Abstract
Human Immune Deficiency Virus-1 (HIV-1) infection can induce severe and debilitating neurological problems, including behavioral abnormalities, motor dysfunction, and dementia. HIV can persistently infect astrocytes, during which viral accessory proteins are produced that are unaffected by current antiretroviral therapy. The effect of these proteins on astrocyte function remains unknown. Astrocytes are the predominant cells within the brain; thus, disruption of astrocyte function could influence the neuropathogenesis of HIV infection. To explore further these effects, we constitutively expressed HIV-Tat protein in astrocytes. Since the nuclear presence of Tat protein leads to alteration of host gene expression, we further analyzed the effects of Tat on host gene transcripts. Endothelin-1 (ET-1) was a significantly elevated transcript as verified by reverse transcription-polymerase chain reaction (RT-PCR), and it was subsequently released extracellularly in Tat-expressing and HIV-infected astrocytes. ET-1 expression was also prominent in reactive astrocytes and neurons in brain tissues from basal ganglia and frontal lobes of HIV encephalitic patients. HIV-Tat regulated ET-1 at the transcriptional level through NF-κB (NF-κB)-responsive sites in the ET-1 promoter. Intriguingly, simvastatin (10 µM) down-regulated HIV-Tat-induced ET-1 and also inhibited activation of NF-κB in astrocytes. Our findings suggest that ET-1 may be critical in mediating the neuropathogenesis of HIV dementia and that statins may have therapeutic potential in these patients.
Keywords: HIV-neuropathogenesis, nuclear Tat, ET-1 transcriptional regulation, NF-κB, simvastatin
Before the introduction of highly active antiretroviral therapy (HAART), ~30% of HIV-infected patients developed cognitive, behavioral, and motor symptoms, collectively known as HIV-associated dementia (HAD) (1). However, with the continued use of HAART, the severe form of HAD has dramatically decreased. Instead, a more subtle form of central nervous system (CNS) dysfunction, minor cognitive motor disorder, is more common in HAART-treated patients (2, 3). With currently available antiretroviral drugs, viral transcription and translation proceed normally in the infected cells, but infection of new cells is prevented. Whether or not this unproductive HIV infection plays a role in the pathogenesis of the minor cognitive impairment remains unclear.
HIV has been shown to remain in the brain resident cells productively or unproductively. Brain cells, including astrocytes, microglia, and perivascular macrophages, are susceptible to HIV infection (4); however, the latter two types are most productively infected (5). Astrocytes are the most numerous cell type within the brain, outnumbering neurons by 10:1, and provide an important reservoir for HIV infection within the brain (6–9). The virus causes a noncytopathic infection in these cells (10, 11). HIV infection in astrocytes may lead to production of accessory viral proteins but may not produce fully replicative viral particles, resulting in a restricted or unproductive infection (9, 12–5). In contrast, some studies, including ours, showed productive HIV infection in human astrocytes in vitro (16, 17).
During HIV infection, Tat is the first viral protein produced and it subsequently induces the production of other early as well as late transcripts in the HIV life cycle. It is also produced after cytokine stimulation of persistently infected astrocytes (12, 13). In patients with HIV encephalitis, the cytokines are chronically activated (18). To date most studies on Tat have been performed with extracellular Tat. The effects of intracellular Tat on cell function are not well understood. Therefore, to explore further the potential effects of Tat on astrocyte function, we expressed Tat protein intracellularly in human astrocytes. Using microarray analysis, we found endothelin-1 (ET-1) transcripts among prominently deregulated (elevated) ones in astrocytes expressing Tat. ET-1 is widely expressed in the brain (19), particularly in neurons at presynaptic and postsynaptic regions, and therefore is considered to be an important neurotransmitter-neurohormone. However, its role in the pathogenesis of HAD is not known (20). Hence, in this study we focused on the mechanism of ET-1 regulation by HIV/Tat in neuroglial cells.
MATERIALS AND METHODS
Primary human fetal brain cell culture, cell lines, and reagents
Primary human fetal astrocytes were cultured from human fetal tissues as described previously (10). Human fetal brain specimens were obtained from fetuses of 8–14 wk gestational age. Briefly, the meninges were carefully removed, and brain tissue in Dulbecco’s modified Eagle medium (DMEM) with 5% heat-inactivated FBS and 1% antibiotic solution (penicillin G 104 units/ml, streptomycin 10 mg/ml, and amphotericin B 25 µg/ml) was mechanically dissociated by using an 18-gauge needle and syringe. The dissociated cells were cultured in DMEM with 10% FCS and antibiotics for at least 2 mo and passaged two to three times before use. SVGA, a human fetal astrocytic cell line (21), SVGA reporter cells, and 293T cells were maintained in DMEM with 2 mM L-glutamine, 10% FBS, and antibiotic. Human recombinant IL-6 and IL-8; TNF-α and ET-1 were purchased from eBioscience (San Diego, CA, USA) and R&D Systems (Minneapolis, MN, USA), respectively
HIV-infected brain specimens and immunohistochemistry
Brain tissue from HIV-infected patients with dementia (n=13; MSK>1), without dementia (n=10; MSK=0), and HIV (−) normal (n=6) were obtained from National Neuro AIDS Tissue Consortium (NNTC, Bethesda, MD, USA) and Johns Hopkins School of Medicine Brain Bank. Sections, 5–10 µm, were cut from the frontal lobe and/or basal ganglia and processed for immunohistochemistry. Antigens were retrieved by pressure cooker treatment in 0.01 M citrate buffer (pH 6.0). After H2O2 treatment and permeabilization with Triton X-100 (0.4%; v/v), tisssue sections were overlaid with polyclonal ET-1 antibody (Ab) (Peninsula Labs, Torrance, CA, USA) at 1:150 dilution and kept overnight at 4°C. Goat-anti-rabbit conjugated to peroxidase was used as a secondary Ab, with diaminobenzidine as chromogen. The sections were counterstained with hematoxylin. Images were captured by a CCD camera with a Nikon microscope.
Plasmid constructs
HIV proviral DNA NL4–3 was obtained from the National Institutes of Health (NIH) AIDS reagent facility (Bethesda, MD, USA). Two-exon HIV Tat (101 amino acids, full-length) from HXB-2b was cloned into pCDNA vector at the BamH1/EcoRI sites by using PCR-amplified product, and the sequence was verified by DNA sequencing (22). The wild-type (WT)- and mutant (48–56 amino acids deleted)-Tat genes were cloned inframe upstream of the GFP gene in pEGFP expression vector (22). The long terminal repeat (LTR)-GFP reporter was generated by cloning HIV-1 LTR into the pEGFP vector from which the cytomegalovirus (CMV) promoter has been deleted. ET-1 promoter 480, 640, and NF-κB encompassing regions were amplified by PCR using primers containing restriction sites, in a PCR mix containing 25 pmol of each primer, 0.1 µM dNTP mix, 1.25 U of vent polymerase, and 1.2 mM Mg2+ in 50 µl reaction vol. The following cycle program was used: 96°C/3 min, [96°C/45– sec, 55°C/15 s, and 70°C/30 s to 2.0 min] for 11 cycles and [96°C/45, 57°C/15 s, 72°C/30 s to 2.0 min] for 20 cycles and extension 72°C/7 min. The gel-purified fragments were cloned into a CMV promoter-deleted pEGFP vector at SalI/Sma (ET-480 and ET-640 promoter fragments), while the NF-κB-containing promoter fragment of ~1.5 kb was cloned at XhoI/SalI. The cloned sequences were verified by double-strand DNA sequencing and have been submitted to the Gene Bank (accession # DQ496112). The following cloning primers were used for amplification of ET-1 promoter:
ET 480–1, 5′-GCGTCGACCTGCCCCCGAATTGTCAG-3′ and
ET 480–2, 5′-CGCCCGGGAGATCTCAAAGCGATCCT-3′;
ET 640–1, 5′-GCGTCGACAGACTGGCATTGTCCTAAACGAGCTCT-3′ and
ET 640–2, 5′-CGCCCGGGAGATCTCAAAGCGATCCTTCAGCCCA-3′ and
ET-NF-κB-1, 5′-CGCTCGAGGTTTGGTCAAAGTTGCC-3′ and
ET-NF-κB-2, 5′-CGGTCGACTTTCCCTATCACTGATG-3′.
Transfection of plasmids and siRNA into astrocytes
Primary astrocytes, the astrocytic cells (SVGA), and 293T cells were grown in DMEM containing 10% fetal calf serum, 0.15% sodium bicarbonate, and 1% antibiotic solution (penicillin G 104 units/ml, streptomycin 10 mg/ml, and amphotericin B 25 µg/ml). The cells were transfected with the Tat expression vector, HIV proviral DNA, or reporter constructs by using lipofectamine 2000 (Invitrogen, Inc., Carlsbad, CA, USA), and yielded a transfection efficiency above 80% even with primary astrocytes (22). The plasmid concentrations used in our studies ranged from 0.2 to 15 µg per culture vessel, depending on the well size (6– and 12-well plates, and 35-mm and 60-mm dishes). Green fluorescence was quantified using a fluorimeter (Molecular Devices, Sunnyvale, CA, USA).
The siRNA were designed by web-based siRNA converter programs (Ambion, Austin, TX, USA, and Dharmacon, Lafayette, CO, USA) and sequences were synthesized by Dharmacon. Sequences for the tat siRNA were positioned between 57–75 in the gene, sense, 5′-CUGCUUGUACCAAUUGCUAtt-3′, and antisense 5′-UAGCAAUUGGUACAAGCAGtt-3′. We used a GFP siRNA (cat. # P-002102–01-05) and scrambled siRNA sequence from Dharmacon as a control in our studies (cat. # D-001600–01-05). Transfection of siRNA was performed together with plasmids. We used lipofectamine for siRNA transfection.
