule-1 (ICAM-1) from host cells is packaged by HIV-1 in the viral membrane (Beausejour and Tremblay, 2004; Fortin et al., 1997; Liao et al., 2000; Paquette et al., 1998) and has been shown to be involved in HIV-1 entry through CD11a (LFA-1) as a compensatory viral entry mechanism (Fortin et al., 1998; Goudsmit and Smit, 1990). In the current study, a transcript message for LFA-1 was detectable by RT-PCR (Fig. S1), but was undetectable by flow cytometric analysis on HFA and SVGA (Table S2). Subsequently, flow cytometry investigations of astrocytes for DC-SIGN and mannose receptors (MR) were also futile (Table S2).
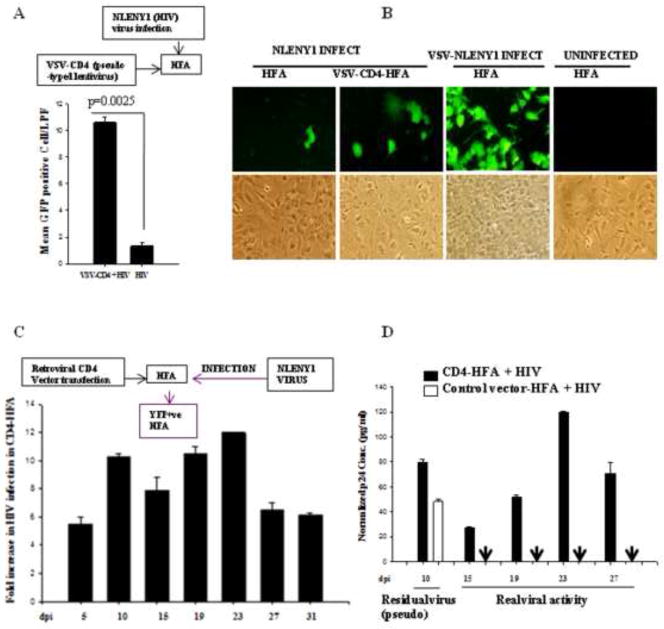
Given that HFA, naturally express CXCR4 chemokine receptor, we ectopically (transiently) expressed CD4-receptor on HFA to determine whether viral entry is the first restriction. Lentiviral- or transfection-mediated expression of CD4 on HFA was followed by NLENY1 virus infection. Viral replication was monitored by intracellular GFP expression (Fig. 2A, B) or p24 in culture supernatants for 3–4 weeks (Fig. 2C, D). The efficiency of viral infection was 5–12 fold higher in CD4-expressing astrocytes than in control vector-transfected astrocytes (Fig. 2A, C), with an incubation period similar to that of wild-type HFA (Fig. 2D). p24 levels in the culture supernatants also suggested productive infection, but failed in wild-type HFA infected with HIV-1 except between days 3–10, which was found to be residual (pseudo) viral activity that did not correlate with GFP expression (Fig. 2D). These observations indicated that HFA can retain HIV-1 without viral replication for at least 10 days post infection, and this process is independent of CD4, CXCR4, CD11a, and mannose receptors (Figs. S1, S2 and Table 1). Overall, these observations suggested the presence of a viral entry block, although one that was not complete for productive HIV-1 infection.
Figure 2. Ectopic CD4 expression in astrocytes increase HIV-1 infection.
(A) HFA were transduced with either CD4-expressing or empty lentiviral particles, and 24 h later, were infected with NLENY1 virus. In parallel, mock-infected HFA cultures were used as control. Infected cultures were followed for 15 days. YFP-positive (HIV) HFA were counted in 10 low-power fields (lpf) and the mean plotted (p ≤ 0025). (B) YFP-positive (HIV-1 infected) cells in CD4 expressing and wild-type HFA were observed by UV microscope at 15 days after infection. (C) HFA were transfected with CD4-expression vector and 48 h later infected with NLENY1 (1.0 μg per mL p24). HIV-1 infected HFA or CD4-expressing HFA were monitored by counting YFP-positive cells upto Day 31 after infection in the entire dish in duplicate every 5th day and fold-increase was calculated. (D) HIV-1 infected CD4-expressing HFA secrete p24 in extracellular culture medium. HFA-transfected cells with empty or CD4-expressing vectors were infected with NLENY1 virus (1 μg/ml). Cultures were monitored up to 27 days to determine YFP expression by fluorescence and p24 in culture supernatants by ELISA.
Table 1.
Principal features of HIV-1 infection in primary human astrocytes
|
Permissiveness of astrocytes to HIV-1 infection in co-cultures
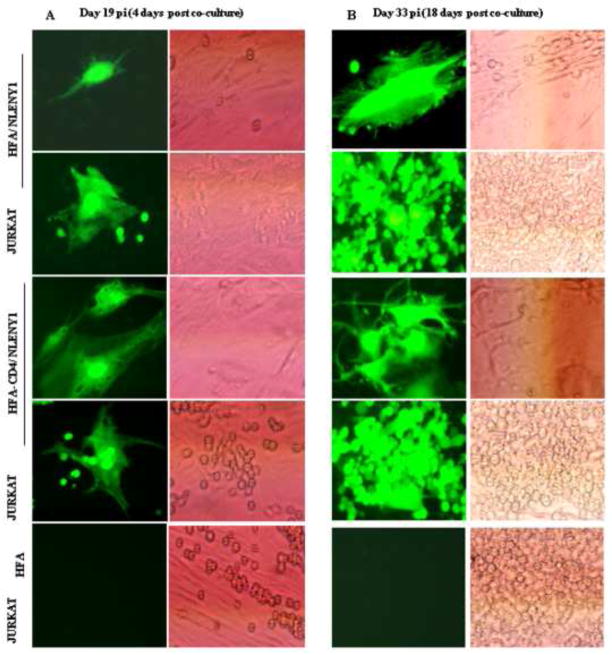
Since cell-free HIV-1 particles produced extremely weak infection, we explored whether HIV-1-infected lymphocytic (Jurkat) cells can efficiently transinfect primary astrocytes in co-culture. We first confirmed that HIV-1-infected astrocytes produce infectious virus particles, then infected HFA with HIV-1 and co-cultured them with uninfected Jurkat cells, monitoring infection by p24 antigen in the supernatants. For specificity, we delayed the start of co-culture until Day 15 after infection to ensure that any residual input viral activity retained on astrocyte surfaces had been completely washed out and that residual p24 levels were, by that time, below the detection limit (Fig. 2D). Virus transinfection was bolstered by HIV-1 infected HFA and CD4-HFA upon co-culture with uninfected lymphocytic cells at Day 4 and Day 18 after co-culture (19 and at 33 days post infection of HFA) (Fig. 3A, B). Infected lymphocytes were seen near or on top of infected YFP-expressing (infected) astrocytes (Fig. 3) more often in CD4-HFA, but by Day 18, almost all lymphocytic cells had become infected in both CD4 and non-CD4 expressing HIV-1-infected astrocytes (Fig. 3B). Live fluorescence microscopy showed that HIV-1 infected (green fluorescent) astrocytes did not transinfect neighboring astrocytes in 33 days of follow-up and that cytolysis of infected astrocytes was not apparent (Fig. 3; Table 1).
Figure 3. NLENY1-virus-infected HFA transinfect lymphocytes.
(A–B) HFA were transfected either with empty or CD4-expressing vectors and, 24 h later, infected with NLENY1 virus. After extensive washing at 15 days after infection, HFA were co-cultured with uninfected lymphocytic cells. (A–B) Cultures were observed for YFP-positive lymphocytic cells. NLENY1-infected HFA (19 or 33 days; A, B) showing transinfection to lymphocytic cells at (A) 4 and (B) 18 days after co-culture.
We further tested transinfection in HFA upon co-culture with HIV-1-infected lymphocytic cells. Jurkat cells were infected with NLENY1 virus and, at the peak of infection (7 dpi), co-cultured with HFA for two weeks. The transmission of infection to HFA was identified by green fluorescence in large leaf-like cells (astrocytes). On live fluorescence microscopy, we observed minimum HIV-1 infection in co-cultured HFA, as in cell-free virus-infected cultures (Fig. S2). Moreover, mixed brain cultures showed similar HIV-1 infection in astrocytes (Fig. S2). Overall, trans-infection from lymphocytic cells to astrocytes in co-culture was similar to that of cell-free virus-infected astrocytes.
HIV-1 enters via endocytosis in astrocytes
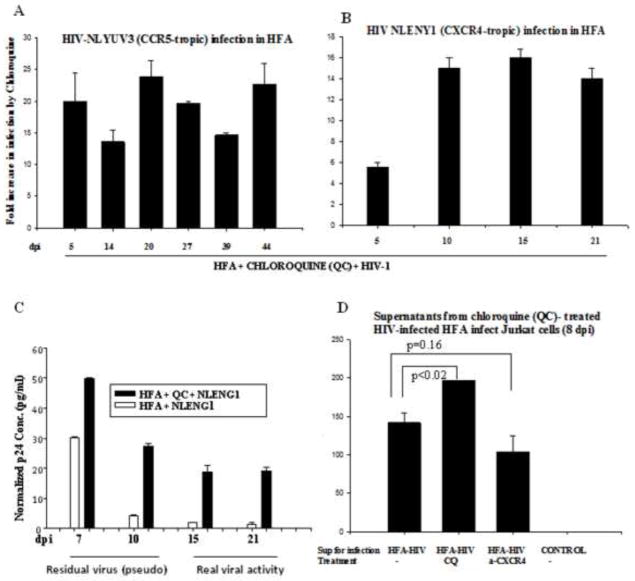
Given that virus particles are taken up by astrocytes without nurturing for several days (Clarke et al., 2006; Vijaykumar et al., 2008), we investigated whether natural HIV-1 infection occurs through endocytosis. To study endocytosis-mediated viral entry, we used endosomal modulators and proteasome inhibitors. Intriguingly, a several-fold increase of HIV-1 infection in HFA upon treatment with endosomal modulators was bolstered (HFA free of microglia) equally by both T-tropic and M-tropic viruses, suggesting pH-dependent endosomal-mediated viral entry (Fig. 4A–D). We anticipated that chloroquine and bafilomycin A would confer their effects through changes in endosomal function (an increase in lysosomal pH and inhibition of endolysosomal fusion), as shown in our earlier study (Vijaykumar et al., 2008). Validating the occurrence of viral replication after HIV DNA integration, we found, in some experiments, that siRNA (Table S3) for TNPO3 (Zhang et al., 2010) and emerin (a nuclear membrane protein involved in HIV-1 import), 2-days prior to infection, blocked HIV-integration by TNPO3, but not by emerin in chloroquine-treated HIV-1 infected cultures (data not shown). Further, like ectopic CD4-expressing HFA, chloroquine-treated HIV-1-infected HFA showed viral kinetics such as a pseudoviral p24 peak (residual) at 7–10 dpi; beyond 10 days, however, detectable real viral activity in culture supernatants correlated with fluorescence data (Fig. 4A–C). Authentic and pseudoviral activity was validated after 5 to 20 days of HIV-1 infection by YFP-positive astrocytes in combination with p24 ELISA on culture supernatants. The supernatants collected from chloroquine-treated, but not untreated HIV-1-infected HFA, successfully infected lymphocytic cells (Fig. 4D). Further, supernatants from anti-CXCR4-HIV-infected HFA showed infection of lymphocytic cells (Fig. 4D), indicating that CXCR4-antibody (10 μg/ml) or AMD (CXCR4 blocker) did not circumvent X4-HIV infection in astrocytes. Subsequently, mannan pretreatment (30 mg/ml) of HFA also did not block HIV-1 entry (data not shown).
