Abstract
Single-molecule fluorescence spectroscopy has proven to be instrumental in understanding a wide range of biological phenomena at the nanoscale. Important examples of what this technique can yield to biological sciences are the mechanistic insights on protein-protein and protein-nucleic acid interactions. When interactions of proteins are probed at the single-molecule level, the proteins or their substrates are often immobilized on a glass surface, which allows for a long-term observation. This immobilization scheme may introduce unwanted surface artifacts. Therefore, it is essential to passivate the glass surface to make it inert. Surface coating using polyethylene glycol (PEG) stands out for its high performance in preventing proteins from non-specifically interacting with a glass surface. However, the polymer coating procedure is difficult, due to the complication arising from a series of surface treatments and the stringent requirement that a surface needs to be free of any fluorescent molecules at the end of the procedure. Here, we provide a robust protocol with step-by-step instructions. It covers surface cleaning including piranha etching, surface functionalization with amine groups, and finally PEG coating. To obtain a high density of a PEG layer, we introduce a new strategy of treating the surface with PEG molecules over two rounds, which remarkably improves the quality of passivation. We provide representative results as well as practical advice for each critical step so that anyone can achieve the high quality surface passivation.
Keywords: Chemistry, Issue 86, single-molecule spectroscopy, polymer, polyethylene glycol (PEG), piranha etching, amino-silanization, surface passivation, fluorescence, glass surface coating.
Introduction
When performing a single-molecule protein study it is essential to achieve a high quality of surface passivation so that the experiment is free from any surface-induced protein malfunction or denaturation 1,2. While a glass surface treated with surfactants, such as bovine serum albumin, is commonly used for single-molecule nucleic acid studies 3, the degree of its passivation is not high enough for protein studies. A glass surface coated with polymer (polyethylene glycol, PEG) is superior in the passivation performance 4-6. Thereby, it has become universally used for single-molecule protein studies ever since it was introduced to a single-molecule fluorescence study 7-10. The polymer coating procedure requires multiple surface treatments 7,11,12. Therefore, it is difficult to follow the whole procedure without detailed instructions. Often the degree of surface passivation varies depending on which protocol is followed. Here we present a robust protocol with step-by-step instructions, which will remove one of the major bottlenecks of single-molecule protein studies. See Figure 1 for overview.
Protocol
1. Slide Preparation and Cleaning
A microfluidic chamber is composed of a quartz slide and a coverslip. In prism-type total internal reflection fluorescence (TIRF) microscopy, the surface of the quartz slide is imaged. Therefore, it is important to clean a quartz slide thoroughly using H2O, acetone, KOH, and piranha solutions. Th is multi-step cleaning eliminates fluorescent organic molecules on a surface which interfere with single molecule fluorescence measurements. Additionally, the piranha etching makes the quartz surface hydrophilic by generating hydroxyl groups. The free hydroxyl groups are essential for the amino-silanization reaction in Step 3.
- Drilling quartz slides. Drill a pair of holes into a quartz slide with a 3/4 mm diamond drill bit (Figure 2a). If multiple channels are desired, drill more pairs of holes (Figure 2b). These holes are used for injecting solutions into a microfluidic chamber in Step 7.
- Keep the drill bit wet in H2O during the drilling. H2O performs as a lubricant, which increases the lifetime of the drill bit.
- Occasional wiping the tip of the drill bit helps drilling holes.
- After drilling holes, mark one side of the slide for easy identification during the PEGylation process. The markers can be used as reference to avoid confusion later on. For marking, a diamond drill bit can be used. Do not use any pen since the ink might get on the surface.
Cleaning with H2O. Place the slides in a glass staining jar. Typically 5 to 15 slides can be placed in a single jar. Rinse the slides with MilliQ H2O. Repeat it 3 times and sonicate the slides with MilliQ H2O for 5 min to remove dirt. Dispose the water and rinse the slides again 3 times with MilliQ H2O. Note that 130 W is the sonication power used for this protocol. Higher sonication power might decrease the lifetime of the quartz slides.
Cleaning with acetone. Replace the MilliQ H2O with acetone. Sonicate the slides with acetone for 20 min or longer.
