Abstract
Medulloblastoma (MB) expresses Src kinase, while aurora kinase A overexpression correlates with poor survival. We thus investigated novel combination treatment with dasatinib and AT9283, inhibitors of Src and aurora kinase, respectively, on MB growth in vitro and in vivo. Treatment with each drug significantly reduced cell viability and combined treatment markedly potentiated this response. AT9283 induced p53 expression, autophagy, and G2/M cell-cycle arrest, while combined treatment induced S phase arrest. Dasatinib treatment caused tumor regression in vivo. Activated Src was detected in 44% MB analyzed. We conclude that further evaluation of this combination therapy for MB is highly warranted.
Keywords: medulloblastoma, dasatinib, aurora kinase, survival
1. Introduction
Medulloblastoma (MB) is a highly invasive malignant brain tumor [1]. There are four molecularly-defined subgroups: Wnt, having a good prognosis, sonic-hedgehog (Shh), with intermediate prognosis, and Group 3 and 4, having the worst prognosis [2–4]. However, no molecular alteration is predictive for dissemination and thus all patients continue to receive craniospinal irradiation to protect against metastasis. Due to the excessive neurocognitive toxicity of this treatment, alternative strategies are highly desired [5].
Overexpression of platelet-derived growth factor receptor (PDGFR) has been associated with metastatic MB [6], and PDGFR activity promotes MB migration [7]. We recently showed that PDGFR-induced migration is regulated by Src [8], which is implicated in invadopodia formation and breast cancer metastasis [9]. Rossi et al. demonstrated that treatment of MB with pyrimidine derivatives impedes tumor growth by inhibiting Src [10]. Dasatinib, a potent Src inhibitor, crosses the blood-brain-barrier and is well tolerated in pediatric patients [11]. In glioblastoma, dasatinib exhibits antiproliferative and anti-migratory activity in vivo, and is in clinical investigation for its efficacy against solid tumors [12, 13].
Aurora kinases play an essential role in cell division, including centrosome function, spindle assembly, chromosome segregation, and cytokinesis [14, 15]. In MB, overexpression of aurora kinase A (AurkA) is an independent predictor of poor prognosis [16], and AurkA inhibition with either C1368 or MLN8237 enhances MB cytotoxicity and chemosensitivity [17, 18]. AT9283, a novel multi-targeted aurora kinase inhibitor with activity against AurkA and AurkB [19], induces aneuploid cells with decreased survival [20, 21]. Based on this evidence, we hypothesized that the combined inhibition of Src and aurora kinases may act in a potentiating manner to effectively block MB growth and migration. We therefore investigated the effects of dasatinib and AT9283, alone and in combination, against a panel of MB cells in vitro, and the efficacy of dasatinib treatment of MB in vivo.
2. Materials and Methods
2.1 Cell culture and reagents
DAOY and D556 human MB cells were investigated (ATCC, Manassas, VA). DAOY is Shh-activated, p53-mutated [4]. D556 is MYC amplified, similar to Group 3 MB [4]. PS125 mouse MB cells were derived from the SMOA1 mouse, a p53 wild-type Shh MB murine model [22, 23]. Dasatinib was provided by Bristol Myers Squibb (New York City, NY). AT9283 was purchased from Selleck Pharmaceuticals (Houston, TX).
2.2 Dasatinib and AT9283 drug treatment
For in vitro studies, dasatinib (10mg/mL) was dissolved in DMSO and concentrations of 1, 5, 10, 50 and 100nM were added for 1–24 hours to cells grown to 80% confluence. For in vivo studies, dasatinib (0.01 mL/g) was dissolved in citrate buffer and administered by oral gavage. Mice were treated daily for 5 consecutive days per week for 4 weeks with dasatinib (15 mg/kg/day) or vehicle control. AT9283 was formulated in 10% DMSO, 20% water, and 70% 2-hydroxypropyl-β-cyclodextrin (25% w/v aqueous). For in vitro studies, AT9283 (10nM) was added for 1–24 hours to cells grown to 80% confluence.