SVGA-Tat, ET-1 promoter, and HIV-LTR reporter cell lines
SVGA cells were transfected either with Tat or control vector plasmids, and stable cells were cloned. Both low and high Tat-expressing cells were established. Tat expression was demonstrated in the high Tat-expressing cells by immunofluorescence and Western blotting and in the low Tat-expressing cells by RT-PCR and HIV-LTR transactivation assay (22). Plasmids LTR-GFP, ET-640-GFP, or ET-NF-κB-GFP were stably introduced by transfection in SVGA cells. The stable cells were selected with G418 after 48 h of transfection of the reporter plasmids and maintained for 6–8 wk. The clones derived from single cells were expanded and verified using Tat or proviral HIV DNA vectors. These transgenic astrocytic cells were used for monitoring transcriptional regulatory activities of Tat on the HIV-LTR or ET-1 promoter.
DNA microarray and RT-PCR
Total RNA from Tat and control cells [plasmid construct DNA (pcDNA) vector] was extracted as reported (22). Briefly, in each case, total RNA was extracted from 3–4 × 106 cells by Trizol reagent (Invitrogen) and treated with RNase-free DNase. RNA (5 µg) from three independent samples were used for Affymetrix gene chip (containing 22,000 human gene arrays) experiments, and values were analyzed statistically. In RT-PCR, 1 µg of RNA from each sample was reverse-transcribed into cDNA by using random hexamer primers and 200 U of superscript reverse transcriptase (Invitrogen) at 45°C for 1 h and at 70°C for 10 min in 20 µl total vol. One-tenth of the RT product was used in PCR amplification with 25 pmol of each primer, 0.1 mM dNTP mix, 1.25 U of platinum Taq polymerase, and 1.2 mM Mg2+ in 50 µl reaction vol. The cycle program for ET-1 used was 96°C/3 min followed by 42 cycles of 96°C/45 s, 55°C/15 s, 69°C/20 s and a final extension at 72°C for 7 min.
The program for GAPDH was 96°C/3 min/ [96°C/45 s, 57°C/15 s, 69°C/20 s/35 cycles] extension at 72°C for 7 min. The following primers were used:
For Tat: Tat-AC1, 5′-ATGGAGCCAGTAGATCCTAG-3′ and
Tat-AC2, 5′-TCATTGCTTTGATAGAGAAACTTG-3′;
For ET-1 primers: Endothel-1, 5′-GCATCTATTCTCACGGTCTGTTG-3′
and Endothel-2, 5′-GAGCTCCAGAAACAGCAGTCTTA-3′ and
For GAPDH: GAPDH1, 5′-ACCACAGTCCATGCCATCAC-3′ and
GAPDH2 5′-TCCACCACCCTGTTGCTGTA-3′. The amplified products were analyzed on 1% agarose gels with ethidium bromide and visualized by a UV transilluminator.
HIV pseudotyping and infection of astrocytes
HIV proviral DNA NL4–3 was packaged in 293T cells or pseudotyped with VSV-G envelope by using NL4–3 R+ E− HIV proviral DNA, where 10 µg of viral plasmid with 3 µg of VSV-G plasmid DNA were cotransfected using Lipofectamine plus. The next day the transfected cells were washed with fresh medium and incubated in fresh DMEM containing 10% FBS. Seventy-two hours post-transfection; the infected medium was harvested, centrifuged at 1200 rpm for 10 min, and filtered through a 0.20 µm pore size filter. The filtrate was then digested with DNase for 30 min at 37°C, tested for HIV-p24 antigen concentration by quantitative ELISA (Beckman Coulter, Fullerton, CA, USA), and aliquots were stored at −80°C. The virus inoculum at 1000 ng p24 levels was used for infection of primary astrocytes or astrocytic cells.
Cytokine array and ELISA
We investigated cytokine profiles on the supernatants collected from astrocytes expressing Tat or containing empty vector or astrocytes alone, using Human Cytokine Ab Array-V from Ray Biotech (Norcross, GA, USA). In brief, the supernatants from 72 h conditioned media were reacted with the cytokine Ab array membranes overnight at 4°C. The next day, membranes were washed with a solution and reacted with Ab to cytokines conjugated to biotin for 2 h at room temperature followed by washing. The washed membranes were reacted with HRP conjugated streptavidin for 2 h at room temperature. After washing, the membranes were developed using chemiluminescence kit and read in a chemilumnescent imager. The signals were normalized and -fold increases in intensity were determined. Further, ET-1 and IL-6 were quantified using ELISA kits (R&D Systems).
NF-κB activation assay
Tat expressing and HIV-infected astrocytes were either left untreated or were pretreated with 5 µM simvastatin for 72 h. NF-κB activation was determined in the nuclear extracts by using the Transbinding NF-κB p50 Assay Kit (Panomics, Fremont, CA, USA). Further, we also demonstrated activated NF-κB p65 fragment by immunostaining with rabbit polyclonal NF-κB p65 fragment antibodies (1/200 dilution) obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Immunofluorescence
ET-1, Tat, p24, and GFAP expression levels were visualized by immunofluorescence with monoclonal antibodies. Antip24 (AIDS reagent facility, NIH) and anti-Tat Ab (National Institute of Biological Standards and Control, Herts, UK), anti-ET-1 (polyclonal from Peninsula Labs, USA. and monoclonal from Affinity Bioreagents, Golden, CO, USA), anti GFAP (monoclonal, clone G-A-5 from Sigma, St. Louis, MO, USA) and antinucleophosmin B23 (monoclonal) were obtained from Abcam (Cambridge, MA, USA). HIV-infected or HIV-proviral-DNA transfected astrocytes were fixed in 2% (w/v) paraformaldehyde in PBS for 15 min and permeabilized with 0.2% (v/v) TritonX-100 (PBS) for 11 min, followed by blocking with 5% (w/v) BSA in PBS for 1 h. The cells were treated with primary antibodies at optimal working dilutions (ET-1 1/100, Tat 1/400, p24 1/200, GFAP 1/500, and nucleophosmin at 1/75) and kept at 4°C overnight in a moist chamber. Thereafter, they were washed three times with PBS (10 min each, 37°C) and treated with secondary antibodies labeled either with Alexa 568 or 488 (1/500 dilution) for 30 min at room temperature. After washing three times with PBS, they were stained for nuclei with 4′,6′-diam idino-2-phenylidole (DAPI) or Hoechst (in PBS) during the last washing for 10 min and finally rinsed with PBS and mounted with a medium containing an antifading agent (Biomeda, Foster City, CA, USA). The stained specimens were stored at 4°C or −20°C in the dark until examined. The specimens were visualized with a UV microscope with single or double filters, and images were captured with a CCD camera.
Treatment of Tat-expressing cells with statins
Simvastatin and Lovastatin were obtained from Calbiochem (San Diego, CA, USA); stocks of both drugs were prepared in water and stored at −20°C. Both the drugs were first tested for cytotoxicity using microscopy (rounding of and detachment of cells) and by MTT assay at different concentrations (5, 10, and 25 µM) on astrocytic cells and primary neuro-glial cells. Tat-expressing cells, SVGA empty control vector cells, or stable SVGA ET-1 promoter cells, on transient transfection with Tat expression vector, were treated with statins at 10 µM concentrations at −24 (before transfection or 24 h before trypsinization and seeding of stable Tat-expressing cells), +24, +48, and +72 h, and the supernatants were collected and analyzed for ET-1 protein by quantitative ELISA (R&D Systems). For ET-1 promoter studies, cells were seeded into 6-, 12-, or 24-well plates, cotransfected with ET-1 promoter contructs (ET-480, ET-640, and ET-NF-KB) and Tat plasmids, and at 6 h post-transfection, treated with statins and then monitored for 72 h. The specificity was checked by using Tat-siRNA as a specific inhibitor of Tat. The experiments were repeated 3–5 times. The green fluorescence was either photographed or read in a fluorimeter (Molecular Devices)
Statistical analysis
The data were represented as mean ± sem and analyzed statistically using Student’s t test for significance.
RESULTS
HIV Tat up-regulates ET-1 mRNA in human astrocytes
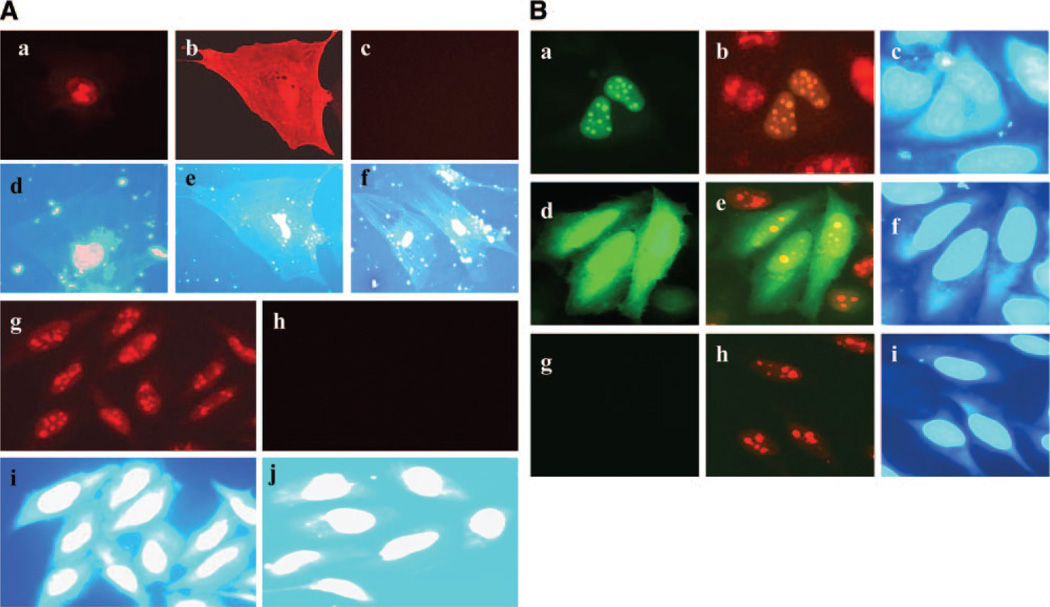
HIV Tat was expressed in the nucleus on transfection in astrocytes (Fig. 1A). In particular, Tat expression was found in the nucleus and nucleolus because of the NLS domain at positions 48–56 (Fig. 1Aa, g, Ba, b). Subsequent verification through deletion of NLS domain in Tat-GFP construct revealed diffuse expression of mutant Tat throughout the cell (Fig. 1Ab, Bd). Tat is required in the nucleus as an HIV-LTR transcriptional elongator and as a foreign protein in the nucleus will inevitably affect host gene expression as well. To gain insight into the effects of nuclear expressed Tat on astrocyte function, we established transgenic astrocytic cell lines in which Tat was expressed constitutively (SVGA-Tat). We established a high Tat expressing SVGA cell line in which Tat could be detected by immunofluorescence (Fig. 1Ag) and a low Tat expressing SVGA cell line in which Tat was detectable only through transactivation assay (HIV-LTR-GFP). Further, to determine whether Tat was biologically active in these stable cells, we transfected them with an HIV-LTR-GFP reporter contruct (HIV-LTR promoter-linked to GFP gene). The presence of green fluorescence on LTR transactivation indicated production of biologically active Tat (Fig. 2A). This effect was specific for Tat-expressing cells, because fluorescence was absent in control empty vector-SVGA cells (SVGA-neo) (Fig. 2A).