Rinsing with H2O. Discard the acetone and rinse the slides with MilliQ H2O. Repeat it for 3 times in order to remove any acetone residue.
Cleaning with KOH. Replace the water with 1 M KOH and sonicate the slides for 20 min or longer. Excessive etching (e.g. overnight KOH treatment) will enhance the quality of the surface, but will introduce scratches, which might interfere with fluorescence imaging.
Rinsing with H2O. Rinse the slides with MilliQ H2O for 3 times to remove traces of KOH.
- Piranha etching.
- Transfer the slides to a Duran slide holder or a custom-made Teflon holder and place them in a beaker (1 L) that is located in a chemical hood.
- Fill the beaker with 450 ml of H2SO4.
- Add 150 ml of H2O2 for 3:1 ratio between H2SO4 and H2O2. Once the solution starts to boil spontaneously, the temperature increases over 90 °C. Make sure that the H2O2 solution is at room temperature before starting the reaction. Otherwise, the temperature of the boiling solution may be lower than 90 °C.
- Stir the solution for proper mixing and let the beaker undisturbed for 20 min.
- Take the slides out of the piranha solution together with the slide holder, and put them in a slide holder containing MilliQ H2O. Meanwhile, discard the piranha solution into a designated waste bottle once it reaches room temperature.
- Rinse the slides 3 times with MilliQ H2O. Extra care should be taken in further steps to avoid any possible contamination of slides. Continue to Step 3. It is advised to immediately proceed with the following steps. * Caution: The piranha solution is extremely reactive. When handling this solution extra caution should be taken. In addition, when by mistake mixed with organic solvents such as acetone, it may cause an explosion.
2. Coverslip Cleaning
A microfluidic chamber is composed of a quartz slide and a cover s lip. When a prism-type TIRF microscope is used, the surface of a coverslip is not imaged . Therefore, it is enough to clean a coverslip only with H2O and KOH. In case a coverslip needs to be imaged (e.g. via the objective-type TIRF microscopy), it is recommended to treat the coverslips with piranha solution (Step 1.7). Note that if PEGylation of the coverslip surface is not of high quality, it might act as a sink for proteins and give rise to variations in the protein concentration.
Rinsing with H2O. Place coverslips (24 x 30, 24 x 40, or 24 x 50 mm2) in a glass staining jar. Typically 5 to 15 coverslips can be placed in a single jar. Rinse the coverslips 3 times with the MilliQ H2O.
Cleaning with KOH. Replace the water with 1M KOH and sonicate the coverslips for 20 min or longer.
Rinsing with H2O. Rinse the coverslips 3 times with MilliQ H2O to remove traces of KOH. Continue to Step 3.
3. Amino-silanization of Slides and Coverslips
Functionalizing the surface of the quartz slides and the coverslips with amine group via the amino-silanization chemistry. Methanol is used as a solvent and acetic acid as a catalyst for the amino-silanization reaction.
Rinsing with methanol. Replace the MilliQ H2O in the staining dishes (from Steps 1 and 2) with methanol. Keep the slides and the coverslips in methanol until Step 3.3. Do not store the slides and the coverslips in methanol for an unnecessarily long period of time (e.g. several hours) since impurities in methanol will adsorb to the surface.
- Preparing amino-silanization solution.
- Rinse a Pyrex flask several times with methanol. Sonicate the flask with methanol for 5 min or longer. It is advised to have a dedicated flask that is maintained clean.
- Pour 100 ml of methanol into the flask.
- Add 5 ml of acetic acid.
- Add 3 ml of APTES (3-aminopropyl trimethoxysilane) and gently mix by shaking it.
- Amino-silanization. Replace the methanol in the staining dishes that contain the slides and the coverslips with the aminosilanization reaction mixture.
- Incubate for 20-30 min. During incubation, sonicate once for 1 min.
Rinsing with methanol. Replace the aminosilanization reaction with methanol. Discard the methanol and add a fresh methanol solution. Repeat this procedure three times.
4. Surface Passivation Using Polymer (The First Round)
Passivating the amine-coated surface of quartz slides and coverslips by conjugating NHS-ester polyethylene glycol (PEG). This reaction is carried out with the saturating concentration of PEG solution at pH 8.5 overnight.