2.3 Western blot
Western blot of whole cell lysates was performed with the primary antibodies: phospho-Src (Y416), total Src, LC3-II, caspase 3, GADPH, and p53 (Cell Signaling Technology, Danvers, MA); phospho-histone H3 (Millipore, Temecula, CA); and PARP (BD Biosciences, Franklin Lakes, NJ). Goat or rabbit anti-mouse horseradish peroxidase secondary antibodies (Santa Cruz, CA) were used to detect immunoreactive bands by ECL.
2.4 Cell viability and proliferation assay
Cells (1500 cells/well) were seeded into 96-well microplates. After drug treatment for 24 h, 10 uL of pre-made MTT reagent (5mg Thiazolyl Blue Tetrazolium Bromide per 10 ml PBS) was added for 4 h at 37°C, then 100 uL of 0.04% HCl in isopropanaolol was added and the spectrophotometric absorbance (570 nm) was measured using a Synergy Mx microplate reader. Each experiment was performed using 8 separate wells per condition in four separate studies.
2.5 Apoptosis assays
Western blot of whole cell lysates was performed using primary antibodies to caspase 3 and PARP. Etoposide (VP16) treatment (25 μM) for 4 h was used as a positive control. Cells were suspended in 1x binding buffer (BD Biosciences, Franklin Lakes, NJ) at 1×106 cells/mL and Annexin V and propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ) were added and analyzed by flow cytometry.
2.6 Cell cycle analysis
Cells were serum-starved overnight and then harvested at 24, 48, and 72 h after drug treatment, treated with ribonuclease to ensure that only DNA was stained, and 200 uL PI added. Samples were analyzed by BD FACS Canto II Flow Cytometer (BD, Franklin Lakes, NJ) and cell cycle was measured using the FlowJo program. Calculations were based on the Dean-Jett-Fox method.
2.7 Autophagy assay
Western blot of whole cell lysates was performed with the primary antibodies to LC3-II (Cell Signaling Technology, Danvers, MA). Rapamycin (10 nM) treatment for 16 h was used as a positive control for autophagy.
2.8 Migration “scratch” assay
Cells (2.5 ×105 cells/well) seeded in 6 well plates were incubated overnight at 37°C. A p1000 pipet tip was used to scrape the cell monolayer to create a “scratch” and growth medium added containing corresponding drug treatment or vehicle control. Cells were photographed under light microscopy at 0, 24, and 48 h after the scratch. Changes in scratch widths were calculated at the 24 and 48 h. Each experiment per condition was performed in four separate studies.
2.9 Mouse flank syngeneic MB tumor model
Wild type C7BL/6 mice (Jackson Laboratory, Sacramento, CA) were implanted with PS125 mouse Shh MB cells (8×106) mixed 1:1 with Matrigel (BD Biosciences, San Jose, CA) into the mouse flank. Drug or vehicle control treatment started the day after implant. Tumors were measured by caliper by a blinded investigator twice weekly for 4 weeks. Mice were weighed and observed daily for signs of drug toxicity. Each group contained 6 separate mice per condition.
2.10 Medulloblastoma tumor specimens
Medulloblastoma frozen tumors (n= 29) were consented for and obtained from the Children's Healthcare of Atlanta (CHOA) tumor tissue repository. Normal control fetal cerebellar tissue was obtained from Emory University Hospital Department of Pathology. The research protocols were approved by the Institutional Review Boards of CHOA and Emory University. All tumor specimens were studied as de-identified by a board certified neuropathologist (MS).
2.11 mRNA expression profiling
mRNA extracted from MB samples using Trizol (Invitrogen, Carlsbad, CA) was profiled by AROS Biosciences on the Affymetrix human genome U133 Plus 2.0 array with the 3' IVT Express Labeling Kit (Affymetrix, Santa Clara, CA). CEL files were preprocessed using RMA [24] and probesets collapsed to genes using the Genepattern software suite (www.broadinstitute.org/cancer/software/genepattern/). Samples were assigned to molecular subgroups as previously described, using a classifier based on support-vector machines [25]. The relative mean expression level for each gene is calculated by the Affymetrix software.