Figure 1.
Tat expression in astrocytes. A) Primary fetal astrocytes (PFA) were transfected with Tat constructs and immunostained with mAb to Tat. a) WT Tat shows nuclear expression; (b) mutant Tat (48–56), show expression throughout the cell. c) PFA transfected with empty control vector. d–f) Show nuclear staining with DAPI and correspond to (a–c). g) SVGA cells were stably transfected and immunostained for Tat. WT Tat expressed in the nucleus; (h) absence of immunostaining for Tat in cells transfected with empty vector (control). i, j) nuclear staining with DAPI, corresponding to (g) and (h). B) SVGA cells transiently transfected with Tat 101-GFP or mutant Tat (48–56)-GFP and immunostained for nucleoli using nucleophosmin mAb. a) Tat-GFP (green) expression in the nucleus, (b) same field as in (a) Tat-GFP (green) and nucleophosmin (red) showing colocalization of Tat and nucleoli, (c) DAPI nuclear stain corresponds to (a and b); (d) mutant Tat (48–56)-GFP shows diffuse expression (green) throughout the cells, (e) same as (d) showing mutant Tat-GFP (green) and nucleoli (red), have lost nucleolar distribution of Tat, (f) DAPI nuclear staining corresponds to (d and e); (g) control cells transfected with vector DNA and in (h) same as (g) showing nucleolar staining; (i) DAPI nuclear staining for (g) and (h).
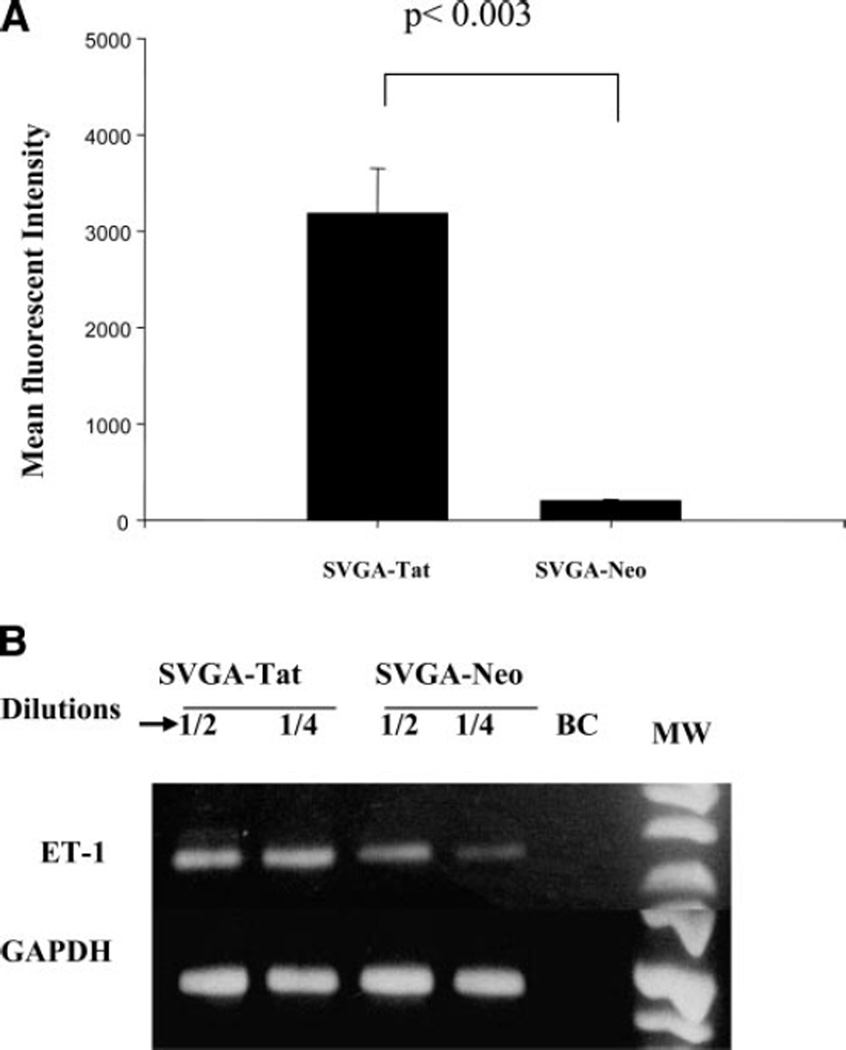
Figure 2.
ET-1 mRNA up-regulation in Tat expressing astrocytes. A) SVGA cells stably transfected with Tat (SVGA-Tat) or control DNA vector cells (SVGA-neo) were transiently transfected with HIV-LTR-GFP. Expression of GFP on LTR transactivation is seen in the SVGA-Tat cells (biologically active), but not in SVGA-neo cells (P<0.003, n=5). These cell lines were used for microarray studies and subsequent studies on ET-1. B) Semiquantitative RT-PCR analysis for ET-1 in SVGA-Tat or SVGA-neo cells showing increased ET-1 mRNA levels: upper bands lane, ET-1 at 2-fold dilutions; the lower bands lane, GAPDH signals (housekeeping gene) correspond to the same dilutions as in ET-1 lane. BC is a RT-PCR control where no cDNA template was added.
To determine whether Tat expression affected subtle astrocyte functions, we compared gene expression in SVGA-Tat and SVGA-neo cells by microarray analysis. Interestingly, among many other genes, endothelin-1 (ET-1) and IL-8 mRNA were significantly up-regulated (Table 1). We further confirmed up-regulation of ET-1 transcripts by semiquantitative RT-PCR (Fig. 2B). Since ET-1 has been implicated in the neuropathogenesis of HIV, and its levels have been shown to be increased in cerebrospinal fluid (CSF) of HIV-infected patients (23), we further explored the regulation of ET-1 in HIV-infected neuroglial cells.
TABLE 1.
Up-regulation of mRNA/proteins (few of the selected) in stable Tat-expressing astrocytic cells
| Gene name | Gene array (Affymatrix) | RT-PCR (Signal) | ELISA (Quantitative) | Ab Array | Simvas/Lovastatin (Inhibition) |
|---|---|---|---|---|---|
| ET-1 | ++ | Elevated | Elevated | ND | + |
| IL-6 | ND | Elevated | ++ | − | |
| IL-8 | +++ | ND | ND | ++ | − |
| EGR-1 | ++ | ND | ND | ND | ND |
++ = 2−3 fold; +++ = 3−5 fold; ND = not done; (+) = inhibition; (−) = no effect
Up-regulation of ET-1 protein in HIV-infected or Tat-expressing astrocytes
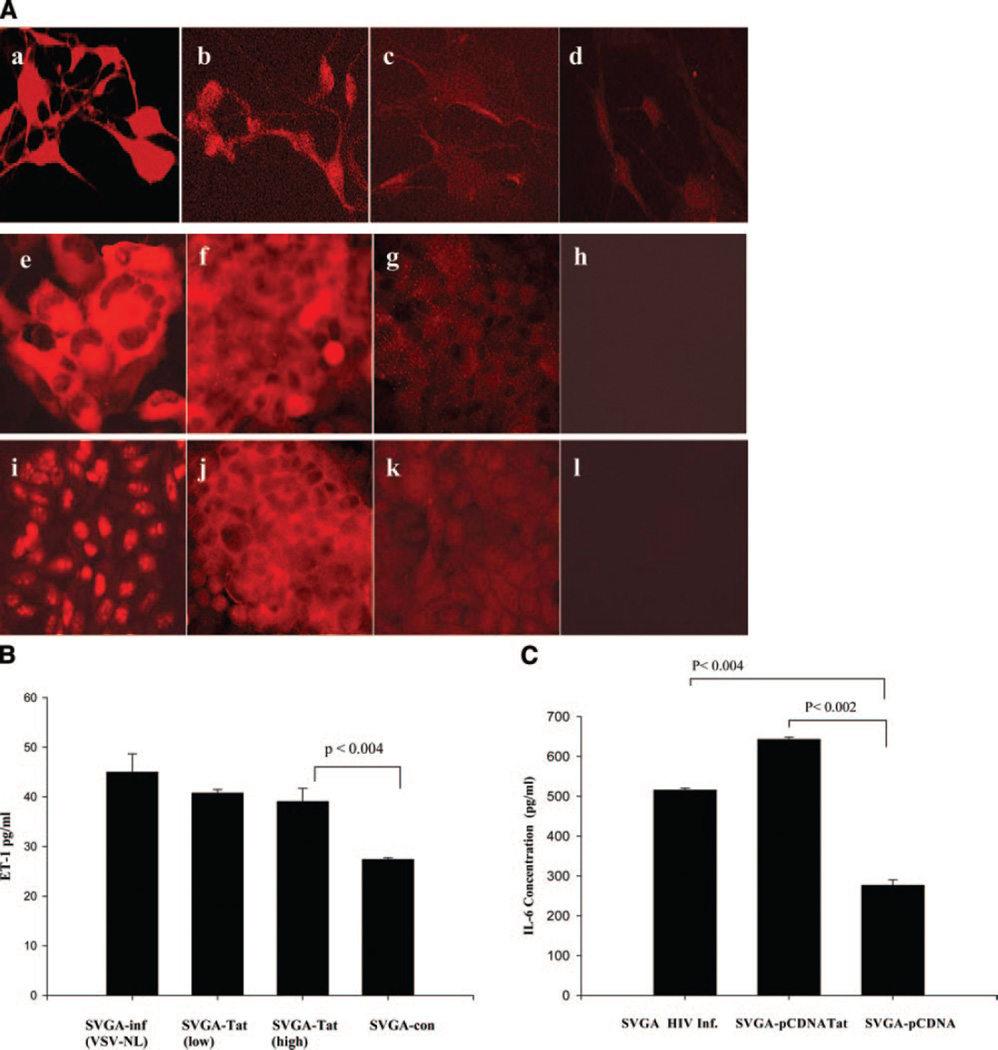
Since glial cells comprise the largest cell population in the brain and are also infected in HIV patients, we examined whether ET-1 was deregulated in these cells. Using a monoclonal antibody (mAb) specific to ET-1, we detected low levels of immunostaining in normal astrocytes. ET-1 was present in the cytoplasm and cell membrane of astrocytes (Fig. 3A). Nevertheless, HIV-infected primary astrocytes (Fig. 3Ab) or astrocytic cells (Fig. 3Af) and Tat-expressing astrocytes (Fig. 3Aj) revealed ET-1 expression, which was found to be more intense than in uninfected or mock-transfected astrocytes (Fig. 3Ac, d, g, h, k, i). Since ET-1 is released extracellularly, we quantified ET-1 in culture supernatants of Tat-expressing or HIV-infected astrocytes by ELISA. Figure 3B shows that high levels of ET-1 were found in both HIV-infected and Tat-expressing cells compared with control cells transfected with control vector alone. Further, cytokine Ab array and ELISA revealed marked increases in IL-6 (Fig. 3C, Table 1) and IL-8 in Tat-expressing astrocytes (Table 1).