Drying slides and coverslips. Dry the slides and the coverslips using N2 gas and place them in clean pipette boxes in such a way that the side which has to be PEGylated is facing up. The pipette box is partially filled with MilliQ H2O. This moist environment prevents PEGylation solution from drying out during the overnight incubation.
Preparing reaction buffer. Prepare 0.1 M of fresh sodium bicarbonate buffer (pH 8.5) by dissolving 84 mg of sodium bicarbonate in 10 ml of MilliQ H2O. There is no need of adjusting pH. This solution may be stored frozen for the next use (e.g. Step 6.1).
- Preparing PEGylation solution.
- Prepare a PEG mixture of 0.2 mg biotinylated NHS-ester PEG (5,000 Da) 13 and 8 mg of NHS-ester mPEG (5,000 Da) in a 1.5 ml tube. When preparing N number of slides, prepare N times larger amount of the PEG mixture in a single tube.
- Add 64 µl (or N times 64 µl for N number of slides) of the freshly prepared buffer (Step 4.2). Pipette it up and down in order to dissolve them completely.
- Centrifuge with 16,100 x g for 1 min to remove air bubbles. Do not pipette afterwards. Otherwise, air bubbles will form which interfere with PEGylation at Step 4.4.
- PEGylation.
- Drop 70 µl of the PEGylation mixture to a dried quartz slide from Step 4.1. Use 90 µl in case of 24 x 50 mm2 coverslip.
- Gently place a dried coverslip from Step 4.1 over the solution.
- To have a uniform and high quality of PEGylation, take care not to introduce air bubbles. Due to surface tension, most of the air bubbles may spontaneously leave the reaction volume within five minutes due to surface tension.
- Incubate the slides in a dark and humid environment. The half-life of NHS-ester PEG that we use at pH 8 is about one hour. At a minimum, incubate them for 2 hr. Overnight incubation leads to the higher quality of PEGylation (data not shown).
5. Long-term Storage
Storing the PEGylated slides and coverslips in N2 at -20 ºC.
Drying slides and coverslips. Carefully disassemble the slide and the coverslip by sliding the coverslip to a side, rinse them with MilliQ H2O, and dry them with N2.
- Storing slides and coverslips. For immediate use, follow the procedure for the second round of PEGylation (Step 6). In order to store for a longer period of time, follow the next steps.
- Place a pair of the slide and the coverslip in a 50 ml tube such that the PEGylated surfaces are facing away from each other.
- Partially close the tube, vacuum the tube, and then fill it with N2. These steps help preserving the PEGylated surface for a long period of time. Screw the tube tightly and store it at -20 °C. The quality of the slides remains good for up to 3 months (Figure 3a, right).
6. Surface Passivation Using Polymer (The Second Round)
Additional round of PEGylation to make the PEG layer denser and also to quench any remaining amine groups on the surface. The use of short NHS-ester PEG molecules (333 Da) may be effective in penetrating into an existing PEG layer. It is recommended to do this second round of PEGylation right before using a slide.
Preparing reaction buffer. Prepare 0.1 M of fresh sodium bicarbonate buffer (pH 8.5). The frozen solution from Step 4.2 can also be used.
Preparing PEGylation solution. Dissolve 7 µl of 250 mM MS4-PEG in 63 μl of the sodium bicarbonate buffer.
PEGylation. Follow the same procedure as in Step 4.4. Incubate for 30 min up to overnight.
Drying slides and coverslips. Disassemble the pair of the slide and the coverslip, rinse them with MilliQ H2O, dry them with N2, and keep them in a clean pipette box. Proceed for assembling a microfluidic chamber in Step 7.
7. Assembling a Microfluidic Chamber
Assembling a microfluidic chamber using a pair of a PEGylated quartz slide and a coverslip. Double-sided sticky tape is used as a spacer. The chamber is sealed with Epoxy glue and, solutions are introduced through the holes in the quartz slide.
Place a quartz slide on a flat surface with the PEGylated side facing up.
Make a channel (width 5 to 7 mm) diagonally on the PEGylated surface by putting double-sided sticky tapes over the slide. Make sure that the holes are positioned at the center of the channel. Take care not to spoil the PEGylated surface during the process of making a chamber. It is possible to make a multi-channel slide by placing and sticking the double-sided sticky tapes differently (see Figure 2).