2.12 Tissue microarray immunohistochemistry
Tissue microarrays (TMA) were constructed from the MB tumor tissue blocks corresponding to the 29 frozen MB tissue samples utilized for gene expressing profiling. Standard immunohistochemistry (IHC) of each TMA was performed to assess expression of activated Src in MB using the primary antibodies for p-Src (Y418) and p-Src (Y529) (Millipore, Temecula, CA). Negative and positive controls were fetal cerebellum and adult colon adenocarcinoma, respectively. Each tissue sample was blindly scored for positivity or negativity grading established by a neuropathologist (MS).
2.13 Statistical analysis
For all above mentioned bioassays, a p value was calculated using Student's t-test and an alpha level of 0.05.
3. Results
3.1 Dasatinib treatment abolishes Src phosphorylation, but induces histone H3 phosphorylation (p-HH3), while AT9283 treatment inhibits dasatinib-induced p-HH3
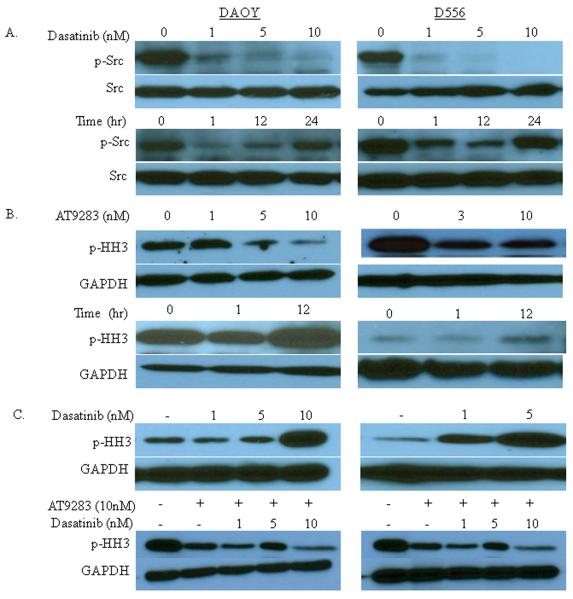
Dasatinib (1nM) markedly decreased p-Src levels (Y416; phosphorylation at this site is essential for Src activity), with near ablation of detectable p-Src following treatment with 5 and 10nM (Fig. 1A, upper panels). The inhibition on Src activation was observed to last at least 12 hours after treatment, with a return to near basal level Src activity by 24 hours after treatment (Fig. 1A, lower panels). There was no difference in the duration of p-Src suppression at higher concentrations tested (data not shown). Dasatinib can also inhibit BCR-ABL, c-KIT and PDGFR; however, since DAOY and D556 cells do not harbor the BCR-ABL mutation and the amount of dasatinib required to inhibit c-KIT and PDGFR is higher (50–100nM) than that needed for Src inhibition (1–10nM), the biological effects of dasatinib treatment in our investigations using the lower dose range can be ascribed to Src inhibition.
Figure 1.
Dasatinib treatment abolishes Src phosphorylation, but induces histone H3 phosphorylation (p-HH3), while AT9283 treatment inhibits dasatinib-induced p-HH3. DAOY and D556 cells grown in culture were treated with dasatinib and AT9283 and then the level of the respective phosphorylated effector proteins, p-Src and p-HH3, were detected by Western blot of whole cell lysates. Total Src and GAPDH served as internal protein loading controls for each target, respectively. A) p-Src levels are shown at 1 hour after treatment with increasing concentrations of dasatinib (upper panels) and at the indicated timepoints after treatment with 1 nM dasatinib (lower panels). B) p-HH3 levels are shown at 1 hour after treatment with increasing concentrations of AT9283 (upper panels) and at the indicated timepoints after treatment with 10 nM AT9283 (lower panels). C) p-HH3 levels are shown at 1 hour after treatment with increasing concentrations of dasatinib alone (upper panels) and in combination with 10 nM AT9283 (lower panels).