Figure 4. Endosomal modulators (lysosomotropic agents) regulate HIV-1 infection in astrocytes.
(A–B) Two-month old HFA seeded overnight in 6-well plates were either pre- or post treated with chloroquine (2.5–5.0 μM) for 1 h, then infected for 2 h with either NLYUV3 (M-tropic) (A) or NLENY1(T-tropic) (B) with 1.0 μg/ml of p24 equivalent. Infection was monitored by YFP fluorescence in entire well in duplicate and plotted as fold-increase from untreated infected cultures. (C) HIV-1 infected HFA left untreated or treated with chloroquine were followed for 21 days. ELISA for viral p24 protein on culture supernatants from HIV-1 infected HFA was positive up to 10 days in both chloroquine (QC)-treated and untreated HIV-1-infected cultures. In first 10 days there was a pseudo-p24 peak that did not correlate with fluorescence microscopic data. (D) Untreated HFA or HFA treated with chloroquine or CXCR4 antibody (α-CXCR4), was infected with NLENY1 virus (1 μg/mL p24). Fifteen days later, supernatants were used to infect normal Jurkat cells. Eight days later, supernatants from infected Jurkat cells were collected and analyzed for p24 by ELISA. Uninfected control cultures were used to normalize p24 levels.
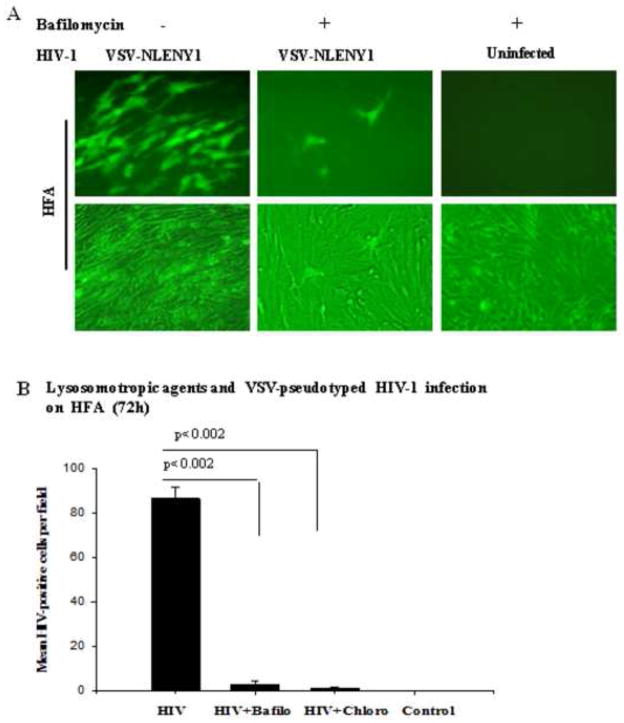
In our earlier studies, although chloroquine behaved as an agonist on chronically HIV-1-infected astrocytes, ensuring that chloroquine abrogates degradation of viral components, it was not involved in direct activation of HIV-1 LTR (Vijaykumar et al., 2008). To confirm that chloroquine in astrocytes modulates the intracellular environment for permissiveness to HIV-1 infection, we established a robust model of HIV-1 infection. Chloroquine-pretreated HFA were infected with VSV-pseudotyped NLENY1 virus, then allowed to stabilize for 5 days to eliminate residual VSV-based viral particles. Chloroquine treatment was then resumed with an initial dose of 5 μM for 3 days, followed by 2.5 μM for another 7 days or the duration of follow-up. A change of medium with added chloroquine was done at day-5 intervals, at which time supernatants were collected for p24 and infection. In parallel, experiments were done with another endocytosis modulator, bafilomycin-A. Chloroquine and bafilomycin A treatment severely impaired VSV-NLENY1 replication in HFA (Fig. 5A, B), as had been found earlier (Schaeffer et al., 2004; Vijaykumar et al., 2008). In contrast, wild-type HIV-1 infection led to a several-fold increase in viral replication in the presence of chloroquine (Fig. 5B). These observations suggested that adequate numbers of HIV-1 particles were entrapped within endosomes in astrocytes but could establish little productive infection owing to increasing degradation or limited escape of virus particles from endosomes.
Figure 5. Lysosomotropic agents inhibit VSV-pseudotyped HIV-1 infection in HFA.
(A) HFA seeded overnight in six-well culture plates were infected with VSV-NLENY1 virus for 2 h, then washed two times with medium. Infected or uninfected cultures were left untreated or treated either with chloroquine (10 μM) or bafilomycin A (100 nM). Cultures were observed for 7 days for fluorescence; YFP-positive cells were observed by UV microscope and photographed. (B) HIV-positive fluorescent (YFP+) cells from (A) were counted in 7–9 fields in duplicate and plotted as mean ± SEM (p ≤ 0.002).
HIV-1 entry via endocytosis in astrocytes involves Rab
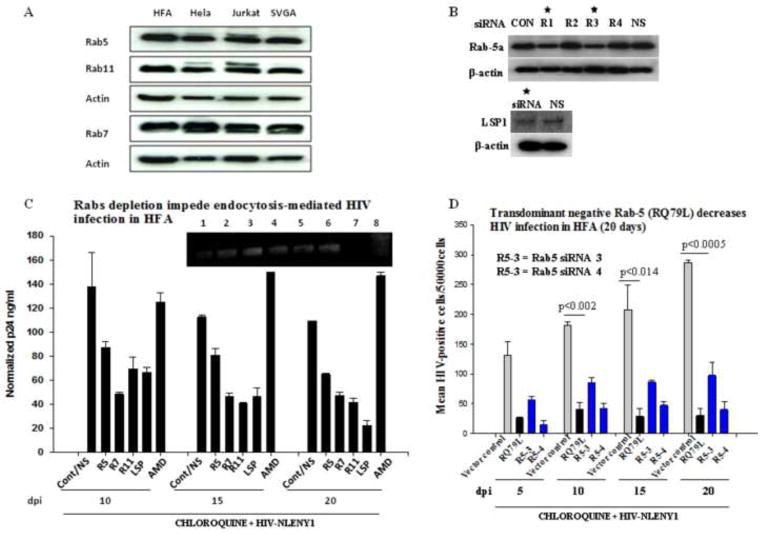
To examine the endocytosis-mediated-HIV-1 entry mechanism in astrocytes, we investigated the role of Rab proteins. We depleted endogenous Rabs (Rab -5a, -7 and -11a) expression in HFA by RNAi. Rab-5 is a key regulator of early endosomes; Rab-7 and -11 are involved in late endosomal processes such as fusion and sorting (Galvez et al., 2012; Mohrmann and van der Sluijs, 1999; Seachrist and Ferguson, 2003; Zerial and McBride, 2001). Natural levels of Rabs were checked in astrocytes (HFA and SVGA) and HIV-1 permissive cells (lymphocytic and HeLa cells) and found to be equally expressed (Fig. 6A). Several siRNAs were tested for their efficacy in depleting Rabs (5, 7 and 11), LSP1, TNPO3, and emerin (Figs. 6B and S3; Table S3). We used the selected siRNAs at 200 nM concentrations. LSP1 and TNPO3 siRNAs were used as positive controls to show HIV-1 inhibition.
Figure 6. Depletion of Rab proteins in astrocytes obliterates endocytosis-mediated HIV-1 infection.
(A) Astrocytes (HFA and SVGA) and HIV-permissive cells (lymphocytic and HeLa cells) grown for 48 h were processed for Western blot using monoclonal antibodies against Rab-5, -7 and -11. (B) Screenings of several siRNAs for depletion of each Rab and LSP1 (50–200 nM) were investigated on HFA by Western blotting. HFA were transfected with 200 nM each siRNA and, 72 h later lysates were analyzed by Western blotting for Rabs. Beta actin was used as a loading control. LSP1 siRNA was tested as a positive control to inhibit HIV-1 and non specific (NS) siRNA as a negative control. (C) Seeded HFA were transfected with 200 nM Rab specific siRNAs in parallel with LSP-siRNA and non-specific siRNA as controls. Then, one set of HFA was treated with CXCR4 blocker AMD3100 (AMD) before HIV-1 infection. At 72 h after siRNA transfection, HFA were infected with NLENY1 virus for 2 h. After washing, all cultures were treated with chloroquine (10 μM). At 48 h after infection (5 days after initial siRNA transfection), infected HFA were re-transfected with the respective siRNAs. p24 levels in culture supernatants collected at 10, 15, and 20 days after infection were analyzed by ELISA (n=4). HFA at 20 days after infection were tested for viral DNA integration by Alu-HIV LTR PCR (inset C). (D) HFA in 6-well culture plates were transfected with control vector, Rab 5a transdominant negative vectors, or Rab 5a siRNA #3 and #4 and, 72 h later infected with NLENY1. After washing, cultures were treated with chloroquine. Cultures were followed for 20 days after infection. YFP-positive HFA were counted in the entire well in duplicate and plotted (n=2).