Gently place a PEGylated coverslip on top to complete the chamber. The PEGylated side should be facing down.
Seal the chamber by pressing the coverslip over the area where double-sided tapes are placed. Do it gently but thoroughly so that the chamber becomes water-tightly sealed.
Close the edges of the chamber with Epoxy glue.
Immobilize Streptavidin or Neutravidin on the biotinylated PEG layer by adding 50 µl of 0.1 mg/ml of Streptavidin or Neutravidin solution in T50 (10 mM Tris-HCl [pH8.0], 50 mM NaCl buffer) using a p200 pipette. After 1 min of incubation, flush with 100 µl of T50 buffer.
Add biotinylated biological molecules for your single-molecule imaging.
8. Slide Recycling
Recycling quartz slides. Used slides are recycled by taking off the coverslips and the double-sided sticky tapes.
After use, store the chambers in tap water. Long-term incubation in water eases disassembly of the chambers.
Boil the chambers in tap water using a microwave. Use a Pyrex beaker. Boil for 10 min or longer.
Take off the coverslips and the double-sided sticky tapes using a razor blade. Do not press a quartz slide perpendicular to its plane. Otherwise, it breaks. Keep the razor blade away from the channel. Otherwise, it introduces scratches on the channel.
Rinse the slides using a household detergent by rubbing them with fingers.
Place the slides in a glass staining jar. Typically 5 to 15 slides can be placed in a single jar. Add 10% household detergent and sonicate the slides for 20 min or longer. Rinse the slides with a large quantity of tab water.
Go to Step 1.2.
Representative Results
After the microfluidic chamber assembly (Step 7.1 - 7.6) and before carrying out Step 7.7, it is advised to carry out the quality control of the PEGylated surface.
If the surface passivation has been done successfully, there are less than 10 non-specifically adsorbed proteins per imaging area (25 mm x 25 mm) observed when 1-10 nM fluorescently labeled protein is added into the chamber (Figure 3a, left).
When any of the cleaning or reaction steps has not been properly carried out, the number of non-specifically adsorbed proteins significantly increases, and the CCD screen may be saturated by fluorescence signals. For example, if the piranha etching is skipped, there is 100 times a larger amount of non-specific adsorption observed (Figure 3a; compare left with middle). When the second PEGylation step is skipped, there was approximately 3 times a larger amount of non-specific adsorption observed (Figure 3b). A low degree of PEGylation is observed when expired chemicals (e.g. APTES stored at room temperature for several months) are used (data not shown). The quality of the surface also drops down when a significant amount of time has passed by since it was PEGylated (Figure 3a; compare left with right).
To our best knowledge, it is the first time that the two rounds of PEGylation are introduced for single-molecule studies. The two rounds of PEGylation guarantee the highest quality of PEG layer formation (Figure 3b). The superior nature of the double PEGylation is prominently shown in the movies (Compare Movie 1a with Movie 1b). In these movies, the background signals from fluorescent molecules in solution are observed to be much weaker when the double PEGylation was used, which indicates that proteins are repelled more effectively by the doubly PEGylated layer. Though this two-step process is strongly advised, the second PEGylation step might be skipped if your experiment is tolerable to non-optimal passivation.
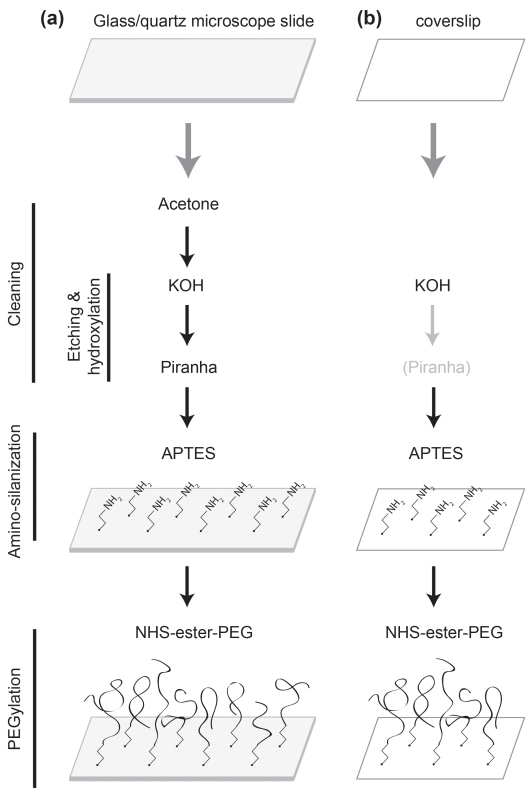 Figure 1: Schematic of the surface treatments. (a) A microscope slide is cleaned with acetone, KOH, and piranha solutions. It is functionalized with APTES and PEGylated with NHS-ester PEG. (b) A coverslip is cleaned with KOH, as well as with the piranha solution if necessary. It is functionalized with APTES and PEGylated with NHS-ester PEG.