Phospho-histone H3 (p-HH3) is a readout for mitosis and aurora kinase (Aurk) activity, such that decreased p-HH3 levels directly correspond to inhibition of Aurk activity. Treatment with a single AT9283 dose of 3nM and 5nM markedly suppresses p-HH3 in D556 and DAOY cells, respectively; however, 10nM maximally inhibits p-HH3 in Daoy cells (Fig. 1B, upper panels), with minimal further suppression at higher concentrations (data not shown). Interestingly, p-HH3 levels appear to exhibit a “rebound” effect above basal activity at 12 hours after drug treatment (Fig. 1B, lower panels).
To determine cross-reactivity, we tested the effect of each drug on the other's functional readout target (i.e. the effect of dasatinib on p-HH3, and the effect of AT9283 on p-Src). As expected, AT9283 had no effect on Src phosphorylation (data not shown); however, we surprisingly observed a reciprocal dose-dependent induction of p-HH3 1 hour after treatment with dasatinib, which to our knowledge has not been reported. The induction of p-HH3 was maximal at the concentrations of dasatinib (5–10nM) required to maximally suppress Src activation (Fig. 1C, upper panels). We observed that 1 hour co-treatment with AT9283 (10nM) abolished this induction of p-HH3 by dasatinib (Fig. 1C, lower panels).
3.2 Dasatinib or AT9283 treatment decreases proliferation and combined treatment with dasatinib and AT9283 potentiates the individual drug responses
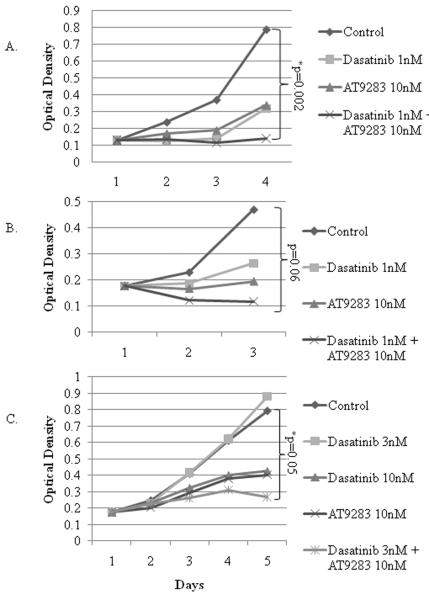
Each drug inhibited the proliferation of DAOY (Fig. 2A) and D556 (Fig. 2B); however, the combined treatment ablated the proliferative capacity of both cells, and resulted in the loss of D556 viability (Figs. 2A and B). Murine PS125 Shh MB cells, which similarly exhibit p-Src and p-HH3 suppression in response to dasatinib and AT9283 treatment, respectively (data not shown), also demonstrated enhanced suppression of proliferation with combined treatment (Fig. 2C). Notably, a synergistic, rather than a potentiated or additive effect, was observed with AT9283 in combination with dasatinib (3nM) treatment of PS125 cells, as evidenced by dasatinib (3nM) alone having no effect on viability (Fig. 2C). Altering treatment timing by administering dasatinib first, followed by AT9283 one hour later, and vice versa, did not enhance or diminish the suppressive effect on proliferation and viability (data not shown).
Figure 2.
Dasatinib or AT9283 treatment decreases cell proliferation and combined treatment with dasatinib and AT9283 potentiates the individual drug responses. A) DAOY, B) D556, and C) murine PS125 cells were grown in culture and treated with a single dose of dasatinib (1nM for DAOY and D556; 3nM or 10nM for PS125 cells), AT9283 (10nM), dasatinib and AT9283 combined, or vehicle control, and then proliferation was measured daily by the MTT assay (optical density, OD, measured at 570nm) for 3–5 days after treatment. Graphs show the average daily OD per treatment condition. *, p-value < or = 0.05.