Figure 3.
HIV-Tat induces ET-1 and IL-6 production in astrocytic cells. A) ET-1 expression in HIV-infected and Tat-expressing astrocytes. a) Primary astrocytes infected with VSV pseudotyped HIV (NL4–3 strain) showing p24 antigen immunostaining (marker for productive HIV infection); (b) ET-1 expression in HIV-infected astrocytes; (c) ET-1 expression in uninfected astrocytes; (d) an isotype Ab control; (e) SVGA cells infected with pseudotyped-HIV showing immunostaining for p24 antigen; (f) HIV-infected SVGA cells show immunostaining for ET-1. g) Mock-infected SVGA cells immunostained for ET-1 and (h) an isotype Ab control. i) Stable Tat expression in SVGA cells (j) SVGA-Tat cells showing ET-1 expression similar to HIV-infected SVGA; (k) ET-1 expression in control SVGA-neo cells; and (l) an isotype Ab control. B) ET-1 levels were measured (ELISA) in 72 h culture supernatants of SVGA cells expressing low levels of Tat, high levels of Tat, HIV-infected SVGA cells, or control SVGA-neo cells. Tat expression or HIV infection shows marked increases in ET-1 production over basal ET-1 levels (data represent mean ± sem, P<0.004, n=4). (C) IL-6 production in HIV- infected or Tat-expressing astrocytes. Significant amount of IL-6 was produced in HIV-infected (P<0.004) or Tat expressing (P<0.002) astrocytes compared with control SVGA-neo cells, n = 2.
ET-1 expression in HIV-infected brain tissue
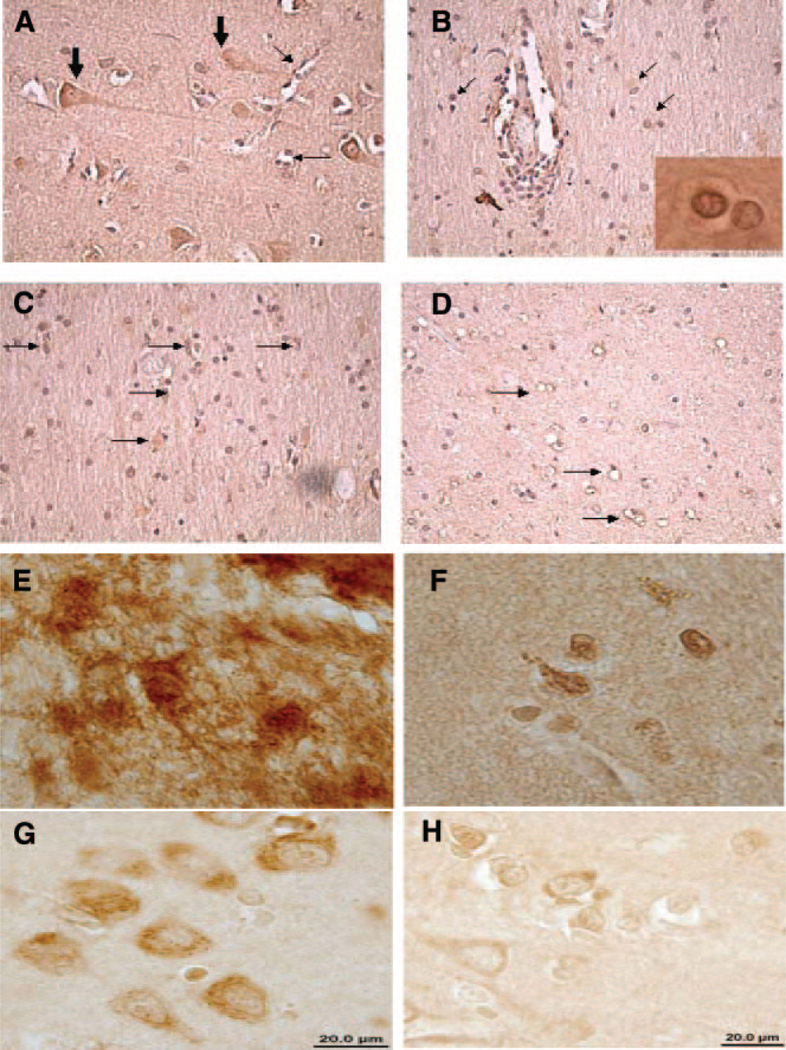
To determine ET-1 expression in HIV-infected brain tissues from postmortem specimens, we immunostained brain tissues from HIV-demented, -nondemented patients, and uninfected individuals with Ab to ET-1, and results were evaluated blindly by two independent investigators. We observed immunoreactivity for ET-1 in neuronal and astrocyte cell populations and in endothelial cells (Fig. 4A–D) in the basal ganglia and frontal lobe. Interestingly, ET-1 expression was more pronounced in the frontal lobe (Table 2). In neurons and endothelial cells, immunoreactivity was mainly cytoplasmic, whereas in astrocytes immunoreactivity was localized largely in astrocytic processes and cytoplasm (Fig. 4E–H), which is also similar to in vitro observations on SVGA-Tat or HIV-infected SVGA cells (Fig. 3A). In some areas of the brain, midfrontal gyrus, and basal ganglia, immunoreactivity was also seen in the nucleus. Comparison of immunoreactivity pattern of tissues from HIV-demented patients with that of HIV-negative controls suggests that ET-1 is markedly increased in reactive astrocytes (Table 2), but no major differences were seen in neuronal or endothelial staining patterns. Minimum immunoreactivity for ET-1 in astrocytes was observed in HIV (−) subjects and only mild to minimal staining was noted in the HIV-infected nondemented patients. Significant immunoreactivity, however, was present in the astrocytes of 4 patients with HIV dementia. Another 3 patients with HIV dementia had mild increases and 4 patients had minimal changes in ET-1 immunoreactivity while the remaining 2 patients had no immunoreactivity (Table-2).
Figure 4.
ET-1 expression in HIV encephalitis (HIVE). A) Prominent neuronal (large arrows) and endothelial cell staining (small arrows) is noted in frontal lobe of a patient with HIVE. This pattern was also seen in HIV (−) patients. B) Glial cell staining (arrows) is seen close to perivascular infiltrates in a patient with HIVE. Insert shows nuclear staining in some glial cells. C) Prominent glial cell staining (arrows) in frontal white matter of a patient with HIVE. D) Only endothelial cell staining in the frontal white matter of a HIV (−) patient. High-power magnification: (E) Astrocytes with massive ET-1 expression from HIV-infected brain tissue. F) ET-1 expression in HIV-negative brain tissue with nuclear staining in astrocytes. G) Cytoplasmic ET-1 expression in neurons from HIV-infected brain tissue; and (H) ET-1 expression in neurons from HIV-negative brain tissue.
TABLE 2.
Endothelin-1 expression in HIV-demented and -nondemented brain tissue using immunohistochemistry
| Patient# | Stage | Brain Region |
ET-1 Staining (Astrocytes) |
|---|---|---|---|
| 1. CA 200 | HAD | BG | − |
| FL | + | ||
| 2. CB 109 | HAD | BG | − |
| FL | + | ||
| 3. CB 194 | HAD | BG | + |
| FL | ++ | ||
| 4. CC 120 | HAD | BG | − |
| FL | +++ | ||
| 5. 1019 | HAD | BG | − |
| FL | − | ||
| 6. 6007 | HAD | FL | + |
| 7. 6037 | HAD | BG | − |
| FL | − | ||
| 8. 6050 | HAD | FL | +++ |
| 9. 6068 | HAD | FL | + |
| 10. 010015 | HAD | BG | +++ |
| FL | +++ | ||
| 11. 7100147470 | HAD | BG | + |
| FL | ++ | ||
| 12. 7100178083 | HAD | BG | ++ |
| FL | ++ | ||
| 13. 7100616568 | HAD | BG | + |
| FL | +++ | ||
| ND | |||
| 1. CE 104 | ND | BG | − |
| FL | −/+ | ||
| 2. 266 | ND | BG | + |
| 3. 291 | ND | BG | ++ |
| 4. 408 | ND | BG | + |
| FL | ++ | ||
| 5. 432 | ND | BG | + |
| FL | + | ||
| 6. 489 | ND | BG | + |
| 7. 556 | ND | FL | + |
| 8. 6800048087 | ND | BG | + |
| 9. 6800278276 | ND | BG | ++ |
| FL | −/+ | ||
| 10. 7100246771 | ND | FL | −/+ |
| Control HIV – | |||
| 1. 497 | HIV- | FL | −/+ |
| 2. 523 | HIV- | FL | ++ |
| 3. 526 | HIV- | FL | + |
| 4. 529 | HIV- | FL | + |
| 5. 543 | HIV- | FL | + |
| 6. 544 | HIV- | FL | + |
HAD = HIV dementia; ND = Nondemented; BG = Basal ganglia; FL = Frontal lobe; +++ = >10 cells; ++ = 5−10 cells; and + = <5 cells/×40 magnification; −/+ = doubtful, and−= negative.