In 20 days of follow-up, RNAi-mediated ablation of Rab-5, -7, and -11 demonstrated their indispensible role in endocytosis-mediated HIV-1 infection (Fig. 6C). Again, CXCR4 blocker (AMD) did not impair HIV-1 entry into astrocytes. In addition, ablating LSP1 by siRNA (Fig. 6B, D) severely impaired HIV-1 infection in astrocytes with lysosomotropic agents at 10, 15, and 20 dpi (Fig. 6C), indicating the intricate role of LSP1 in HIV-1 infection. LSP1 siRNA was used as a positive control to inhibit HIV-1 infection. Establishing that viral activity occurred postintegration, Alu-LTR integration-PCR showed viral DNA integration at 5, 10, 15, and 20 days after infection (Fig. 6C, inset). Rab-7 and -11 had a far greater impact than did Rab-5 in attenuating viral infection in astrocytes, but this could have been caused by variation in siRNA silencing efficacy. The use of a transdominant-negative Rab-5 expression construct resulted in a significant decrease in HIV-1 infection, thus corroborating the action of Rabs in endocytosis-mediated HIV-1 entry into astrocytes (Fig. 6D). Together, these findings indicate that although the endosomal route is indispensable for HIV-1 infection in astrocytes, it is, at the same time, detrimental, because endosomal internal machinery is least conducive to successful establishment of viral infection.
Effect of proteasome and autophagy inhibitors on HIV-1 infection in astrocytes
As we have shown, endocytosis-mediated HIV-1 entry is the least productive route, although a several-fold increase in viral infection in astrocytes occurred in response to treatment with lysosomotropic agents. However, the overall increase in infection did not surpass 0.5%. Given that HIV-1, apart from the CD4 and either the CCR5 or CXCR4 entry process, enters monocytes, macrophages, and dendritic cells through DC-SIGN and mannose receptor (MR), a small fraction of the virus is degraded in lysosomes. Some of the virus in endosomes recycled to the surface, but the major portion degraded through the proteasome pathway (Cambi et al., 2009; Muratori et al., 2009; Nguyen and Hildreth, 2003). Therefore, we explored the role of the proteasome pathway in HIV-1 infection in astrocytes.
The proteasome inhibitor MG132 and autophagy modulator slightly increased HIV-1 infection in HFA, but not to the extent of that done by endosomal inhibitors (Fig. S4A, inset). Leupeptin, filipin and FAMK did not affect HIV-1 infection (Fig. S4B). Some HIV-1 (particles) enter via endocytic fusion, but some also enters via the proteasome pathway, and hence may be the reason for the low rate of infection in astrocytes. In addition, astrocytes acquire HIV-1 through incomplete plasma membrane fusion. Although this HIV is released within 10 days without actually entering astrocytes, intracellular restrictions other than endosomal and proteasome cannot be ruled out. Thus, we suspected that after HIV-1 entry, viral nucleic acid is degraded by nucleases in astrocytes, resulting in weak infection. To elucidate the role of nucleases in restricted viral infection, we treated primary astrocytes with nuclease inhibitor ATA, then infected them with HIV-1. There was no evidence that nucleases were involved in restricted HIV-1 infection in astrocytes (Fig. S4C).
Intracellular environment in astrocytes conducive to HIV-1
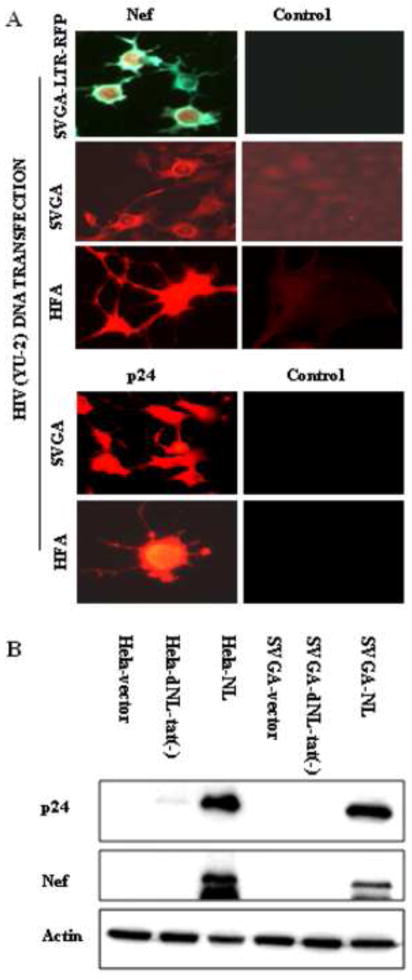
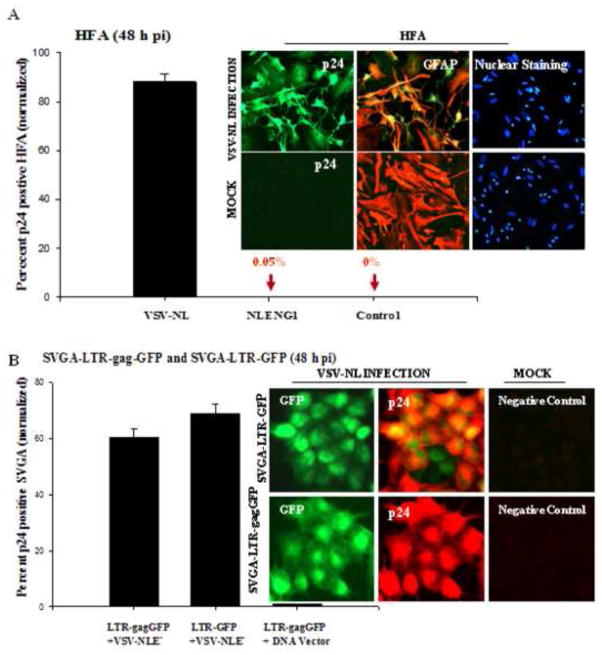
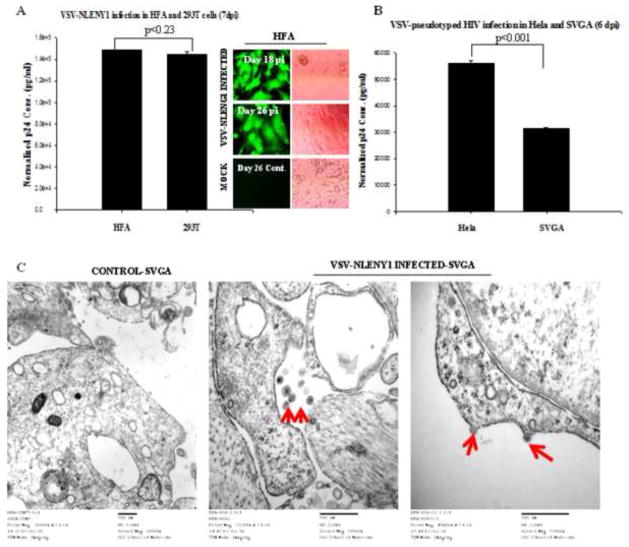
Since HIV-1 infection in astrocytes was limited by viral entry restrictions and endosomal degradation of virus particles, we explored whether transfected HIV-1 infectious molecular clones could replicate efficiently and yield infectious virus particles. Transfection of SVGA-LTR-GFP, SVGA-LTR-gag-GFP/ LTR-RFP cells, or HFA with either YU-2 or pNL4-3 full-length HIV-1 clones led to robust production of the early gene products (proteins) Tat, Rev, and Nef, but not by Tat-mutated HIV-1 molecular clone (Fig. 7A upper panel, 7B). Robust p24 production in astrocytes similar to that in Hela cells occurred at 72 h after transfection of HIV-1 infectious molecular clones (Fig. 7A lower panel, 7B). To corroborate our transfection data (Fig. 7A, B), we performed HIV-1 infection experiments. VSV-G envelope-pseudotyped wild-type HIV-1 either NL4-3 or recombinant NLENY1 was used in experiments on HFA, SVGA-LTRGFP, or SVGA-LTR gag-GFP-RRE cells. In addition to extracellularly released viral protein p24, we used GFP fluorescence as a marker of infection. HIV infection studies on HFA and reporter astrocytes demonstrated robust viral infection in GFAP-positive HFA, but not in uninfected controls (Fig. 8A, inset). Similarly, robust HIV infection was seen in SVGA-LTR-GFP and SVGA-LTR-gag-GFP, suggesting that Tat, Rev, and late viral proteins (p24) were expressed robustly (Fig. 8B, inset). To corroborate these findings (Fig. 8A, B), we infected HFA, SVGA, and HIV-permissive HeLa and 293T cells with VSV-pseudotyped-NL4-3 virus. This resulted in highly productive infection in HFA and 293T cells (Fig. 9A), as well as SVGA and Hela cells (Fig. 9B). Further, there was no difference in productive HIV-1 infection between HFA and 293T cells (Fig. 9, p < 0.23).
Figure 7. Conducive intracellular environment for HIV-1 replication in astrocytes.
(A) Late HIV-1 gene expression in HFA and SVGA upon transfection with HIV-1 infectious molecular clone. SVGA, SVGA-LTR-RFP reporter cells, or HFA were transfected with molecular HIV-1 clone (YU 2). In controls, empty vector DNA was transfected. At 48 h after transfection, cells were immunostained with monoclonal antibody for Nef (green) and observed under a UV microscope using a double filter (green/red). Left top panel: Nef immunostaining in SVGA-reporter cells; Left second panel: Nef expression in SVGA cells. Left third panel: Nef expression in HFA. Left fourth and bottom panels: HIV-1 late gene (p24) expression in SVGA and HFA. Cultured SVGA and HFA in flaskets were transfected with HIV-1 infectious molecular clone (YU-2) and, 48 h later, immunostained for p24 using monoclonal antibody (NIH). Panels on the right show p24 antibody on normal astrocytes as control. (B) Cultured SVGA and HeLa cells in six-well plates were transfected with 1 μg HIV-1 molecular clone of NL4-3, mutant NL4-3 (Δtat), or empty control vector; 72 h later, cell lysates were prepared. After protein normalization, Western blot analysis for viral Nef and p24 proteins was done in parallel with analysis for beta actin on the same blots.
Figure 8. Robust HIV-1 late gene expression in astrocytes.