3.3 Dasatinib or AT9283 treatment induces apoptosis and combined treatment with dasatinib and AT9283 potentiates the individual drug responses
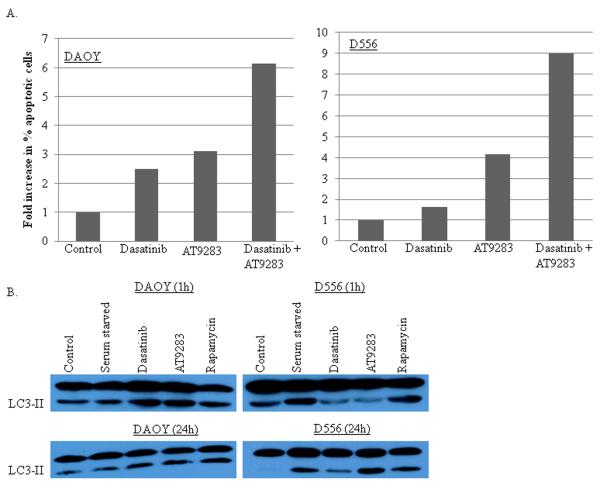
There was no change in the levels of cleaved PARP at 1 hour after treatment with either drug alone or in combination; however, at 16 and 24 hours after treatment with AT9283 or dasatinib, cleaved PARP levels increased in D556, but not in DAOY (not shown). There was also no change in the level of cleaved caspase 3 at 1 hour after drug treatment of DAOY or D556, or after 24 hours compared to positive control treatment with etoposide (not shown). However, DAOY cells revealed a 2- and 3-fold increase in annexin V-positive cells 72 hours after treatment with dasatinib or AT9283, respectively, and a 6-fold increase in annexinV-positive cells 72 hours after treatment with the combination of dasatinib and AT9283 compared to control (Fig. 3A). In D556 at 72 hours after treatment, nearly a 5-fold increase in annexin V-positive cells was observed with AT9283, and a 10-fold increase after treatment with the combination of dasatinib and AT9283 (Fig. 3A). Dasatinib alone was not associated with increased annexin-V positive D556 cells.
Figure 3.
Dasatinib or AT9283 treatment induces apoptosis and autophagy, while combined treatment with dasatinib and AT9283 markedly potentiates apoptosis. DAOY and D556 cells were grown in culture and treated for 1 hour with dasatinib (1nM), AT9283 (10nM), dasatinib and AT9283 combined, or vehicle control, and then cells were assayed for apoptosis by annexin V labeling at 72 hours after treatment, and for evidence of autophagy by Western blot for the autophagy marker LC3-II at 1 hour and 24 hours after treatment. For the autophagy assays, rapamycin (10nM) treatment for 16 hours was used for a positive control. A) Graphs show the average fold-change in the percentage of annexin V-positive apoptotic cells per treatment condition compared to pre-treatment for DAOY and D556 cells. B) Representative changes in the levels of the autophagy protein marker LC3-II are shown at 1 hour and 24 hours after the individual drug treatments.
3.4 Dasatinib and AT9283 treatment induces autophagy and p53 expression
Conflicting data suggests that autophagy in cancer cells can play both an anti-tumorigenic role, by promoting chemotherapy and radiation cytotoxicity, as well as a pro-tumorigenic role, by minimizing metabolic stress and facilitating resistance to anti-cancer therapies [26]. One hour after treatment with a single dose of dasatinib (1nM) or AT9283 (10nM) resulted in an increase in the autophagy marker LC3-II in DAOY, but not in D556 (Fig. 3B). LC3-II returned to near basal level in DAOY after 24 hours (Fig. 3B). In contrast, D556 cells displayed increased LC3-II levels at 16 (not shown) and 24 hours after treatment, with AT9283 (Fig. 3B). Combined treatment with dasatinib and AT9283 did not significantly change the level of LC3-II compared to the individual drug treatments (not shown).
Since p53 regulates cell-cycle progression, and is mutated in DAOY cells, we investigated the level of p53 protein in these MB cells following the drug treatments. As expected, dasatinib and AT9283 treatment had no effect on DAOY mutant p53 levels (not shown), but in D556 the level of wild-type p53 increased over time in response to drug treatment, with maximal levels reached by 16 and 24 hours (Supplementary Fig. S1).