Figure 1: Schematic of the surface treatments. (a) A microscope slide is cleaned with acetone, KOH, and piranha solutions. It is functionalized with APTES and PEGylated with NHS-ester PEG. (b) A coverslip is cleaned with KOH, as well as with the piranha solution if necessary. It is functionalized with APTES and PEGylated with NHS-ester PEG.
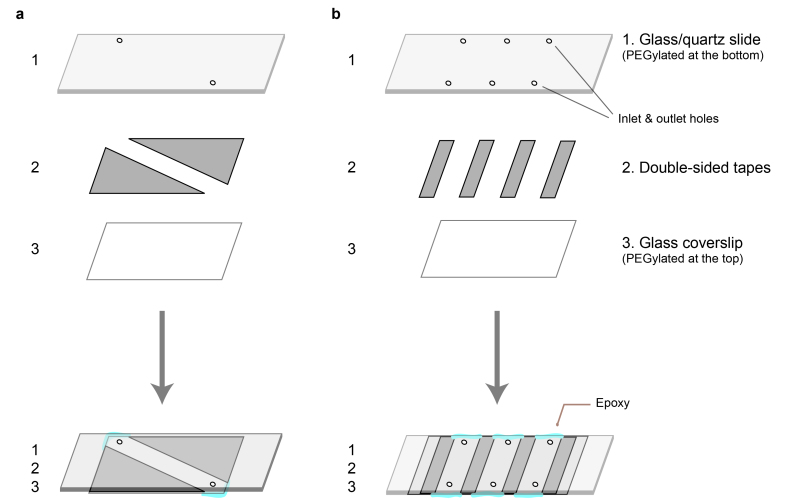 Figure 2: Microfluidic chamber. (a) A single-channel chamber. The microscope slide has two holes drilled. It is assembled with a coverslip using two slices of a double-sided sticky tape. (b) A three-channel chamber. The microscope slide has six holes drilled. It is assembled with a coverslip using four slices of a double-sided sticky tape.
Figure 2: Microfluidic chamber. (a) A single-channel chamber. The microscope slide has two holes drilled. It is assembled with a coverslip using two slices of a double-sided sticky tape. (b) A three-channel chamber. The microscope slide has six holes drilled. It is assembled with a coverslip using four slices of a double-sided sticky tape.