(A) HFA cultured for 48 h were infected with VSV-pseudotyped NL4-3 virus (200 ng/ mL p24) and, 48 h after infection, were immunostained for GFAP, p24 and nuclei. Images were captured under a fluorescence microscope (inset). Cells positive for both GFAP (red) and p24 (green) were randomly counted in 10 fields with total nuclear counts (blue) in duplicate sets; mean ± SEM were plotted using a sigma plot. (B) VSV-pseudotyped HIV-1 infection in SVGA-LTR reporter cells: Stable SVGA-LTR GFP or LTR-gagGFP-RRE reporter cells were seeded overnight in six-well culture plates. Next day, cells were infected with VSV-NLE−R+ virus for 2 h (200 ng/ mL p24) and washed. At 48 h after infection, cells were immunostained for p24 (red) and nuclei (blue). Dual-positive cells (green and red) were counted in ten random fields in duplicate cultures and mean positive cells ± SEM were plotted.
Figure 9. Productive HIV-1 infection in primary astrocytes.
(A) HFA and 293T cells were infected with VSV-pseudotyped NLENY1 virus (100 ng/ mL p24). In parallel, untreated HFA and 293T cells were used as mock controls for normalization of p24 levels in the supernatants. Infected and uninfected culture supernatants from HFA and 293T cells at 7 days after infection were monitored for p24 by ELISA (p ≤ 0.23). Infected HFA were observed under UV microscope (inset). (B) Hela and SVGA cells were infected with VSV-pseudotyped-NL4-3 (100 ng/mL p24). Six days later, supernatants from infected and uninfected cells were monitored for p24 by ELISA (p ≤ 0.001). (C) HFA were infected with VSV-pseudotyped HIV-1 and, 48 h later, processed for transmission electron microscopy (TEM). Mock-infected HFA were used as control. Virus particles of 150–250 nm were observed intracellularly or at the membranes (40,000 magnifications).
Transmission electron microscopic (TEM) examination of HIV-1-infected HFA showed competent 150–250 nm viral particles inside infected, but not uninfected astrocytes (Fig. 9C). There also was viral budding from the membranes of these infected astrocytes (Fig. 9C third panel), as in earlier studies of other cell types (Mergener et al., 1992; Mlcochova et al., 2013; Sundquist and Krausslich, 2012). Thus, upon successful viral entry via pH-dependent endocytosis (VSV-HIV), normal virus reverse transcription, integration, transcription, and production of infectious virus particles occurred in astrocytes (Figs. 7–9).
HIV-1 produces persistent infection in astrocytes
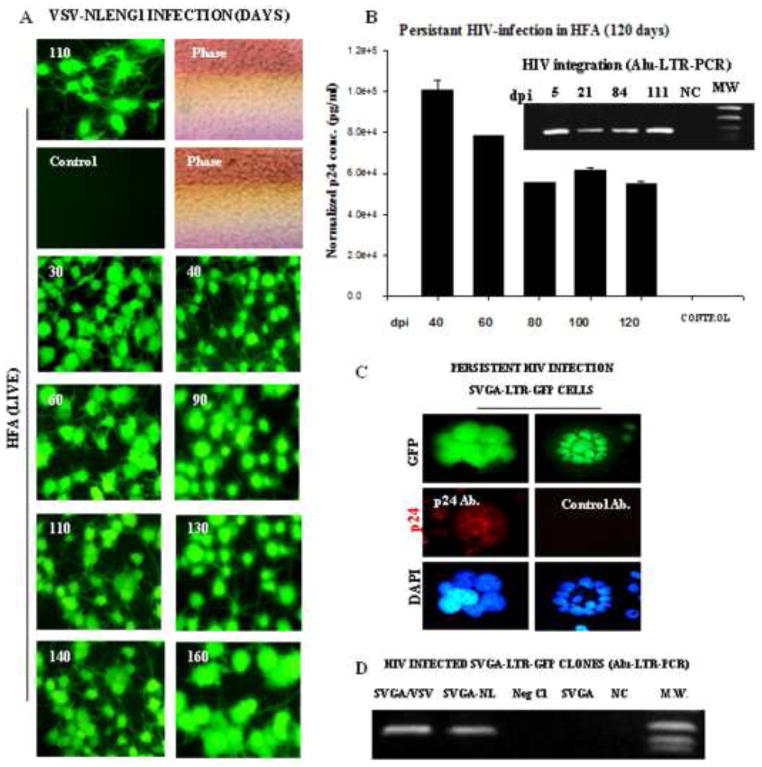
To investigate the persistence of productive infection, we followed VSV NLENY1 virus- infected HFA from Day 26 through Day 160 using viral p24 and YFP fluorescence as markers every 10 days. We observed robust YFP expression (Fig. 10A) and high levels of p24 in HIV-infected HFA (Fig. 10B). The p24 levels in HIV-infected astrocytes declined sequentially through 160 days, but still were maintained at high levels (Fig. 10B). Infected HFA did not transinfect bystander astrocytes, as shown by the fact that number of uninfected cells was continuously seen on follow-up (Fig. 10A), despite a high viral load (Fig. 10B). In contrast, 100% of Jurkat cells became infected by 10 days after infection. At 111 days postinfection of HFA, the Alu-HIV-LTR PCR showed HIV-1 integration (Fig. 10B inset), suggesting viral replication from an integrated DNA state. To validate these observations and replicate the infection in astrocytic cells, we generated VSV- NL4-3.HSA.R+E− virus-infected-SVGA-LTR-GFP clones. In follow-up, these cultures continued to display green fluorescence and the green cell population was subcloned further. The sustained green fluorescence in SVGA-LTR-GFP cultures was an indication of HIV-LTR transactivation by Tat (Fig. 10C). Immunostaining these persistently HIV-1-infected fluorescent cells for p24 was positive (Fig. 10C), indicating a productive infection.
Figure 10. Persistent HIV-1 infection in astrocytes.
HFA in T-25 flasks were infected with VSV-NLENY1 virus and followed for 160 days. HIV-1 infection was monitored by YFP expression in infected HFA and p24 secretion in culture supernatants (A, B). (A) VSV-NLENY1- infected HFA showing YFP expression (live). (B) Viral kinetics in long-term (40 to 120 days) HIV-1 infected HFA is shown. Control (NC) is a mock-infected HFA. Alu-HIV-LTR PCR demonstrated HIV viral genome integration in infected HFA from 5 to 111 days after infection (inset B). (C) Persistent infection of HIV-1 in astrocytic (SVGA) cells: SVGA-LTR-GFP reporter cells were infected with HIV-1 NL4-3 and GFP-expressing cells were cloned. Clonal population of persistently HIV-1 (NLE−R+)-infected SVGA-LTR-GFP cells (chronic infection) showing sustained green fluorescence and p24 expression by immunofluorescence. (D) HIV-1 DNA integration by Alu-HIV-LTR PCR in NLE−R+ SVGA-LTRGFP infected cells is shown.
To rule out Tat and Rev transcription from unintegrated circular HIV-1 DNA (Kelly et al., 2008), clones of SVGA-LTR-GFP/VSV-NL were verified for viral integration by nested Alu-HIV-LTR PCR (Fig. 10D). Given that the HIV-1 virus used for infection (reporter astrocytes) was mutated in the envelope region (NL4-3.HSA.R+E−) (He et al., 1995), we tested the ability of persistently infected astrocytes to produce infectious virus by transfecting these green fluorescent cells with VSV-G envelope plasmid to rescue infectious virus particles. We analyzed the VSV-NL culture fluids for p24 and infection of Jurkat cells, finding that cultures were negative for p24 and that there was no infection in Jurkat cells (data not shown). However, we used the envelope-mutated NL4-3 virus pseudotyped with VSV-G envelope, which may not have been efficiently rescued by ectopic-VSV expression in persistently infected green cells. Overall, our observations on HIV-1 infected HFA and SVGA cells indicated the persistence of productive HIV-1 infection.
Un-inducible persistent HIV-1 infection in astrocytes
Since our observations on persistent HIV-1-infected SVGA-LTR-gag-GFP or LTR-GFP reporter cells demonstrated sustained production of Tat and Rev, as well as low intracellular p24, we investigated whether low p24 expression was caused by under-expression of Tat or Rev or was a partially latent state. Also, HFA that has been persistently infected for 160 days has high levels of extracellular p24. Given the fact that HIV reverts to a latent state in other cell types, we hoped to determine whether the same phenomenon occurs in long-term infected astrocytes. We pseudotyped HIV-1 (NLENY1) with VSV-envelope and used this to infect SVGA cells. For 21 days, we followed the infected cells to determine p24 expression. Persistently infected green fluorescent cells were sorted by FACS, then cultured and subcloned into nonfluorescent and fluorescent populations.
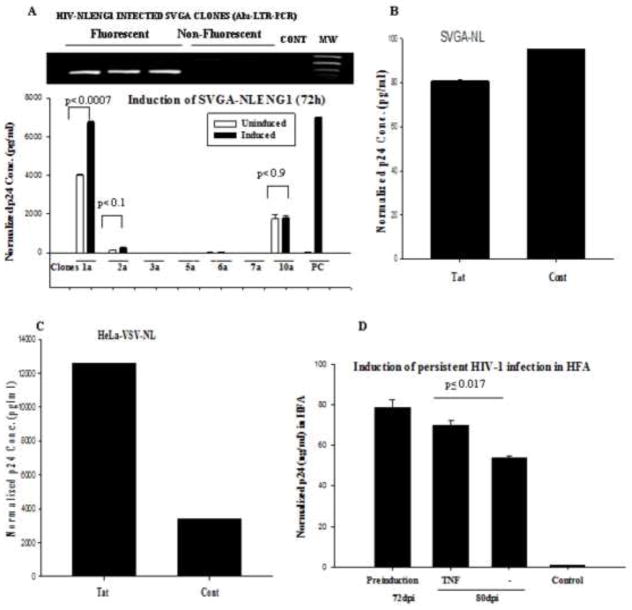
The clones were verified by viral DNA integration using standard Alu-integration PCR strategy. The infected fluorescent (YFP+) cells showed HIV-1 DNA integration (Fig. 11A inset). Also, p24 in culture supernatants was positive in four fluorescent clones (Fig. 11A), while nonfluorescent clones were negative. We initially induced 7 clones of SVGA-NLENY1 with TNF-α and monitored p24 levels in the supernatants. However, we could not ascertain HIV-reactivation beyond basal levels except in clone 1a and control (positive) latent HIV-1-infected monocytic THP89GFP cells (Fig. 11A). Persistently HIV-1-infected astrocytic clones induced by TSA, TNF-α, Tat, or IL-1β did not show activity of LTR and p24 above basal levels (Figs. 11B and S5). Therefore, we established persistently infected Hela (Hela-VSV-HIV) cells, using VSV-NLENY1 virus (Table S4), and investigated the regulation of HIV-1 using p24 assay.