3.5 Dasatinib and AT9283 treatment induces G2/M and S phase cell-cycle arrest
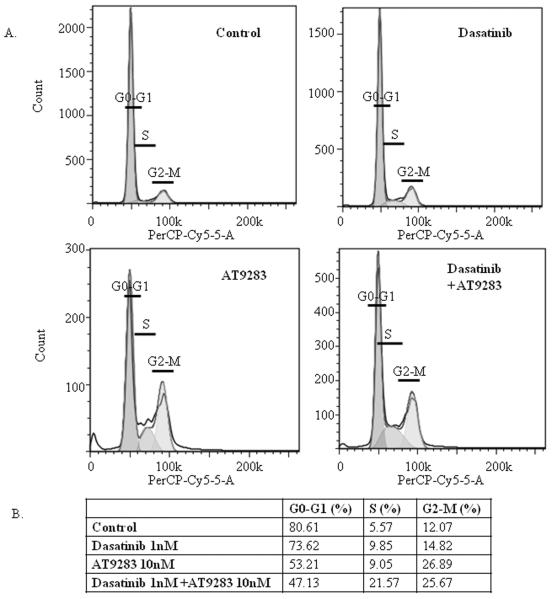
In D556, AT9283 treatment induced G2/M phase cell-cycle arrest that was maximal at 72 hours (Fig. 4), while dasatinib induced a moderate S phase cell-cycle arrest that was markedly potentiated by the addition of AT9283, and similarly maximal at 72 hours (Fig. 4). There did not appear to be any effect of treatment on cell-cycle progression of DAOY cells.
Figure 4.
Dasatinib and AT9283 combined treatment induces G2/M and S phase cell-cycle arrest. DAOY and D556 cells were serum-starved overnight and then treated with a single dose of dasatinib (1nM), AT9283 (10nM), dasatinib and AT9283 combined, or vehicle control, with 10% FBS, and then cell cycle analysis was performed by flow cytometry of PI-labeled cells at 24, 48, and 72 hours after treatment, without drug replenishment after the initial treatment at hour 0. Results shown are representative cell-cycle plots of D556 cells at 72 hours after the respective treatments, with the mean percentage (%) of D556 cells in each phase of the cell cycle at 72 hours after the treatments indicated, as measured using the FlowJo program.
3.6 Dasatinib or AT9283 treatment inhibits migration and combined treatment markedly potentiates the individual drug treatment responses
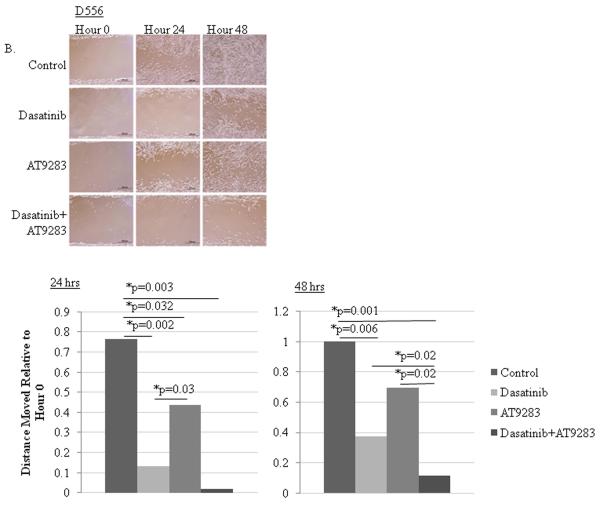
Migration was measured by scratch assay at 24 and 48 hour time points after initiation of the wound. Since the time to undergo a full cell-cycle is approximately 30 hours for these cells, the 24 hour time point is more of a measure of cell migration while the 48 hour time point will reflect more of the combined effect of each drug on viability, proliferation and migration. Dasatinib potently inhibited DAOY (Fig. 5A) and D556 (Fig. 5B) cell movement at 24 and 48 hours. AT9283 significantly inhibited DAOY movement at 48 hours and D556 at 24 hours. The addition of AT9283 to dasatinib markedly potentiated the inhibition observed at 48 hours in both cells and at 24 hours in D556.
Figure 5.
Dasatinib or AT9283 treatment inhibits migration and combined treatment markedly potentiates the individual drug treatment responses. DAOY and D556 cells were grown to confluency on culture plates, a scratch wound was made, and the cells were treated with a single dose of dasatinib (1nM), AT9283 (10nM), dasatinib and AT9283 combined, or vehicle control, and then cell movement across the scratch was measured at 24 and 48 hours following treatment at hour 0. Drug treatment was not replenished after hour 0. Results of the scratch assays are shown for A) DAOY and B) D556 cells by their corresponding representative photomicrographs displayed in the upper panels and bar graphs demonstrating the relative ratio of the mean cumulative movement relative to hour 0, as displayed in the lower panels, for each of the treatment conditions and indicated time points after treatment. *p-value < 0.05.