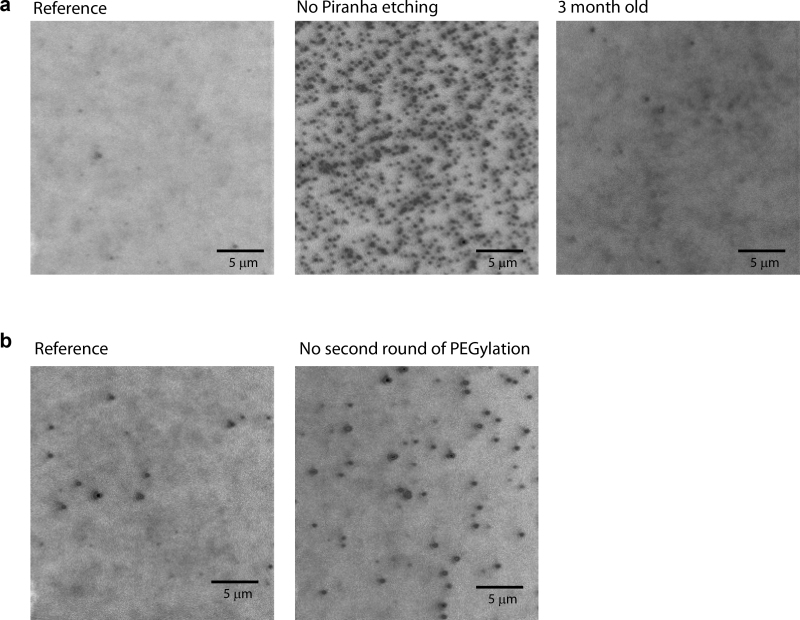 Figure 3: CCD images taken with dye-labeled proteins in solution. (a) CCD images were taken by prism-type total internal reflection fluorescence microscopy using a 60X objective lens with 10 nM Cy3-labeld Rep in solution. (Left) A surface was prepared following the protocol in this article. (Middle) A surface was prepared following the protocol in this article, but piranha etching was skipped. (Right) A surface was prepared following the protocol in this article and stored for 3 months at -20 ºC under nitrogen. (b) CCD images taken with 10 nM Cy3-labeld Rep in solution. (Left) A surface was prepared following the protocol in this article. (Right) A surface was prepared following the protocol in this article, but the second round of PEGylation was skipped. Scale bar = 5 µm.
Figure 3: CCD images taken with dye-labeled proteins in solution. (a) CCD images were taken by prism-type total internal reflection fluorescence microscopy using a 60X objective lens with 10 nM Cy3-labeld Rep in solution. (Left) A surface was prepared following the protocol in this article. (Middle) A surface was prepared following the protocol in this article, but piranha etching was skipped. (Right) A surface was prepared following the protocol in this article and stored for 3 months at -20 ºC under nitrogen. (b) CCD images taken with 10 nM Cy3-labeld Rep in solution. (Left) A surface was prepared following the protocol in this article. (Right) A surface was prepared following the protocol in this article, but the second round of PEGylation was skipped. Scale bar = 5 µm.
Movie 1: CCD movies taken with dye-labeled proteins in solution. CCD movies were taken by prism-type total internal reflection fluorescence microscopy using a 60X objective lens with 10 nM Cy3-labeld Rep in solution. The time resolution is 100 msec. (a) A surface was prepared following the protocol in this article. (b) A surface was prepared following the protocol in this article, but the second round of PEGylation was skipped. Click here to view Movie 1a and click here to view Movie 1b.
Discussion
Critical steps within this protocol
It is essential to make the surface hydrophilic before the amino-silanization reaction. This was achieved through the piranha etching which generates the free hydroxyl groups on a glass/quartz surface. It is recommended not to keep the piranha etched surface exposed to either H2O or air for a long period of time since the hydrophilicity of the surface goes down gradually.
The NHS-ester PEG molecules are reactive. It is advised to make aliquots and store them under nitrogen in -20 ºC. The shelf life of the APTES chemical at the room temperature is short. It is advised to replace it with a new one every month.
Modifications of this protocol
When you cannot use the piranha etching for any practical reason, you may etch using KOH for a long period of time (e.g. overnight), which will also make hydroxyl groups exposed. The pitfall of this alternative approach is that the slide becomes unusable after a few recycles due to severe scratches.
It is often practiced to burn a glass/quartz surface using propane torch, which is effective in eliminating fluorescent organic materials 7. This procedure was not included in this protocol because it is redundant to the piranha etching. Note that this procedure will potentially lead to oxidation of the hydroxyl groups. Therefore, this procedure should not be carried out once a surface was etched using KOH or piranha solution.
When a buffer with pH lower than 7 is used for single-molecule imaging, the conformation of PEG changes from “mushroom” to “brush,” which reduces the degree of passivation. Therefore, when pH lower than 7.0 is used, it is recommended to further passivate the surface using disuccinimidyl tartarate 7.
Perspectives
This work has provided a robust protocol for achieving high quality surface passivation. This protocol will be useful for single-molecule fluorescence studies that are involved with proteins 14 as well as protein complexes within cell extracts and immunoprecipitates 15. It will be widely used for other single-molecule techniques such as force spectroscopy and torque spectroscopy 16. It will be also useful for preventing cells from adsorbing to a surface 17.