Figure 11. Reactivation of persistent HIV-1 infection in astrocytes.
(A) Seven persistently HIV-1 infected clones of astrocytic cells (SVGA-NLENY1) were treated with TNF-α (10 ng/mL) for 72 h. In parallel, latently HIV-1 infected THP89-GFP cells were used a positive control. The supernatants were monitored for p24 by ELISA. One clone was inducible (p < 0.05); 6 were not (p ≤ 0.1, 0.9). (B–C) Persistently VSV-pseudotyped NLENY1-infected SVGA and Hela cells were cultured in six well plates and transfected with Tat or control vectors (0.5 μg per well). After 72 h, supernatants were analyzed for p24 in SVGA-NLENY1 (p ≤ 0.905) and Hela-NLENY1 cells (p ≤ 0.000005). (D) Persistently HIV-1-infected HFA were cultured overnight, then treated with TNF-α. At 48 h after treatment, supernatants from HFA were tested for p24 by ELISA. Controls were untreated HIV-1 infected HFA or TNF-a treated uninfected HFA.
To explore the potential of Tat in the reactivation of HIV, we ectopically expressed Tat in persistently infected SVGA-VSV-NLENY1 and Hela-VSV-NLENY1 clones. p24 levels in the supernatants after 72 h on SVGA-HIV-YFP cultures did not suggest induction or reactivation as compared to control latent HIV-1 infected Hela cells (Fig. 11B, C). To corroborate the astrocytic data, we investigated the role of TNF-α in long-term HIV-1- infected HFA. After 72 days, we treated long-term HIV-1- infected HFA with TNF-α. It should be noted that long-term HFA infection always led to high levels of virus in the supernatants; indubitably, these HIV-1 infected cultures were unresponsive to TNF-α (Fig. 11D). Moreover, no effect occurred upon overexpression of Tat and Rev (data not shown), suggesting persistent productive infection without a latent state.
Persistently HIV-1 infected astrocytes transinfect lymphocytes but not astrocytes
Having found weak but persistent HIV-1 infection in astrocytes, we investigated whether persistently HIV-1-infected astrocytes can transinfect astrocytes and lymphocytes. HIV-1 infected SVGA cells with weak p24 expression were chosen for transinfection studies. Transfected SVGA cells with HIV-1 infectious molecular clone (NLENY1) were cultured for two weeks and the fluorescent cell population was cloned, then sub-cloned multiple times after 8 weeks of culture and investigated for viral replication. Even after repeated cloning, cell clones still progressively lost green fluorescence. Later, we found that this was a consequence of cell death and the emergence of a nongreen cell population seen only in astrocytic and not persistently infected HFA.
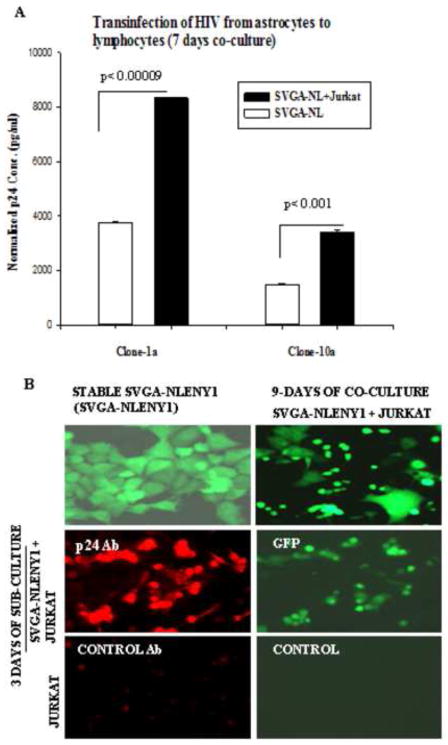
Transinfection was successful in the co-culture of stable HIV-1 YFP-positive clones Cl-1a and -10a of SVGA (SVGA-NL) with Jurkat cells; p24 levels after co-culture were significantly elevated (Fig. 12A, p ≤ 0.009, p ≤ 0.001). In other SVGA-NL clones, p24 was negative on immunostaining, but fluorescence for YFP was positive; the absence of p24 was confirmed in culture supernatants. Intriguingly, on co-culture of uninfected lymphocytic cells with stable SVGA-NL-AC clone, transinfection after 9–30 days of co-culture was shown by the appearance of YFP fluorescence and production of p24 in lymphocytic cells (Figs. 12B and S6). Indeed, viral reactivation was ruled out by fact that TNF-α could not induce p24 in these clones, as shown earlier. Although co-stimulation upon co-culture, especially involving the CD40-CD154 pathway, might have been possible, we ruled this out because IL-6, IL-8, and IFN-γ had no direct role in viral replication (Mehla et al., 2012). Also, persistently HIV-1-infected HFA at 160 days after infection still had uninfected astrocytes. Thus, our observations indicated that extremely weak HIV-1 infection occurs by endocytosis and that infected astrocytes can serve as long-term and productive viral reservoir (Table 1). However, the significance of low HIV-1 infection in astrocytes contributing to neurological complications is unclear.
Figure 12. Persistently HIV-1 infected astrocytes transinfect lymphocytes.
(A) Persistently NLENY1-infected SVGA clone-1a or clone-10a was co-cultured with Jurkat cells for 7 days (cell-free wild-type HIV-1 normally infects Jurkat cells between 3–7 days). In controls, SVGA-pcDNA (stable) cells were co-cultured with uninfected lymphocytic cells; persistently infected HIV-1-infected cells served as base control. (B) The supernatants from (A) were analyzed for p24 by ELISA (p < 0.001, p < 0.0009).
Discussion
The paucity of data on HIV-1 infection in astrocytes is presumably a consequence of the complexity of infection and failure to detect authentic viral infection. Although earlier studies showed astrocytes to be nonpermissive to HIV-1 infection (Gorry et al., 1998; Gorry et al., 1999; Neumann et al., 1995), several studies have now shown that HIV-1 infection in astrocytes is productive (Brack-Werner, 1999; Churchill et al., 2006; Churchill et al., 2009; Wiley and Achim, 1997). In the current study, irrespective of viral tropism, we have shown an extremely low rate of natural HIV-1 infection in astrocytes, which was independent of known receptors and co-receptors. Intriguingly, ectopic CD4 expression on HFA demonstrated a ~16–80 fold increase in HIV-1 infection, with a peak at Day 12 postinfection. Our observations like those of others (Canki et al., 2001; Liu et al., 2004; Schweighardt and Atwood, 2001; Schweighardt et al., 2001), indicate that HIV-1 entry into astrocytes is restricted by the absence of classical CD4-receptor. However, we found no postviral entry restrictions; the details will be published separately.
Canki et al. (2001), have shown efficient production of 9-, 4- and 2-kb viral mRNA transcripts and production of p24 in VSV-pseudotyped HIV-1-infected astrocytes. This is similar to our observations on Tat, Rev, Nef, and p24 translated products. Our use of NLENY1 viruses or fluorescent astrocytic reporter cells unambiguously revealed extremely low productive infection by T- or M-tropic viruses, which was far below the limit of the p24 detection assay (ELISA). We found that viral activity in astrocytes, which was detectable up to 10 days after infection, was actually residual, as has been suggested for other cell types (Bounou et al., 2004). It seems that the residual viral activity is more prominent in astrocytes than in Hela or epithelial cells (Bounou et al., 2004; Gorry et al., 1999).
On follow-up of HIV-1 infection in astrocytes from Day 0 to 21 postinfection, we found that p24 levels peaked at Day 3, declined by Day 10, and became undetectable at 3 weeks. These findings were similar to those of other studies (Canki et al., 2001; Vijaykumar et al., 2008). Intriguingly, on monitoring the live fluorescence of infected astrocyte cultures, HIV-1 infection was first observed on Day 5 and peaked on Day 12 postinfection, which did not correlate with extracellular p24 levels for the initial 10 days. This suggested that pseudo-viral activity occurred during the first 10 days of infection. Similar results were obtained by infecting ectopically-CD4-expressing HFA or astrocytic reporter cells, suggesting an incubation period of 4–5 days in more than 20 experiments.
Thus, we provide exquisite evidence that infection between Days 1 and 10 after HIV-1 infection of astrocytes is, in fact, a result of the adsorbed viral inoculum, not the true replicated virus that is released extracellularly. In contrast, previous studies have shown peak infection in astrocytes on Days 3 or 7 (p24 ELISA), with infection entering latency by Day 10 (Tornatore et al., 1991). Clarke et al., (2006), demonstrated that astrocyte-bound virus is initially released during the first three days after infection, with no evidence of viral DNA integration. They attributed the early HIV-1 transcript synthesis to prepackaging of multiply spliced HIV-1 mRNA transcripts during viral assembly in the permissive cells, and hence, translation of these transcripts after virus uptake in astrocytes without viral replication. In the same study, Clarke and coworkers demonstrated that no viral RNA reverse-transcription occurred in astrocytes.
In contrast, our use of stringent recombinant HIV-1 YFP virus infection (fluorescence) on primary astrocytes (depleted for microglia) demonstrated authentic HIV-1 infection (fluorescence). This was supported by viral DNA integration, an increase in viral infection after treatment with lysosomotropic agents, and specific inhibition of viral activity, demonstrated by targeting viral integration or viral DNA import using TNPO3 siRNAs. We therefore, argue that if translation of prepackaged early viral transcripts occurs as shown by Clarke et al. (2006), the translated products will be far below the threshold levels required for HIV-LTR transcription, as well as below the limit of detection (protein). Nonetheless, we observed intense green fluorescence in sporadically infected astrocytes for three weeks after infection, after which these GFP-positive astrocytes, but not nongreen cells in the same cultures, were capable of infecting Jurkat cells in co-cultures.