HIV Tat programs ET-1 transcription in astrocytes
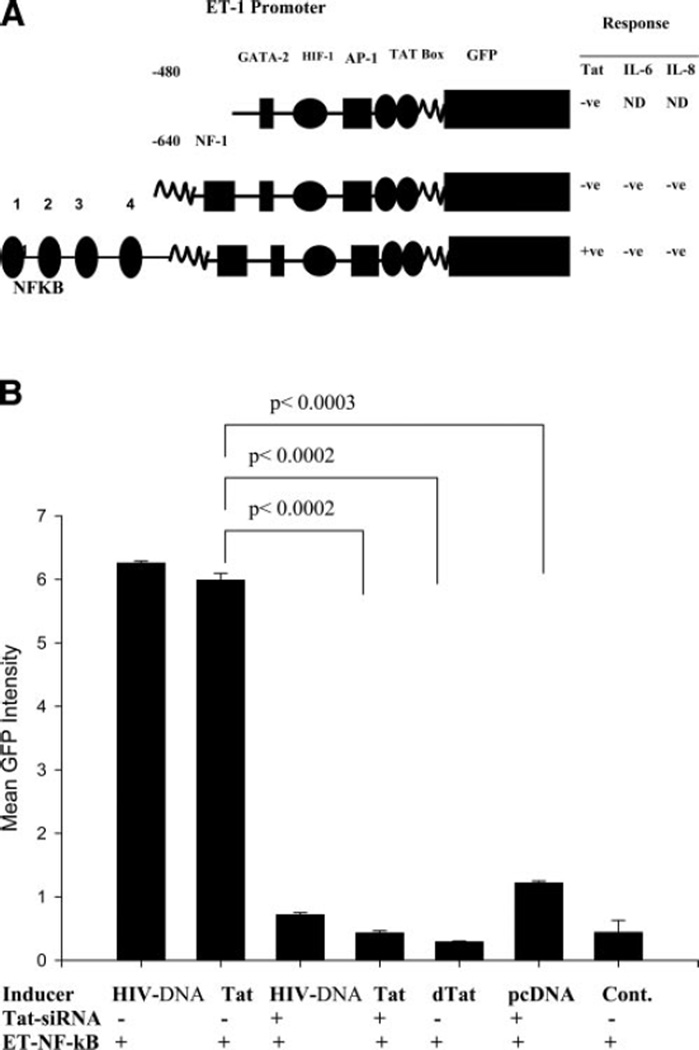
To explore the mechanism of ET-1 regulation in HIV-infected astrocytes, we cloned the human ET-1 promoter and inserted it upstream of the GFP gene. Two constructs were generated, one of them contained the minimum essential promoter region ET-480-GFP, and the other, ET-640-GFP, contained additional transcription factor binding sites (Fig. 5A). To reduce nonspecific induction and transient transfection errors, we introduced the ET-GFP promoter constructs into SVGA cells and stable transgenic clones were selected for negative GFP fluorescence by single-cell cloning. The promoter constructs ET-480 and ET-640-GFP were not responsive to Tat (Fig. 5A). Hence, a third ET-1 construct of 2.264 kb was developed encompassing an additional 1.426-kb fragment that included four NF-κB binding consensus sites (ET-NF-κB, Fig. 5A). Figure 5B shows that HIV proviral DNA or Tat induced ET-NF-κB promoter to a similar degree within 72 h of transfection, while mutant Tat (dTat) showed minimum effect. Further, to demonstrate the specificty, the effects of HIV proviral DNA and tat were blocked with siRNA to tat but not with nonspecific scrambled siRNA sequence, suggesting that the effects were specific (Fig. 5B). The controls were cotransfected with promoter construct and empty vector DNA to rule out any non-specific stimulatory effects. Above transient transfection results were confirmed in stable ET-NF-κB-SVGA cells. Further, Tat Ab was not used in inhibition studies because of the nuclear presence of Tat and indeed inability of Ab to enter the cells.
Figure 5.
Cloning of ET-1 promoter constructs and their response to Tat, IL-6, and IL-8. A) Diagrammatic view of ET-1 promoter constructs 480, 640, and the ET-promoter encompassing 480, 680, and additional 1.4 kb sequences containing NF-κB binding consensus sites (ET-NF-κB) are shown. The promoter constructs were monitored in SVGA cells on co-transfection with Tat expression vector or treatment of stable SVGA-ET640 or SVGA-ET-NF-κB cells with IL-6 or IL-8. Only ET-NF-κB construct was responsive to Tat but not to IL-6 and IL-8 in 72 h of observations. B) ET-1 promoter induction by HIV proviral DNA or Tat expression vectors and their specific inhibition by Tat-siRNA. ET-1 promoter induction was measured by green fluorescence in a fluorimeter. SVGA cells were cotransfected with the ET-NF-κB-GFP promoter construct and either HIV-1 proviral DNA or Tat or mutant Tat (dTat) expression vectors alone or in combination with Tat-siRNA (data represent mean fluorescence ± sem, P<0.0002, n=3).
Effect of IL-6, IL-8, and TNF-α on ET-1 promoter
Since Tat-expressing astrocytes revealed very high levels of IL-6 and IL-8 in cytokine arrays (Table 1) and in microarrays,—and ELISAs, which were investigated for regulation of ET-1 promoter—we performed experiments on stable SVGA-ET- NF-κB promoter cells to explore whether the above cytokines have any role in induction. Even though Tat mediated induction of IL-6 and IL-8 (Fig. 3C, Table 1), no effect of IL-8 on ET-1 promoter was observed, with concentrations up to 1000 ng/ml (Fig. 5A). However, a very small induction was noticed with IL-6 and TNF-α in a fluorimetric assay. But, on monitoring with microscopic GFP fluorescence, induction could not be verified and was presumed to be noninducible in this study.
Effect of IL-6, IL-8, and ET-1 on HIV-LTR promoter
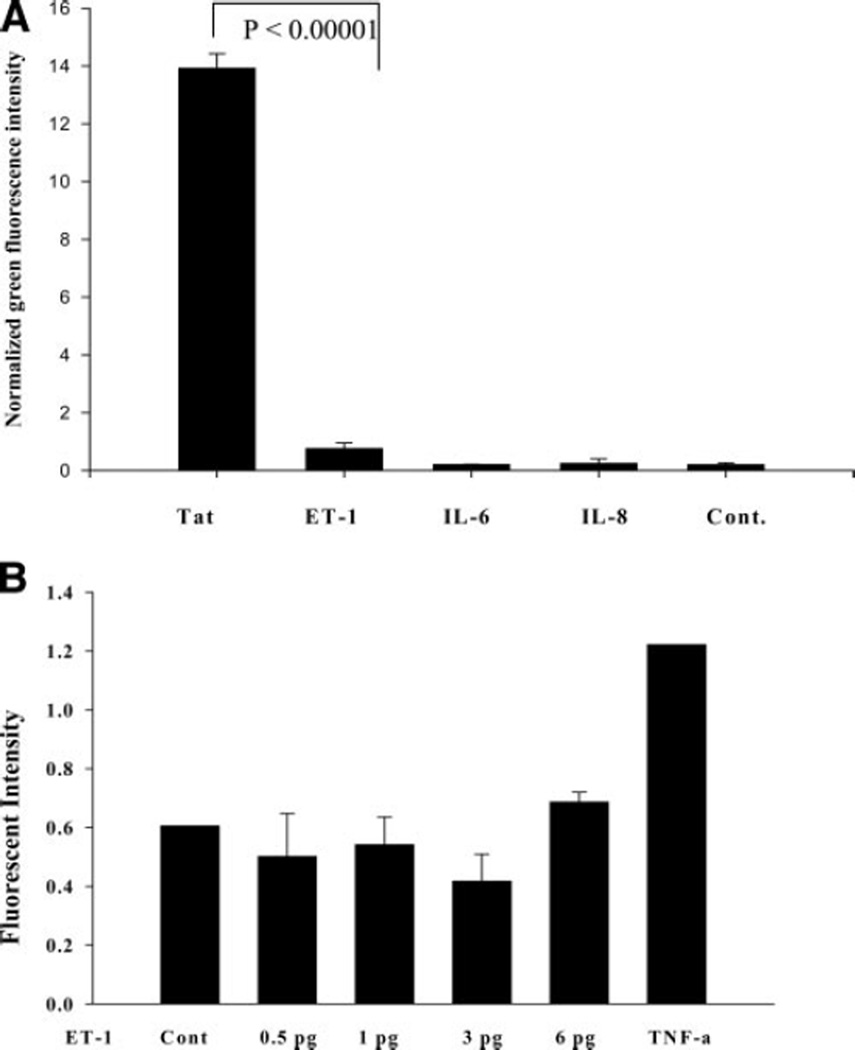
We explored whether IL-6, IL-8, and ET-1 have any effect on activation of the HIV LTR-promoter (HIV-transcription) in astrocytes. On treatment of SVGA-LTR-GFP cells with IL-6, IL-8, or ET-1, no stimulatory effect was detected in comparison to Tat (Fig. 6A). We also verified the effect of ET-1 on latently HIV-infected monocytic cells. Similarly, induction effect could not be seen in this model (Fig. 6B), suggesting that these proinflammatory molecules, at least, do not directly contribute to HIV replication.
Figure 6.
Effect of ET-1, IL-6, and IL-8 on HIV-LTR promoter (transcription) in astrocytes. Stable SVGA-LTR-GFP reporter cells were treated with high concentrations (200 ng/ml) of ET-1 or IL-6 or IL-8 using Tat as positive control (48 h). A) ET-1, IL-6, and IL-8 effect on HIV-LTRGFP astrocytic cells. B) Effect of ET-1 on latent HIV-infected reporter monocytic cells (p89-GFP). p89-GFP monocytic cells were treated with ET-1 for 48 h. TNF-α was used as positive control while untreated cells were negative control.