The protocol provided in this work is demanding for its multi-step procedures. Surface passivation using lipid-PEG 8 and poly-lysine PEG 9 are available as an alternative approach. Since, they do not require any chemical reactions it is easy to implement. However, the degree of passivation is not as high as that achieved through chemical modification of a surface.
Disclosures
We have nothing to disclose.
Acknowledgments
S.D.C., A.C.H., and C.J. were supported by Starting Grants (ERC-StG-2012-309509) through the European Research Council. J.-M.N. was supported by the National Research Foundation (NRF) (2011-0018198) of Korea; and the Pioneer Research Center Program (2012-009586) through the NRF of Korea funded by the Ministry of Science, ICT, and Future Planning (MSIP). This work was also supported by Center for BioNano Health-Guard funded by MSIP of Korea as the Global Frontier Project (H-GUARD_2013-M3A6B2078947). Y.K.L. and J.-H.H. were supported by the Seoul Science Fellowship Program of Seoul City, Korea. The labeled Rep protein was a generous gift from Dr. Sua Myong.
References
- Rasnik I, McKinney SA, Ha T. Surfaces and orientations: much to FRET about. Acc Chem Res. 2005;38(7):542–548. doi: 10.1021/ar040138c. [DOI] [PubMed] [Google Scholar]
- Lamichhane R, Solem A, Black W, Rueda D. Single-molecule FRET of protein-nucleic acid and protein-protein complexes: surface passivation and immobilization. Methods. 2010;52(2):192–200. doi: 10.1016/j.ymeth.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiori N, Meller A. Automated system for single molecule fluorescence measurements of surface-immobilized biomolecules. J Vis Exp. 2009. pp. 1542–1510. [DOI] [PMC free article] [PubMed]
- Kingshott P, Griesser HJ. Surface that resist biodegradation. Curr Opin Solid St M. 1999;4(4):403–412. [Google Scholar]
- Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Annu Rev Mater Sci. 1996;26:365–394. [Google Scholar]
- Zalipsky S, Preface Harris JM. Poly(theylene glycol) 1997. pp. 11–12.
- Selvin PR, Ha T. Single-Molecule Techniques: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. [Google Scholar]
- Finkelstein IJ, Greene EC. Supported lipid bilayers and DNA curtains for high-throughput single-molecule studies. Methods Mol Biol. 2011;745:447–461. doi: 10.1007/978-1-61779-129-1_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenausis GL, et al. Poly(L-lysine)-g-Poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J Phys Chem B. 2000;104(14):3298–3309. [Google Scholar]
- Groll J, Star Moeller M. polymer surface passivation for single-molecule detection. Method Enzymol. 2010;472:1–18. doi: 10.1016/S0076-6879(10)72019-X. [DOI] [PubMed] [Google Scholar]
- Bahmani B, Gupta S, Upadhyayula S, Vullev VI, Anvari B. Effect of polyethylene glycol coatings of uptake of indocyanine green loaded nanocapsules by human spleen macrophages in vitro. J Biomed Opt. 2011;16(5) doi: 10.1117/1.3574761. [DOI] [PubMed] [Google Scholar]
- Kulczyk AW, Tanner NA, Loparo JJ, Richardson CC, van Oijen AM. Direct observation of enzymes replicating DNA using a single-molecule DNA stretching assay. J Vis Exp. 2010. p. e1689. [DOI] [PMC free article] [PubMed]
- Upadhyayula S, et al. Coatings of polyethylene glycol for suppressing adhesion between solid microspheres and flat surfaces. Langmuir. 2012;28(11):5059–5069. doi: 10.1021/la300545v. [DOI] [PubMed] [Google Scholar]
- Dulin D, Lipfert J, Moolman MC, Dekker NH. Studying genomic processes at the single-molecule level: introducing the tools and applications. Nat Rev Genet. 2012;14:9–22. doi: 10.1038/nrg3316. [DOI] [PubMed] [Google Scholar]
- Joo C, Fareh M, Narry Kim V. Bringing single-molecule spectroscopy to macromolecular protein complexes. Trends Biochem Sci. 2012;38(1):30–37. doi: 10.1016/j.tibs.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]