In further experiments, we were unable to detect p24 between Days 10 and 21 post infection except in ectopic CD4-expressing or chloroquine/bafilomycin- or MG132- treated HIV-infected cultures. HIV-1 infected either CD4-negative astrocytes or CD4-expressing-astrocytes transinfected lymphocytes in co-culture. HIV-1 in these co-cultures was detected only in Jurkat cells sitting near or on the top of the infected (GFP) astrocytes. HIV-1 infection in HFA was corroborated by viral integration and secretion in short- and long-term infected cultures for 160 days of follow-up. Our co-culture studies on HIV-1- infected Jurkat cells with normal astrocytic reporter cells (SVGA-LTR-RFP) did not show increased susceptibility to infection. In contrast, co-culture of persistently infected or stable HIV proviral DNA-transfected astrocytes (SVGA-HIV-GFP) demonstrated successful transmission of viral infection to lymphocytic cells, but not astrocytes. Notably, in earlier studies on persistently (latent) HIV-1 infected astrocytes, viral replication had been shown to be reactivated using cytokines such as TNF-α and IL-1β (Tornatore et al., 1991). In stark contrast, our studies demonstrated chronic HIV-1 infection in primary astrocytes for 160 days postinfection with no sign of latency. Similarly, other studies have reported persistent infection in human astrocytes using VSV-pseudotyped HIV-1 viruses (Bencheikh et al., 1999; Canki et al., 2001; Tornatore et al., 1991). These studies have shown detectable p24 levels only up to Day 49 of infection, although the infection of susceptible cells by supernatants from these infected astrocytes was tested only from Days 14 and 21 after infection. In contrast, we found that supernatants collected beyond Days 41, 51, 61, 71, 81, 91, 101, 111, or 120 postinfection of HFA continued to have high viral activity and were able to infect lymphocytic cells within 4 days.
Normally, HIV-1 enters via a pH-independent pathway (natural infection) using a classical receptor-coreceptor mechanism with the occurrence of viral and cell membrane fusion in permissive cells. However, it seems that virus mainly enters astrocytes via a pH-dependent endocytic route and is degraded. This was supported in the current study by treatment of HFA with lysosomotropic agents (chloroquine and bafilomycin), which led to increase in HIV-1 infection, although one that, overall was below 0.5%. Further evidence of an endocytic pathway was corroborated by depletion of Rabs 5, 7 and 11, which suggested the involvement of early and late endosomes in HIV-1 endocytosis. Interestingly, one earlier study showed that Rab5 and 7 are important in the endocytic entry of HIV-1 in placental trophoblasts (Vidricaire and Tremblay, 2005). We found for the first time that Rabs 5, 7 and 11 are implicated in the HIV-1 endocytic entry process in astrocytes. Overall, however, endocytosis of HIV-1 in astrocytes is not efficient, given that endosomal and proteasomal inhibitors could not increase susceptibility to infection beyond 0.5%.
VSV is internalized by endocytic vesicles and, being pH-dependent, needs an acidified environment to reach cytosol. VSV-pseudotyped HIV-1 infection was extremely productive on HFA and SVGA. While productive entry of HIV-1 via classical receptor and co-receptors is pH-independent, vesicular internalization of HIV-1 particles independent of viral receptor in CD4-negative astrocytes is extremely weak. Although most HIV-1 particles internalized by the vesicular pathway appear to be degraded in the lysosome, a small number of virus particles escape the acidic environment in endosome vesicles to enter the cytoplasm before being damaged by acidification. Thus, HIV-1 infection in astrocytes can indeed be productive, but is minimal, as we have shown here (Table 1) and others have corroborated on cells other than astrocytes (Fackler and Peterlin, 2000; Fredericksen et al., 2002; Marechal et al., 2001; Schaeffer et al., 2004) and inhibition of acidification enhances HIV-1 infectivity (Daecke et al., 2005).
In an earlier study, inhibition of vesicular H+-ATPase (vATPase) by bafilomycin or chloroquine increased productive HIV-1 infection in macrophages (Carter et al., 2011). Blocking H+ pumping by vesicular H+-ATPase (vATPase) inhibitor increased pH and reduced endocytic uptake, but did not abolish fusion of vesicle membranes. We found that increasing pH with chloroquine or bafilomycin A, both M and T-tropic viruses, increased HIV-1 infection with long-term persistence and without cell death, indicating the stability of infected astrocytes. Depletion of LSP1 substantially decreases the rate of endocytosis (Walther et al., 2006). LSP1, an F-actin binding protein, has been shown to direct HIV-1 particles to endosomes and proteasomes, and hence facilitates viral degradation (Smith et al., 2007). In this study, we used LSP1 as a control. Its depletion with siRNA decreased HIV-1 infection in astrocytes, suggesting that whatever small amount of viral infection occurred in astrocytes was by endocytosis.
Overall, our findings elucidated the mechanism of HIV-1 infection in astrocytes and demonstrated that natural infection with HIV-1 by endocytosis is minimally productive, but persistent (Table 1). The contribution of HIV-infected astrocytes to overall viral load in the brain seems negligible, but unambiguously may serve as an elusive viral reservoir and present imminent problems in purging the virus from the body.
Materials and Methods
Ethics Statement
Human fetal tissues were obtained following written approval from adult female patients undergoing therapeutic abortion at 10 to 12 weeks gestational age at the University of Washington, Seattle, USA (IRB approval #11449). The use of human fetal tissue was approved by the University of South Carolina (USCeRA#: HSA4636) and is IRB exempt (45 CFR 46.102(d). Peripheral blood mononuclear cells (PBMCs) were obtained from New York Blood Bank (IRB exempt). All cell cultures (primary human and cell lines), HIV infection, and HIV-1 plasmid DNA studies were done according to university guidelines in a biocontainment facility approved by the Institutional Biosafty Committee (IBC) of the University of South Carolina.
Primary human fetal brain and cell culture
Primary human fetal astrocytes (HFA) were cultured from human fetal tissues as described earlier (Chauhan et al., 2007; Chauhan et al., 2003; Nath et al., 1995). HFA, SVGA, SVGA-reporter cells (Table S1) and HeLa, Jurkat, 293T (HEK), and THP89-GFP cells (monocytic cells latently infected with HIV-1 p89GFP) were cultured as described below. Human fetal brain specimens were obtained from the University of Washington, Seattle, where they were collected, following Institutional guidelines, from fetuses at 8–14 weeks of gestational age. Briefly, the brain tissue was mechanically dissociated in Opti-MEM with 5% heat inactivated fetal bovine serum (FBS), and antibiotic solution (penicillin G, 100 units/ml; streptomycin, 100 μg/ml; and amphotericin B, 25 μg/ml). The dissociated cells (without trypsin) were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS and antibiotics for at least one month before use. Further, after 8 weeks in culture, HFA after 3rd or 4th passage were used in experiments requiring a minimally non dividing population of astrocytes and elimination of minor contaminants (neurons and microglia). In some experiments, HFA cultures were further treated with 5 mM LME (Sigma) for 8 h to remove any contaminating microglia (Hamby et al., 2006). The cultures were verified for purity using GFAP, MAP-2, and CD68 markers; no microglia were found, but a few neurons were observed. To reduce the chance of mycoplasma contamination in these cultures derived from aborted human fetuses, plasmocin, an antimycroplasma agent (Invitrogen, CA, USA), was used during the initial culture passages. The cultures that tested negative for Mycoplasma species by polymerase chain reaction were used.
SVGA (human astrocytic cells sub-clone of the SVG cells gift from Dr. Eugene Major, NIH) (Chauhan et al., 2003), SVGA reporter cells and HeLa cells were maintained in DMEM supplemented with 2 mM L-glutamine, 10% FBS, and antibiotic solution. Jurkat and 293T cells, as well as macrophages, were grown in RPMI-1640 supplemented with 10% FBS or 10% pooled human serum as described earlier (Mehla et al., 2012). HIV-1-infected or uninfected astrocytes or stable HIV-1 expression vector transfected SVGA (Table S4) cells were co-cultured either with Jurkat or SVGA-LTR-RFP cells. The co-cultured cells were examined for red or green fluorescence, using a fluorescence microscope. Infected Jurkat cells were identified by green fluorescence, while SVGA-LTR-RFP cells were identified by red and green fluorescence. Only cells with red and green fluorescence were considered as infected cells.
Viral constructs and plasmids
HIV-1 Tat of 101 amino acids and mutant Tat (d-Tat) deleted for 48–56 amino acids were cloned into pCDNA-3 vector (Chauhan et al., 2003; Mehla et al., 2010; Zhang et al., 2010), LTR-gag-GFPRRE obtained from QBIO lab. An HIV-1 LTR GFP was created by cloning HIV-1 LTR into CMV promoter-deleted pEGFP vector (Chauhan et al., 2003). LTR-RFP was created by cloning the RFP gene downstream of HIV-1 LTR in place of the removed CAT gene in a pHIV-LTR-CAT vector. The green fluorescent reporter HIV-1 infectious molecular clones, NLENY1 and NLYUV3-YFP were created by inserting the YFP gene between the viral envelope and nef genes without impairing the viral open-reading frames, as reported earlier (Kutsch et al., 2002). T4-pMV7 (Richard Axel) and VSV-G expression vectors; HIV-1 expression vector pNL4-3 (Malcolm Martin, NIH) (Adachi et al., 1986), pNL-HSA.R+ E- (Nathanial Landau, NYU) (He et al., 1995), pMtat(−) HIV-1 molecular clone mutated for tat (R Gallo, NIH) and infectious molecular clone YU-2 (Li et al., 1992; Li et al., 1991) were obtained from NIH AIDS research and reagent facility. HIV-Ba-L strain was obtained from Dr. Suzanne Gartner, Johns Hopkins University, Baltimore, USA, Rab-5a (wt) and transdominant negative constructs were from Paul D Bieniasz, Rockefeller University, New York. Lentiviral vectors expressing Rev and Tat were created in pLVX backbone (Clontech) after deleting Zs Green1 gene at Age1 and Xho1.
Flow cytometry and reporter cells for HIV-1 Tat/Rev transcriptional regulation
HIV-1 LTR reporters were established in SVGA cells (Chauhan et al., 2003). Stable LTR-GFP, LTR-RFP, or LTR-gag-GFP-RRE plasmids in SVGA (Table S1) or HeLa cells were performed by transfection. The clonally selected single-cell populations were expanded, verified, and stored in liquid nitrogen. HIV-1 LTR transactivation was monitored either by fluorescent microscopy, flow cytometry, or florimeter (Molecular Devices) at an excitation wavelength of 480 nm and emission of 530 nm. The details of flow cytometry are described below.