Statins inhibit Tat-mediated induction of ET-1
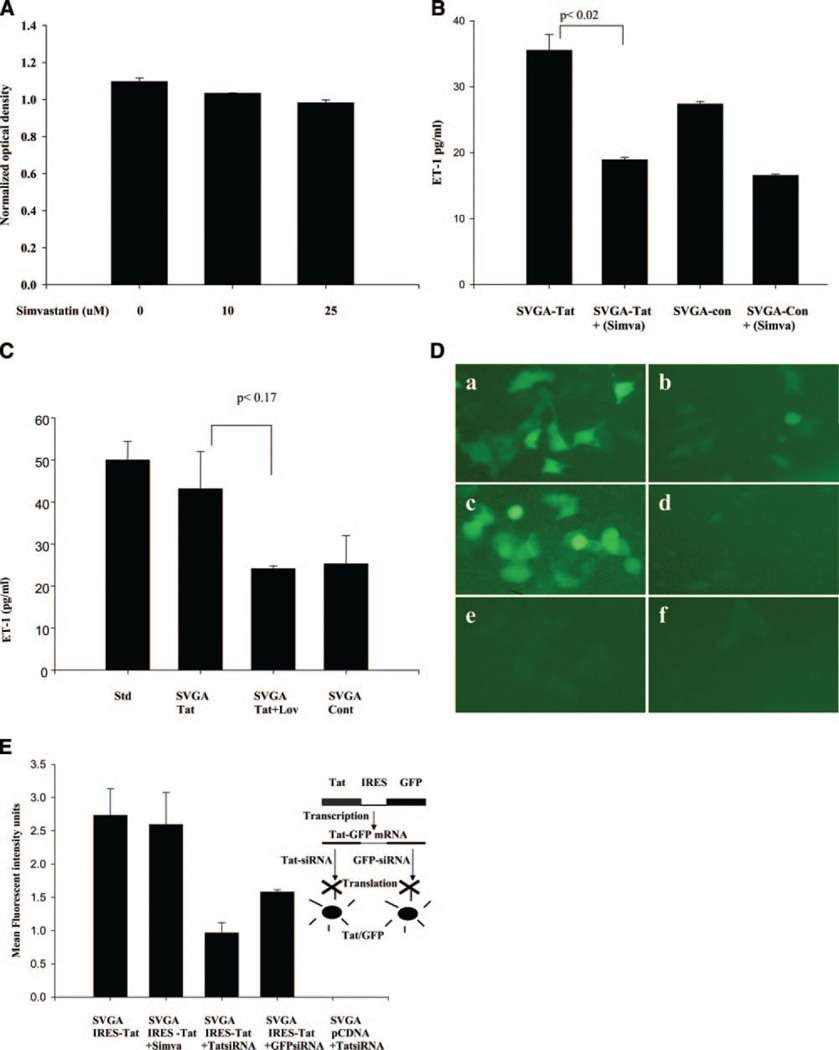
Since statins have been shown to inhibit TNF-α, IL-1β, and NF-κB activation, we investigated their effects on ET-1 production. At 10 µM concentrations, simvastatin and lovastatin showed minmal toxicity to neurons and glial cells on MTT assays (7A), whereas 25 µM revealed ~10% toxicity. Therefore, we studied a range of 5–10 µM concentrations of these statins. Stable SVGA-Tat cells in the presence or absence of simvastatin (10 µM) or lovastatin (10 µM) were monitored for ET-1 levels in the conditioned culture supernatants. Both the statins efficiently inhibited Tat up-regulated ET-1 production (Fig. 7B,C) at 48 and 72 h posttreatment. Interestingly, treatment of control cells with the statin drugs further reduced the ET-1 below basal levels (Fig. 7B).
Figure 7.
Statins inhibit ET-1 production in astrocytes-expressing-Tat stably, measured by quantitative ET-1 ELISA (72 h). A) Simvastatin toxicity was measured by MTT assay. Simvastatin revealed minimum toxicity at 10 and 25 µM concentrations on mixed primary neuronal and glial cell cultures for 24 h. B) Simvastatin (10 µM) significantly inhibited ET-1 production in Tat-expressing cells (data represent mean ± sem, P<0.02, n=2). C) Lovastatin (10 µM) decreased ET-1 levels in Tat-expressing astrocytic cells but the difference was not statistically significant (P<0.17, n=2). D) ET-1 promoter induction by HIV or Tat and its inhibition by simvastatin. SVGA cells were cotransfected with the ET-NF-κB-GFP promoter construct and either Tat or HIV proviral DNA (NL4–3) plasmids followed by addition of 10 µM simvastatin 4 h post-transfection. Cells were screened for green fluorescence by UV microscopy (72 h). a) SVGA cells transfected with ET-NF-κB promoter and Tat plasmid; (b) the same as in (a) but treated with 10 µM simvastatin (c) SVGA cells cotransfected with the ET-NF-κB promoter and HIV proviral DNA plasmids; (d) the same treatment as in (c) plus simvastatin; (e) SVGA cells cotransfected with the ET-NF-κB promoter and empty control vector (pCDNA); and (f) the same as in (e) plus simvastatin; (e) and (f) served as negative controls for (a) and (c). E) Effect of simvastatin on constitutive Tat expression through heterologous (non ET-1 promoter) in astrocytes (72 h). Control experiments for specificity of simvastatin were performed using CMV-promoter. SVGA-Tat cells, wherein Tat and GFP are transcribed into a single mRNA under the control of CMV promoter but encoding two different proteins because of the presence of IRES sequence between two genes, were not inhibited by simvastatin. But specific inhibition of Tat or GFP by Tat or GFP siRNAs was observed.
To explore the effects of statins at the transcriptional level, we studied the effect on ET-1 promoter linked to the GFP gene. We used ET-NF-κB-GFP promoter co-transfected with Tat plasmid into SVGA cells, followed by treatment with simvastatin (10 µM). Strong inhibition of the ET-1 promoter was noted (Fig. 7D), implying that Tat-regulated ET-1 transcription is sensitive to statins. In control experiments, simvastatin treatment had no effect on constitutive Tat-GFP expression through a hetrologous CMV promoter in astrocytes (Fig. 7E), but Tat or GFP specific siRNA efficiently inhibited Tat-GFP expression, thus validating the specificity of statins in our investigations. Furthermore, no effect of simvastatin was observed on IL-6 and IL-8 production in Tat-expressing astrocytes (Table 1), indicating that additional mechanisms probably are involved in ET-1 regulation.
Statins inhibit Tat-mediated activation of NFkB in astrocytes
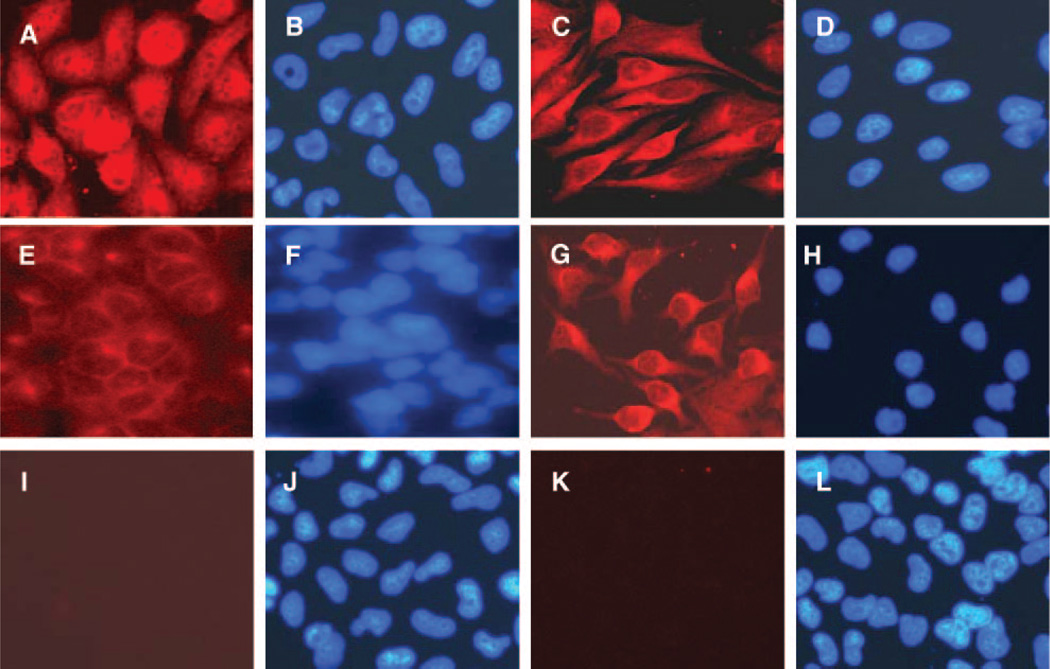
Lastly, we performed studies to determine whether Tat can influence the activation of NF-κB in astrocytes or whether statins have any role in NF-κB regulation. We observed that intracellular Tat expression in astrocytes leads to translocation of NF-kB to the nucleus (Fig. 8A), whereas in control SVGA cells, NF-κB is retained in the cytoplasm (Fig. 8E). Furthermore, pretreatment of Tat-expressing astrocytes with simvastatin (−24 to 72 h) inhibited the activation of NF-κB (Fig. 8C), whereas controls were unaffected (Fig. 8G). The translocation of NF-κB p50 was also verified by ELISA (data not shown). These results lend support to our proposed mechanism for the effects of statins on Tat-mediated induction of ET-1 (Fig. 9).
Figure 8.
Inhibition of Tat-mediated NF-κB activation by simvastatin. A) Stable Tat-expressing astrocytes revealed NF-κB activation (nuclear translocation). B) DAPI nuclear staining corresponding to (A). C) Inhibition of NF-κB activation by simvastatin (pretreated) Tat-expressing SVGA cells; (D) DAPI nuclear staining corresponding to (C); (E) NF-κB cytoplasmic (inactive) staining in control SVGA cells; (F) DAPI nuclear staining corresponding to (E); (G) control SVGA cells showing cytoplasmic NF-κB in the presence of simvastatin; (H) DAPI nuclear staining corresponding to (G); (I) isotype Ab control; (J) DAPI nuclear staining corresponding to (I); (K) isotype Ab control; (L) DAPI nuclear staining corresponding to (K).