3.7 Dasatinib treatment induces tumor regression in a mouse syngeneic MB flank model
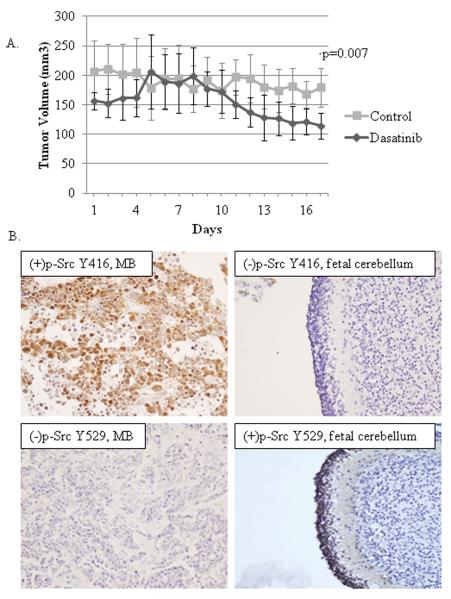
To test whether dasatinib is effective for the treatment of Shh MB in vivo, we implanted PS125 Shh MB tumor cells into the flanks of wild type syngeneic mice and treated them by gavage with dasatinib (15 mg/kg/day) once daily for 5 consecutive days or vehicle control for 4 weeks. The dose administered corresponds to a peak serum drug level at 1 h post-dose of 500nM, or ~50× our optimal in vitro concentration to suppress Src, and a trough level at 24 h post-dose of ~5nM, similar to the optimal concentration determined by our in vitro experiments. The dasatinib group had significantly lower tumor volumes by the end of the 20 day treatment (4 weeks total) course compared to controls (Fig. 6A), with no differences in the average weight between mouse groups and no visible evidence of toxicity. Efforts to compare tumor volumes in mice treated with both agents were attempted numerous times but were unsuccessful due to difficulties in controlling leakage of AT9283 back out through the IP injection site.
Figure 6.
Dasatinib treatment induces tumor regression in a mouse syngeneic MB flank model, and MB tumors ubiquitously express Src, AurkA and AurkB, and exhibit Src activation. A) Plot shows the effect of dasatinib treatment on MB tumor growth over time in mice bearing flank syngeneic MB tumor implants. Cohorts of 6 mice per group were treated with dasatinib or vehicle control by oral gavage once daily for 5 consecutive days at a dose of 15 mg/kg/day for a total of 20 days of treatment. Tumor volume (mm3) over the course of the treatment was measured at the indicated timepoints for each mouse and then averaged for each cohort. *p-value < 0.05. B) Representative photomicrographs show the positive (+) and negative (−) immunohistochemistry staining results for childhood MB tumor and normal fetal cerebellum tissue specimens stained for activated Src, p-Src (Y416), and inactivated Src, p-Src (Y529).
3.8 MB tumors ubiquitously express Src, AurkA and AurkB, and exhibit Src activation
We evaluated 29 childhood MB samples (11 SHH, 7 Group 3, and 11 Group 4) for expression of Src, AurkA and AurkB. Each target was detected in 100% of tumors, with no significant differences in the relative expression level for any marker between Shh and non-Shh tumors (Group 3 and Group 4 tumors) (Supplementary Table S1), or between Group 3 and Group 4 tumors (not shown), although the number of samples for each subgroup may be too small to detect differences. Activated p-Src (Y418) was detected in 44% MB, while fetal cerebellum was negative (Fig. 6B). Additionally, 83% of MB were negative for p-Src (Y529), the inactive conformation state for Src, but control fetal cerebellum was positive for p-Src (Y529) (Fig. 6B), indicating that the majority of MB contain Src in either the activated or “pre-activation” state. There was no difference in the protein expression levels of activated p-Src between Shh and non-Shh or between Group 3 and Group 4 tumors.
