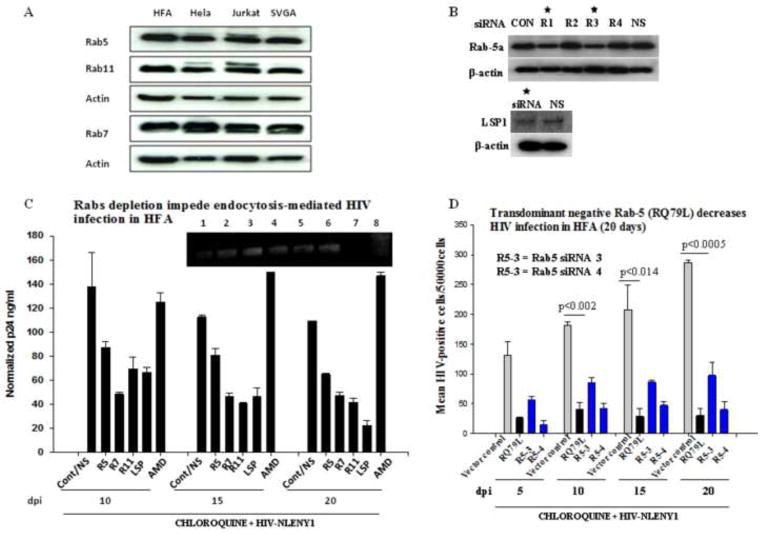
Figure 6. Depletion of Rab proteins in astrocytes obliterates endocytosis-mediated HIV-1 infection.
(A) Astrocytes (HFA and SVGA) and HIV-permissive cells (lymphocytic and HeLa cells) grown for 48 h were processed for Western blot using monoclonal antibodies against Rab-5, -7 and -11. (B) Screenings of several siRNAs for depletion of each Rab and LSP1 (50–200 nM) were investigated on HFA by Western blotting. HFA were transfected with 200 nM each siRNA and, 72 h later lysates were analyzed by Western blotting for Rabs. Beta actin was used as a loading control. LSP1 siRNA was tested as a positive control to inhibit HIV-1 and non specific (NS) siRNA as a negative control. (C) Seeded HFA were transfected with 200 nM Rab specific siRNAs in parallel with LSP-siRNA and non-specific siRNA as controls. Then, one set of HFA was treated with CXCR4 blocker AMD3100 (AMD) before HIV-1 infection. At 72 h after siRNA transfection, HFA were infected with NLENY1 virus for 2 h. After washing, all cultures were treated with chloroquine (10 μM). At 48 h after infection (5 days after initial siRNA transfection), infected HFA were re-transfected with the respective siRNAs. p24 levels in culture supernatants collected at 10, 15, and 20 days after infection were analyzed by ELISA (n=4). HFA at 20 days after infection were tested for viral DNA integration by Alu-HIV LTR PCR (inset C). (D) HFA in 6-well culture plates were transfected with control vector, Rab 5a transdominant negative vectors, or Rab 5a siRNA #3 and #4 and, 72 h later infected with NLENY1. After washing, cultures were treated with chloroquine. Cultures were followed for 20 days after infection. YFP-positive HFA were counted in the entire well in duplicate and plotted (n=2).