HFA and SVGA cells were cultured in T-75 flasks, then treated with 10 mM EDTA for 15 min to detach the monolayers. The cells were washed with 5% FBS in PBS and resuspended in PBS containing 5% FBS, then passed through a nylon gauge (BD, USA) to remove cell clumps. 5 × 105 cells were preincubated with 20% normal human serum or 5% FCS in PBS for 20 min at 25°C. Cells were then washed and directly co-labeled with anti-CXCR4, CCR5, CD4 or CD11a monoclonal antibody (Mab) conjugated to phycoerythrin/FITC or other labels (Pharmingen). As controls, mouse isotype antibodies, immunoglobulin G2a (IgG2a)-PE, IgG1-PerCP, and IgG2b-FITC, were used. After washing, cells were fixed in 2% paraformaldehyde (PFA), then reporter cells in PBS were analyzed on FACS 440 (Becton Dickinson) (Mehla et al., 2010; Zhang et al., 2010).
Reverse genetics and VSV-G pseudotyping
HIV-1 NL4-3, NLENY1, NLYUV3, YU-2, lentiviral pLVX-ZsGreen (Clontech), pLVX-Tat, and pLVX-Rev expression vectors were packaged in 293T cells with native HIV-1 envelope or pseudotyped with VSV-G envelope. Viral packaging was done by using 10–17 μg of each lentiviral construct and 3–4 μg of VSV-G plasmid DNA using Lipofectamine plus or Lipofecamine-2000 (Invitrogen) as described earlier (Mehla et al., 2010; Vijaykumar et al., 2008; Zhang et al., 2010). Lentiviral vectors were packaged using 12 μg pLVX vector, 8 μg gag-pol, 4 μg VSV-G, 3 μg Tat, 4 μg Rev, and 3 μg Vpr plasmids to achieve a workable titer. After 16 h of transfection, cells in 100 mm dishes were washed twice with fresh medium and incubated in RPMI 1640 containing 10% FBS. The culture supernatants were harvested 72–80 h after transfection, centrifuged at 1200 rpm for 15 min, and filtered through a 0.20 μm filter. The filtrate after addition of MgCl2 (4 mM) was digested with 10–50 units of RNase-free DNase (Invitrogen) per 1 μg of plasmid DNA for 30 min at 37°C, aliquoted and stored at −80°C. The viral stocks were quantified for p24 antigen by ELISA (ZeptoMetrix) or titrated for lentiviral particles on reporter cells. A viral inoculum of 50–1000 ng/mL was used for infection of Jurkat, primary macrophages, HFA, and SVGA.
Virus infection
These studies used HFA, monocyte-derived macrophages (MDM), primary lymphocytes, lymphocytic cells (Jurkat), SVGA, SVGA LTR-GFP, SVGA LTR-RFP, SVGA LTR-gag-GFP-RRE, or SVGA LTR-RFP/ LTR gag-GFP-RRE reporter cells (Table S1). HIV-1 strain IIIB and Ba-L virus stocks were prepared in PBMCs and macrophages (Gartner et al., 1986), then titrated by p24 assay. NL-4-3, NLENY1, NLYUV3, YU-2, and VSV-pseudotyped HIV-1 viral stocks were prepared by transfection of 293T cells as described. An equal amount of virus from each strain (50–1000 ng/mL) was used for infection of macrophages, lymphocytes, and astrocytes. About 800 μl of the working viral stock was added to 60%-70% confluent culture plates (6-well) and kept for 2 h at 37°C with gentle mixing every 20 min. After 2 h of incubation, the inoculum was washed two times with DMEM and complete medium was added. On the next day, cultures (HFA) were washed two times and replenished with complete medium. The cultures were followed up for 3–24 weeks by monitoring green fluorescence, cytopathic effect (CPE), and p24 levels by ELISA.
Treatment of HIV-1 infected astrocytes with lysosomotropic agents and autophagy- and proteosomal-inhibitors
50,000–75,000 HFA were seeded in a 6-well tissue culture plates. On the third day, duplicate samples were pretreated for 2 h with chloroquine (2.5–10 μM), bafilomycin-A (100 nM), MG132 (10 μM), 3-MA (10 mM), Filipin (1ug/ml), Leupeptin (10 μM), broad-spectrum caspase inhibitor zVAD-FMK (20 μM), or E64d (10 μM), in parallel with 0.2% DMSO or alcohol. This was followed by HIV-1 infection for 2 h. All the activators and inhibitors are listed in supporting table (Table S5). HFA were washed two times with culture medium to remove residual viral activity and treatment with the respective drugs was resumed for 4 h to 4 days. We also measured the dose response for toxicity of these drugs by Quant-Blue cell viability assay (Bioassay System Hayward, CA) and LDH assay (Mehla et al., 2010; Vijaykumar et al., 2008). The HIV-1 infected HFA cultures treated with above drugs were monitored every five day under fluorescent microscope counting the whole surface area of the wells in duplicate. We calculated the fold increase in infection as compared to untreated-infected controls. The supernatants were collected for determination of p24 levels and infectivity on Jurkat cells.
Transfection of plasmids and siRNA
HFA, SVGA, Hela, and 293T cells were transfected with HIV-1 infectious molecular clones or reporter plasmids using Lipofectamine 2000 (Invitrogen). The transfection efficiency was ~80%, but less in primary astrocytes. The plasmid concentrations used were from 0.5 to 15 μg, depending on the culture plates used.
siRNAs were designed by web-based siRNA programs (Ambion and Dharmacon); sequences were synthesized (Dharmacon) or purchased (Ambion). The sequences for siRNAs are shown in the supporting data (Table S3). Transfection of siRNAs was done together with plasmids wherever necessary. We used lipofectamine-2000 for siRNA transfection and rhodamine (red fluorescent) labeled non-specific siRNA to determine transfection efficiency (Dharmacon and Invitrogen). GFP-siRNA (cat. # P-002102-01-05) and scrambled siRNA sequences (cat. #D-001600-01-05) were purchased from Dharmacon.
Reverse-transcription coupled polymerase chain reaction (RT-PCR)
RT-PCR for CXCR4, CCR5, CD4 and CD11a transcripts were done as described earlier (Chauhan et al., 2007; Mehla et al., 2011; Mehla et al., 2010) with primer sequnecs (Table S6). In each case, total RNA was extracted from 3–4 × 106 cells by Trizol reagent (Invitrogen). The extracted RNA was further purified using RNeasy-plus mini-columns (Qiagen), then treated with DNAse (Chauhan et al., 2007) and quantified by measuring absorbance at OD280. In RT-PCR, 2 μg of RNA from each sample was reverse transcribed into cDNA using random hexamer primers and 200 units of superscript reverse transcriptase (Invitrogen) in a total volume of 20 μl at 45°C for 1 h and at 70°C for 10 min. One-tenth of the RT product was used in PCR amplification with 25–50 pico mole of each primer, 0.1 mM dNTP mix, 1.25 units of platinum Taq polymerase, and 1.2 mM Mg2+ in a 50–μl reaction volume. The cycle program for CD4, CXCR4, CCR5 and CD11a was 96°C for 3 min followed by 42 cycles of [96°C/45 sec, 58°C/15 sec, 72°C/21 sec], and 72°C for 3 min. The program for GAPDH was 96°C/3 min/ [96°C/45 sec, 57°C/15 sec, 69°C/20 sec/] 35 cycles and extension at 72°C for 3 min. The amplified products were analyzed on 1% agarose gels with ethidium bromide and visualized using UV transilluminator. PCR primers in which amplicons have a known single restriction site were used to verify amplified products.
HIV-1 and cellular protein analysis by immunofluorescence and Western blotting
Tat, Nef and p24 expression in HIV-1 infected or transfected astrocytes, Hela, and lymphocytic cells were detected by immunofluorescence using HIV-1 p24 Gag monoclonal antibody (cat # 6458, NIH), Tat, and Nef antibodies (ABI). Infected macrophages or transfected cells were fixed in 2% PFA in PBS (pH 7.2) for 15 min, then permeabilized with 0.2% Triton-X-100 (PBS) for 11 minutes. This was followed by blocking with 3% BSA in PBS for 1 h at 25°C. The treated cells were overlaid with primary antibodies at optimum working dilutions of Tat 1/400, Nef 1/200, and p24 1/200 in a moist chamber at 4°C overnight. After washing four times with PBS (10 min each at 37°C), the secondary antibodies (Molecular probes) labeled with Alexa 568 or 488 (1/500 dilutions) were added to the specimens for 35 min at 25°C in the dark. Subsequently, the specimens were washed four times with PBS, stained with DAPI (PBS) for 10 min during the last wash and finally rinsed with PBS and mounted with anti-fading agent (biomeda). The stained specimens were stored at 4°C or −20°C (long-term) until examined. The specimens were visualized using UV microscope (Nikon) with single or double filters. Images were captured with a digital camera (Nikon).
Western blotting for various gene products, either constitutive or after ablation by siRNAs, was done on astrocytes, Hela cells, Jurkat, and 293T. Each sample of 30–40 μg total protein was analysed on SDS-PAGE and electroblotted to a PVDF membrane. The immunoblotting antibodies for Tat (ABI); Rab-5a, Rab-7 and Rab 11 (all Rab antibodies from Sigma); TNPO3 (abcam); LSP1 and Emerin (LAB VISION); and actin (Sigma). We used these antibodies in combination with HRP-labeled secondary antibodies (BioRad). The blots were exposed to X-ray films and quantified by NIH software.
Viral integration and virus release assay (p24)
Viral integration in HIV-1-infected astrocytes was monitored by Alu-HIV LTR PCR, as described (Mehla et al., 2011; Vijaykumar et al., 2008). Total DNA was extracted using DNAzol reagent (Invitrogen) and amplified by nested PCR using 1.25 units of platinum Taq polymerase, 0.1 mM dNTP mix, 30 pico-moles of each primer with 1.2 mM Mg2+ in a 50 μl reaction. The first round of Alu-LTR PCR was done by external primers (Table S6) using the following program: 96°C/3min [96°C/45sec, 60°C/15 sec, 72°C/59 sec] 35 cycles and extension 72°C/7 min. In the second round, 1–5 μl products from the first amplification were further amplified using nested primers (Table S6) with the following cycle program: 96°C/3min [96°C/45sec, 60°C/15 sec, 72°C/45 sec] 35 cycles and extension 72°C/7min. The bands were analyzed on 1% agarose containing ethidium bromide and visualized by UV.