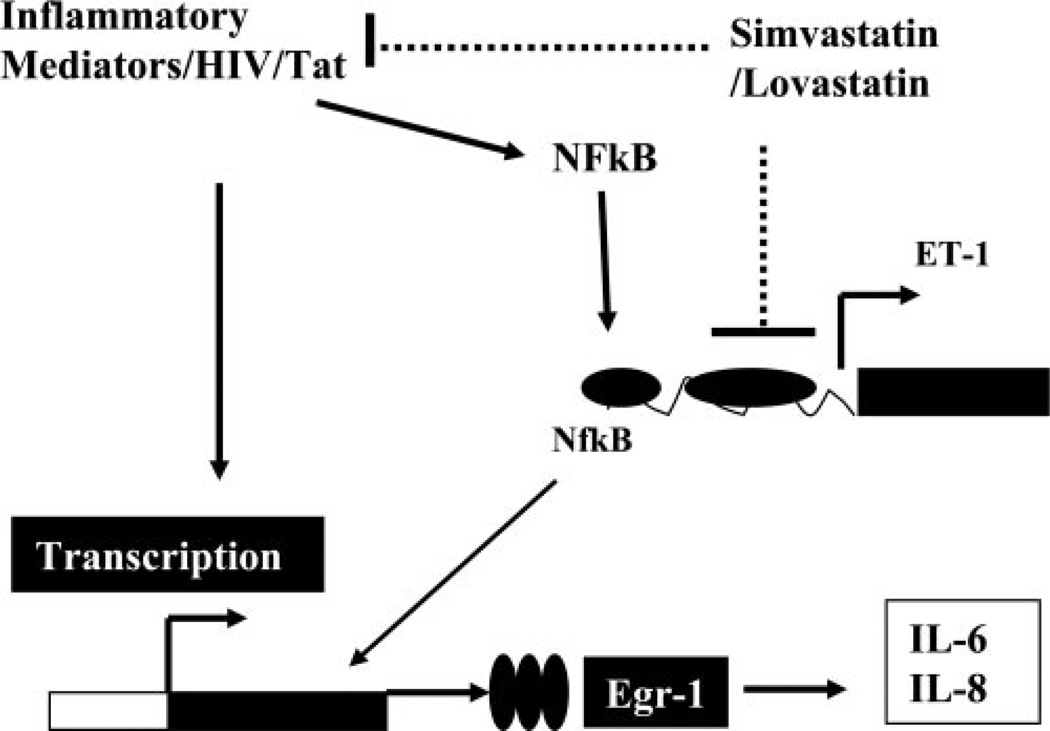
Figure 9.
Schematic diagram showing ET-1 regulation and its inhibition by statins. HIV infection or HIV proteins (Tat) initiate inflammatory response, thereby activating different genes leading to the production of ET-1, IL-6, and IL-8. Statins inhibit ET-1 through NF-κB. Statins might be restricting inflammatory response and hence preventing activation of NF-κB.
DISCUSSION
In our previous study, transplantation of Tat-expressing-astrocytes into the rat brain induced neuronal death, but the effects of Tat on astrocytes were not investigated in detail (24). Moreover, Tat has been exploited more as a recombinant protein extracellularly, but a nuclear protein, as demonstrated in this study and previously by others (25), has not been investigated for its effects on gene deregulation in host cells. We found a number of genes that were deregulated in Tat-expressing astrocytes. Although genes such as MCP-1 and IL-8 have been demonstrated previously to be up-regulated in the brains of HIV-infected patients and their regulation has been studied extensively, we found that another gene, ET-1, was also significantly elevated. ET-1 is well known to exert a variety of effects on the vasculature and other cellular functions.
HIV-Tat was found to be a potent inducer of ET-1 in astrocytes at transcriptional and translational (protein) levels, as seen by its enhanced secretion into the culture supernatants (Figs. 3A, B, 4). Further, to corroborate our in vitro observations, we show that expression of ET-1 was increased in neuroglial cells in patients (postmortem) with HIV dementia, in particular in the frontal lobes and basal ganglia. Interestingly, ET-1 appeared to be more prominent in the frontal lobes than in the basal ganglia (Table 2), a regional distribution similar to that seen in Alzheimer’s disease (26). This persistently elevated expression of ET-1 in the brain of HIV-infected patients is likely to have important pathophysiological consequences. In models of traumatic brain injury, ET-1 is expressed only transiently, for up to 2 d (27). Whereas increased expression of ET-1 intracellularly in astrocytes protects these cells from hypoxic and ischemic insults (28), extracellular ET-1 inhibits gap junctional communications, causes release of the neurotoxin arachidonic acid (29, 30), and alters the expression of several adhesion molecules in astrocytes (31, 32).
In particular, it has been shown that ET-1 was significantly induced in a human blood-brain barrier model of astrocytes and endothelial cells in vitro after exposure to HIV or gp120 (33). Moreover, gp120-mediated induction of ET-1 has been shown in macrophages (34). Similarly, ET-1 levels in cells derived from the CSF were significantly elevated in patients with HIV encephalopathy as compared to patients with multiple sclerosis or bacterial meningitis (35). In particular, levels of ET-1 in CSF correlated significantly with the severity of HIV encephalopathy (23).
ET-1 has also been implicated in causing axonal injury in neurons (36). Its expression in astroglia appears to be limited to the early developmental stage, and to disease states, such as in ischemia (37), traumatic brain injury (27), progressive multifocal leukoencephalopathy (PML), and subacute sclerosing panencephalitis (38). Importantly, up-regulated levels of ET-1 have been shown to decrease neuronal survival through impairment of glutamate uptake in astrocytes by down-regulation of glutamate transporter GLAST/ EAAT-1 (39). Of note, It has been shown that Tat enhances T antigen expression and trnascription of JCV. Therefore, it seems possible that JCV large T antigen and HIV Tat together may have a stimulatory effect on ET-1 (40).
Nevertheless, moderately increased levels of ET-1 over the long term may have a variety of deleterious effects on nervous system function. For example, ET-1 interactions with endothelin A receptors may lead to disruption of the blood-brain barrier (41). HIV-infected astrocytes are most often present at the blood-brain barrier or close to brain capillaries (6, 7). Thus, it is likely that these cells may alter the integrity of the BBB function via ET-1 release and subsequent infilteration of inflammatory cell into the brain. Importantly, ET-1 functions as a chemoattractant for monocytes through MCP-1 and IL-8 has also been described (42) and hence contributes toward HIV-mediated neuropathogenesis. Nevertheless, in our study ET-1, IL-6, and IL-8 levels were elevated in HIV-infected or Tat-expressing astrocytes (supernatants), but no direct involvement of these molecules on HIV transcription or ET production was observed (Fig. 6A); however, their contribution in neuropathognesis with HIV infection cannot be ruled out.
The mechanism(s) by which Tat enhances ET-1 production is unknown. Intriguingly, our findings suggest that the NF-κB binding site region in the ET-1 promoter is critical for Tat-mediated ET-1 production. Also, Tat is known to induce NF-κB activation in astrocytes (43) as well as in other cells (44). Since blocking Tat expression by Tat-siRNA blocked HIV-mediated ET-1 promoter and because in astrocytes Tat expression alone was sufficient to elevate ET-1 production, we propose that Tat mediates ET-1 production in astrocytes. This is the first report, to our knowledge, in which HIV or Tat has been shown to up-regulate ET-1 in astrocytes. Interestingly, the promoter region of ET-1 has sites for hypoxia-inducible factor (45) and four sites for the redox-sensitive NF-κB (46–49). Moreover, it has been shown earlier that increased oxidative stress can induce expression of ET-1 (50). Therefore, these observations suggest that there may actually be increased production of ET-1 during cellular stress, in the presence of cytokines, and in conditions where inflammation may result from ongoing infection(s).
Several blockers of endothelin receptors have been developed, some of which are in current human use for treating pulmonary hypertension (51). Their use for CNS diseases is limited, because of poor penetration across the BBB. Since statins are broad-spectrum inhibitors of inflammation and can cross the blood-brain barrier, we found that simvastatin efficiently inhibited ET-1 production by down-regulating the ET-1 promoter in Tat-expressing astrocytes. Statins have indeed been shown to decrease NF-κB activation (52). Intriguingly, in our study, the presence of NF-κB binding sites in the ET-1 promoter was found to be critical for HIV Tat-mediated induction. Therefore, our study established that only the NF-κB putative binding sites region −882 to −2179) was responsive to Tat and was also sensitive to inhibitory effect of simvastatin through impairment of NF-κB activation. Similarly, simvastatin has been reported to inhibit ET-1 at the transcriptional level, and this involves inactivation of Rho GTPase in endothelial cells (53) as well as inhibition of NF-κB activation (54–57). In particular, Rho GTPase activates NF-κB by phosphorylating inhibitor IkB and promoting its degradation (58).
Statins have also been shown to block HIV entry and exit from susceptible cells owing to the inactivation of Rho GTPase (59). HIV infectivity requires actin-dependent clustering of host lipid raft-associated receptors, a process that is linked to Rho guanosine triphosphatase (GTPase) activation (59). In summary, statins, originally developed as lipid-lowering agents, have recently received wide attention because of their potential anti-inflammatory (60) and neuroprotective effects (61). We thus propose (Fig. 9) that the statin group of compounds might be considered in treatment of HIV dementia.
Acknowledgments
We thank Dr. David Levy for providing latent HIV-infected monocytic cells. We also thank Drs. David Galey for help in analysis of cytokine array, Liping Guo for assistance with immunohistochemistry, Carol Anderson for technical assistance, and Drs. Pamela Talalay and Mahavir Singh for discussion and critical comments on the manuscript. We acknowledge the National Neuro AIDS Tissue Consortium (NNTC, NY, and San Diego, USA) for providing the brain tissues from HIV-infected patients, the NIH AIDS Research and Reference Reagent Program for providing various constructs and NIBS, U.K., for the monoclonal antibody to Tat. The work was supported by NIH grants RO1NS050064 (A.C.), P01MH070056, P01MH070306, and R01NS039253 (A.N.), and K08DA16160 (C.A.P.).