Astrocytes, lymphocytic, Hela, and 293T cells seeded at 60%–70% confluency in culture dishes or plates were transiently transfected with 2.5–7.5 μg of HIV-1-expressing plasmids (YU-2, NL4-3, NLENY1 or pMtat(−), using Lipofectamine 2000 (Invitrogen) as described (Chauhan et al., 2007; Chauhan et al., 2003). The culture fluids at various time points were monitored for p24 in persistently infected astrocytes and stable HIV-1 plasmid transfected clones, infected HFA, SVGA, or lymphocytic cells, or infected primary macrophages. Quantitative viral p24 titration was done by ELISA (ZeptoMetrix). Data were expressed as mean ± SEM. Multiple infection and transfection experiments (n ≥ 5) were done.
Transmission electron microscopy
Primary human astrocytes infected with VSV-pseudotyped HIV-1 were trypsinized at 48 h after infection, suspended in serum-free optiMEM medium (GibcoBRL), and fixed with freshly prepared 3% glutaraldehyde (Electron Microscopy Sciences) in PBS (pH 7.2). After washing twice with PBS, cell pellets were embedded in 2% agar and post fixed with 1% osmium tetraoxide for 1 h. Agar blocks were briefly rinsed with water and dehydrated with a series of ethanol gradients (75%, 95% and 100%), each for 15 min, and infiltrated with ethanol: acetonitrile (1:1) solution for 10 min. Two changes of 2:1 acetonitrile: polybed812 (EMS) for 1 h each were followed by overnight infiltration of polybed812. Further, samples were impregnated with two changes of polyBed812 for 3 h each under vacuum and polymerized at 60°C for 2 days. Ultrathin sections (90 nm) cut using an Ultracut R microtome (Leica) were collected on copper grids and stained with 2% aqueous uranyl acetate for 40 min at 37°C and lead citrate for 6 min. Grids were examined using a JEOL 200CX transmission electron microscope at 120 kV.
Generation of persistently HIV-1 infected and stable HIV-1 full length DNA (infectious clones) transfected astrocytes
Reporter cells (SVGA-LTR-GFP and LTR-gag-GFP RRE) were infected with VSV-pseudotyped NL4-3HSA R+ E− virus (Table S4). In another set, Hela, SVGA, or SVGA-LTR-RFP cells were infected with VSV-pseudotyped NLENY1 virus (Table S4). The persistently infected green fluorescent cells were sorted by fluorescent-activated cell sorting (FACS) at the School of Public Health, the Johns Hopkins University, Baltimore. Stable clones were obtained over 4 months by multi-step cloning process that took advantage of GFP as a marker. After expansion in culture, the FACS-sorted cells were subcloned into bright or weak fluorescent and non fluorescent clones. The integrated HIV-DNA in these clones was detected by Alu-HIV-LTR PCR as described. In a third set, SVGA cells were transfected with HIV-1 NLENY1 infectious molecular clone. The stable transfected cell lines (green) were designated as SVGA-NL. Persistently infected SVGA cell lines were designated as SVGA-LTR-GFP/VSV-NL, SVGA-LTR-gag-GFP/ VSV-NL, SVGA-NLENY1/VSV, and Hela-NL-ENY1/VSV (Table S4). In all these HIV-1 expression plasmids except NL4-3 R+ E−, all HIV-1 genes were intact.
Supplementary Material
Acknowledgments
We thank Dr. David N. Levy (NYU, NY, USA) for HIV-1 NLENY1 and NLYUV3 infectious molecular clones. We acknowledge the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, NIAID for providing various constructs and antibodies, and NIBS, U.K., for the monoclonal antibody to Tat. We also thank flow cytometry core facility, School of Public Health, the Johns Hopkins University, Baltimore, for sorting HIV-1 infected cells and electron microscopy facility of the University of South Carolina School of Medicine, Columbia. The work was supported by NIH (NINDS) grant RO1 NS0064 (AC) and internal funding from the University of South Carolina School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour Y, Tremblay MJ. Interaction between the cytoplasmic domain of ICAM-1 and Pr55Gag leads to acquisition of host ICAM-1 by human immunodeficiency virus type 1. J Virol. 2004;78:11916–11925. doi: 10.1128/JVI.78.21.11916-11925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- Bounou S, Giguere JF, Cantin R, Gilbert C, Imbeault M, Martin G, Tremblay MJ. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. Faseb J. 2004;18:1294–1296. doi: 10.1096/fj.04-1755fje. [DOI] [PubMed] [Google Scholar]
- Boutet A, Salim H, Taoufik Y, Lledo PM, Vincent JD, Delfraissy JF, Tardieu M. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. Aids. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi A, Beeren I, Joosten B, Fransen JA, Figdor CG. The C-type lectin DC-SIGN internalizes soluble antigens and HIV-1 virions via a clathrin-dependent mechanism. Eur J Immunol. 2009;39:1923–1928. doi: 10.1002/eji.200939351. [DOI] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011;409:234–250. doi: 10.1016/j.virol.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Hahn S, Gartner S, Pardo CA, Netesan SK, McArthur J, Nath A. Molecular programming of endothelin-1 in HIV-infected brain: role of Tat in up-regulation of ET-1 and its inhibition by statins. Faseb J. 2007;21:777–789. doi: 10.1096/fj.06-7054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987;61:1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Clarke JN, Lake JA, Burrell CJ, Wesselingh SL, Gorry PR, Li P. Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology. 2006;348:141–155. doi: 10.1016/j.virol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiva K, Khiati A, Hery C, Salim H, Leclerc P, Horellou P, Tardieu M. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS Res Hum Retroviruses. 2006;22:1152–1161. doi: 10.1089/aid.2006.22.1152. [DOI] [PubMed] [Google Scholar]
- Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987;61:3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1:447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. Embo J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JF, Cantin R, Tremblay MJ. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CH, Cottler-Fox M. AIDS in the human brain. Nature. 1986;319:8. doi: 10.1038/319008a0. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Galvez T, Gilleron J, Zerial M, O’Sullivan GA. SnapShot: Mammalian Rab proteins in endocytic trafficking. Cell. 2012;151:234–234 e232. doi: 10.1016/j.cell.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Betts RF, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. Jama. 1986;256:2365–2371. [PubMed] [Google Scholar]
- Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36:681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Howard JL, Churchill MJ, Anderson JL, Cunningham A, Adrian D, McPhee DA, Purcell DF. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, Smit L. CD11a/CD18 (LFA-1) epitopes involved in syncytium formation among CD4+ T-cells following cell free HIV-1 infection. Viral Immunol. 1990;3:289–293. doi: 10.1089/vim.1990.3.289. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Benveniste EN, Shaw GM, Levy DN. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol. 2002;76:8776–8786. doi: 10.1128/JVI.76.17.8776-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985;2:586–588. [PubMed] [Google Scholar]
- Li Y, Hui H, Burgess CJ, Price RW, Sharp PM, Hahn BH, Shaw GM. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Roos JW, Hildreth JE. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res Hum Retroviruses. 2000;16:355–366. doi: 10.1089/088922200309232. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Petrareanu C, Lazar C, Florian P, Branza-Nichita N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J Virol. 2013;87:6415–6427. doi: 10.1128/JVI.00393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou BA, Dermody TS. Transport to late endosomes is required for efficient reovirus infection. J Virol. 2012;86:8346–8358. doi: 10.1128/JVI.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O, Goud B. Rab proteins. Biochim Biophys Acta. 1998;1404:101–112. doi: 10.1016/s0167-4889(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Chauhan A. A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function. PLoS One. 2011;6:e27915. doi: 10.1371/journal.pone.0027915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:239. doi: 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One. 2010;5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergener K, Facke M, Welker R, Brinkmann V, Gelderblom HR, Krausslich HG. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992;186:25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Mlcochova P, Pelchen-Matthews A, Marsh M. Organization and regulation of intracellular plasma membrane-connected HIV-1 assembly compartments in macrophages. BMC Biol. 2013;11:89. doi: 10.1186/1741-7007-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol. 1999;16:81–87. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- Muratori C, Bona R, Ruggiero E, D’Ettorre G, Vullo V, Andreotti M, Federico M. DC contact with HIV-1-infected cells leads to high levels of Env-mediated virion endocytosis coupled with enhanced HIV-1 Ag presentation. Eur J Immunol. 2009;39:404–416. doi: 10.1002/eji.200838751. [DOI] [PubMed] [Google Scholar]
- Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropathol Exp Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Neumann M, Felber BK, Kleinschmidt A, Froese B, Erfle V, Pavlakis GN, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DG, Hildreth JE. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol. 2003;33:483–493. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishibashi K, Miyokawa K, Hokari M, Kanno T, Hirano T, Yamamoto N, Takaku H. HIV-1 suppressive sequences are modulated by Rev transport of unspliced RNA and are required for efficient HIV-1 production. PLoS One. 2012;7:e51393. doi: 10.1371/journal.pone.0051393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette JS, Fortin JF, Blanchard L, Tremblay MJ. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–9336. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Navia BA, Cho ES, Jordan BD, George DC, Price RW. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312:874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79:2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- Sabri F, Chiodi F, Fenyo EM. Lack of correlation between V3 amino acid sequence and syncytium-inducing capacity of some HIV type 1 isolates. AIDS Res Hum Retroviruses. 1996;12:855–858. doi: 10.1089/aid.1996.12.855. [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Soros VB, Greene WC. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J Virol. 2004;78:1375–1383. doi: 10.1128/JVI.78.3.1375-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- Schweighardt B, Shieh JT, Atwood WJ. CD4/CXCR4-independent infection of human astrocytes by a T-tropic strain of HIV-1. J Neurovirol. 2001;7:155–162. doi: 10.1080/13550280152058816. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003;74:225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J, Nabel GJ. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204:421–430. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist WI, Krausslich HG. HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med. 2012;2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, et al. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- Vidricaire G, Tremblay MJ. Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J Immunol. 2005;175:6517–6530. doi: 10.4049/jimmunol.175.10.6517. [DOI] [PubMed] [Google Scholar]
- Vijaykumar TS, Nath A, Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008;381:1–5. doi: 10.1016/j.virol.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim CL. No evidence of significant abortive HIV infection of the brain. Aids. 1997;11:252. [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang R, Mehla R, Chauhan A. Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus -1 preintegration complex (DNA) PLoS One. 2010;5:e15620. doi: 10.1371/journal.pone.0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.