REFERENCES
- 1.Navia BA, Jordan BD, Price RW. Annals Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 2.Sacktor N. J. Neurovirol. 2002;8:115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 3.McArthur JC, McDermott MP, McClernon D, St. Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, et al. Arch. Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 4.Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. AIDS Res. 1984;1:447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Scarano F, Martin-Garcia J. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 7.Tornatore C, Chandra R, Berger JR, Major EO. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 8.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Aids. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, Simmonds P, Bell JE. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol. Appl. Neurobiol. 2003;29:378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 10.Nath A, Hartloper V, Furer M, Fowke KR. J. Neuropathol. Exp. Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Hery C, Wingertsmann L, Levy Y, Gray F, Tardieu M. Neuropathol. Appl. Neurobiol. 1997;23:352–353. doi: 10.1111/j.1365-2990.1997.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 12.Tornatore C, Meyers K, Atwood W, Conant K, Major EO. J. Virol. 1994b;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- 14.Messam CA, Major EO. J. Neurovirol. 2000;6:S90–S94. [PubMed] [Google Scholar]
- 15.Gorry P, Purcell D, Howard J, McPhee D. J. Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- 16.Cheng-Mayer C, Rutka JT, Rosenblum ML, McHugh T, Stites DP, Levy JA. Proc. Natl. Acad. SciUSA. 1987;84:3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewhurst S, Bresser J, Stevenson M, Sakai K, Evinger- Hodges MJ, Volsky DJ. FEBS Lett. 1987;213:138–143. doi: 10.1016/0014-5793(87)81479-5. [DOI] [PubMed] [Google Scholar]
- 18.Wesselingh SL, Glass JD, McArthur JC, Griffin JW, Griffin DE. Advan. Neuroimmunol. 1994;4:199–206. doi: 10.1016/s0960-5428(06)80258-5. [DOI] [PubMed] [Google Scholar]
- 19.Naidoo V, Naidoo S, Mahabeer R, Raidoo DM. J. Chem. Neuroanat. 2004;27:87–98. doi: 10.1016/j.jchemneu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Mosqueda-Garcia R, Inagami T, Appalsamy M, Sugiura M, Robertson RM. Circ. Res. 1993;72:20–35. doi: 10.1161/01.res.72.1.20. [DOI] [PubMed] [Google Scholar]
- 21.Major EO, Miller AE, Mourrain P, Traub RG, de Widt E, Sever J. Proc. Natl. Acad. SciUSA. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major E, Nath A. J. Biol. Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- 23.Rolinski B, Heigermoser A, Lederer E, Bogner JR, Loch O, Goebel FD. Infection. 1999;27:244–247. doi: 10.1007/s150100050020. [DOI] [PubMed] [Google Scholar]
- 24.Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. J. Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauber RH, Pavlakis GN. Virology. 1998;252:126–136. doi: 10.1006/viro.1998.9400. [DOI] [PubMed] [Google Scholar]
- 26.Minami M, Kimura M, Iwamoto N, Arai H. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:509–513. doi: 10.1016/0278-5846(95)00031-p. [DOI] [PubMed] [Google Scholar]
- 27.Petrov T, Steiner J, Braun B, Rafols JA. Neuroscience. 2002;115:275–283. doi: 10.1016/s0306-4522(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 28.Ho MC, Lo AC, Kurihara H, Yu AC, Chung SS, Chung SK. FASEB J. 2001;15:618–626. doi: 10.1096/fj.99-1022com. [DOI] [PubMed] [Google Scholar]
- 29.Tence M, Cordier J, Glowinski J, Premont J. EurJNeurosci. 1992;4:993–999. doi: 10.1111/j.1460-9568.1992.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Sarosi G, Jr, Barnhart D, Yule DI, Mulholland MW. Am. J. Physiol. 1997;272:G1175–G1185. doi: 10.1152/ajpgi.1997.272.5.G1175. [DOI] [PubMed] [Google Scholar]
- 31.Lopez Farre A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, Monton M, Rivas F, Gallego MJ, Egido J, et al. Circulation. 1993;88:1166–1171. doi: 10.1161/01.cir.88.3.1166. [DOI] [PubMed] [Google Scholar]
- 32.Doi M, Shichiri M, Yoshida M, Marumo F, Hirata Y. Hypertens. Res. 2000;23:643–649. doi: 10.1291/hypres.23.643. [DOI] [PubMed] [Google Scholar]
- 33.Didier N, Banks WA, Creminon C, Dereuddre-Bosquet N, Mabondzo A. Neuroreport. 2002;13:1179–1183. doi: 10.1097/00001756-200207020-00022. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenreich H, Rieckmann P, Sinowatz F, Weih KA, Arthur LO, Goebel FD, Burd PR, Coligan JE, Clouse KA. J. Immunol. 1993;150:4601–4609. [PubMed] [Google Scholar]
- 35.Rieckmann P, Albrecht M, Ehrenreich H, Weber T, Michel U. Res. Exp. Med. (Berl) 1995;195:17–29. doi: 10.1007/BF02576770. [DOI] [PubMed] [Google Scholar]
- 36.Hughes PM, Anthony DC, Ruddin M, Botham MS, Rankine EL, Sablone M, Baumann D, Mir AK, Perry VH. J. Neuropathol. Exp. Neurol. 2003;62:1276–1286. doi: 10.1093/jnen/62.12.1276. [DOI] [PubMed] [Google Scholar]
- 37.Tsang MC, Lo AC, Cheung PT, Chung SS, Chung SK. Neuroreport. 2001;12:2265–2270. doi: 10.1097/00001756-200107200-00044. [DOI] [PubMed] [Google Scholar]
- 38.Nie XJ, Olsson Y. Rev. Neurosci. 1996;7:177–186. doi: 10.1515/revneuro.1996.7.3.177. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura S, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N. Glia. 2002;37:178–182. doi: 10.1002/glia.10020. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury M, Taylor JP, Chang CF, Rappaport J, Khalili K. J. Virol. 1992;66:7355–7361. doi: 10.1128/jvi.66.12.7355-7361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, Yoshikawa I, Ohno N, Nakashima T. Pharmacol. Toxicol. 2003;92:21–26. doi: 10.1034/j.1600-0773.2003.920104.x. [DOI] [PubMed] [Google Scholar]
- 42.Ishizawa K, Yoshizumi M, Tsuchiya K, Houchi H, Minakuchi K, Izawa Y, Kanematsu Y, Kagami S, Hirose M, Tamaki T. Hypertens. Res. 2004;27:433–440. doi: 10.1291/hypres.27.433. [DOI] [PubMed] [Google Scholar]
- 43.Conant K, Ma M, Nath A, Major EO. J. Virol. 1996;70:1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demarchi F, Gutierrez MI, Giacca M. J. Virol. 1999;73:7080–7086. doi: 10.1128/jvi.73.8.7080-7086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. J. Biol. Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 46.Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. J. Biol. Chem. 1989;264:14954–14959. [PubMed] [Google Scholar]
- 47.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov, et al. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Diabetes. 2000;49:1561–1570. doi: 10.2337/diabetes.49.9.1561. [DOI] [PubMed] [Google Scholar]
- 49.Woods M, Wood EG, Bardswell SC, Bishop-Bailey D, Barker S, Wort SJ, Mitchell JA, Warner TD. Mol. Pharmacol. 2003;64:923–931. doi: 10.1124/mol.64.4.923. [DOI] [PubMed] [Google Scholar]
- 50.Mattart M, Mazzolai L, Chambaz C, Hayoz D, Brunner HR, Silacci P. Biorheology. 2003;40:289–297. [PubMed] [Google Scholar]
- 51.Braun-Moscovici Y, Nahir AM, Balbir-Gurman A. Semin. Arthritis Rheum. 2004;34:442–453. doi: 10.1016/j.semarthrit.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Inoue I, Itoh F, Aoyagi S, Tazawa S, Kusama H, Akahane M, Mastunaga T, Hayashi K, Awata T, Komoda T, Katayama S. Biochem. Biophys. Res. Commun. 2002;290:131–139. doi: 10.1006/bbrc.2001.6141. [DOI] [PubMed] [Google Scholar]
- 53.Mraiche F, Cena J, Das D, Vollrath B. BrJPharmacol. 2005;144:715–726. doi: 10.1038/sj.bjp.0706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madonna R, Di Napoli P, Massaro M, Grilli A, Felaco M, De Caterina A, Tang D, De Caterina R, Geng YJ. J. Biol. Chem. 2005;280:13503–1311. doi: 10.1074/jbc.M411859200. [DOI] [PubMed] [Google Scholar]
- 55.Lin R, Liu J, Peng N, Yang G, Gan W, Wang W. Biol. Pharm. Bull. 2005;28:1630–1634. doi: 10.1248/bpb.28.1630. [DOI] [PubMed] [Google Scholar]
- 56.Wei CY, Huang KC, Chou YH, Hiseh PF, Lin KH, Lin WW. Mol. Pharmacol. 2006;69:960–967. doi: 10.1124/mol.105.017368. [DOI] [PubMed] [Google Scholar]
- 57.Sironi L, Banfi C, Brioschi M, Gelosa P, Guerrini U, Nobili E, Gianella A, Paoletti R, Tremoli E, Cimino M. Neurobiol. Dis. 2006;22:445–451. doi: 10.1016/j.nbd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Liu CA, Wang MJ, Chi CW, Wu CW, Chen JY. Oncogene. 2004;23:8731–8742. doi: 10.1038/sj.onc.1208106. [DOI] [PubMed] [Google Scholar]
- 59.Del Real G, Jimenez-Baranda S, Mira E, Lacalle RA, Lucas P, Gomez Mouton C, Alegret M, Pena JM, Rodriguez- Zapata M, Alvarez-Mon M, et al. J. Exp. Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeung AC, Tsao P. Circulation. 2002;105:2937–2938. doi: 10.1161/01.cir.0000023397.12047.03. [DOI] [PubMed] [Google Scholar]
- 61.Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. BMC Med. 2004;2:4. doi: 10.1186/1741-7015-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]