4. Discussion
Because of neurotoxicity associated with brain irradiation to control MB metastasis [27], an alternative treatment strategy to target MB growth and migration is desired. Herein, we demonstrate that inhibition of aurora kinase A and B (AurkA and AurkB) by AT9283, and the inhibition of Src by dasatinib, significantly inhibits MB viability, growth and migration in vitro, and that the responses to these treatments are markedly potentiated by combining the two agents. Moreover, we show that dasatinib treatment alone results in MB tumor regression in vivo.
AurkA inhibitors have been clinically investigated in cancer, including relapsed pediatric solid tumors [28]. In MB cells, AurkA inhibition is cytotoxic and enhances chemosensitivity [17, 29]. Inhibition of AurkB has shown preclinical efficacy against leukemia, ovarian, lung, and colorectal cells in vitro and tumor growth in vivo [30–32], as well as early phase clinical efficacy in relapsed adult solid tumors [33]. AT9283, which inhibits AurkA and AurkB, was well tolerated in a Phase I trial for advanced solid malignancies [34]. We observed that AT9283 potently induces G2/M arrest, apoptosis, and strongly induces p53 expression and autophagy, while concomitantly inhibiting MB migration.
Src is a well known oncogenic tyrosine kinase. Similar to two other reports describing Src inhibition in MB [10, 35], we observed that dasatinib induces apoptosis and inhibits growth in vitro [35]. However, we demonstrate for the first time that dasatinib strikingly inhibits migration and concomitantly induces p53 expression and autophagy, albeit less potently than AT9283, and causes MB tumor regression in vivo. Dasatinib is a highly attractive agent for clinical development in pediatric MB since it crosses the blood-brain-barrier and is already known to be well tolerated in children with leukemia and other solid tumors [36, 37].
The combination of Src and Aurk inhibition synergistically inhibits ovarian and colorectal cell growth in vitro [38–40], as well as leukemia and solid tumor growth in vivo [38, 40]. However, our study is the first to demonstrate the efficacy of this molecularly targeted combination for MB, and to our knowledge, is the first preclinical investigation of the specific combination of dasatinb and AT9283. Moreover, we demonstrate that dasatinib treatment at concentrations far below that used for BCR-ABL positive leukemia patients, results in a paradoxical induction of histone-H3 phosphorylation, indicative of active mitosis, which can be ablated by co-treatment with AT9283. We also observed that dasatinib and AT9283 alone had only a modest effect on S phase of the cell-cycle in D556 cells; however, the combination induced a significant S phase growth arrest. These additional findings lend further support for the therapeutic combination of dasatinib and AT9283 for MB.
Finally, we show that each of the investigated targets, Src, AurkA and AurkB, are ubiquitously expressed in all of the major metastasizing MB subgroups (Shh, Group 3 and Group 4) and demonstrate that p-Src (Y416/Y418), which is essential for Src kinase activity, is detectable in 44% of MB, while dephosphorylated Src inhibitory Y527/Y529 is present in 83% MB, and is not associated with a specific molecular subgroup. Although one report suggested that phosphorylation at both the Y416/Y418 and the Y527/Y529 site in MB is indicative of “hyperactive Src” [35], our data show that only one of the 29 specimens tested exhibited positive phosphorylation at both sites. Other evidence points towards dephosphorylation at the Y527/Y529 as a first step in facilitating Src activation via phosphorylation of Y416/Y418 [41]. In keeping with the latter concept, we found that normal fetal cerebellum demonstrates dephosphorylation at the Src activation site (Y416/Y418) and phosphorylation at the presumed inhibitory site (Y527/Y529). Thus, activated Src appears to be a viable MB-specific target, irrespective of the molecular subgroup. Interestingly, we observed less apoptosis and autophagy in DAOY cells in response to any treatment, which may be due to their inherent p53 mutation, delaying the cells' commitment to the apoptotic pathway. In fact, p53 levels increased over time in D556 cells after treatment with each drug alone, and in combination, but there was no change in p53 in DAOY. Although some have shown evidence of apoptosis in DAOY cells after treatment with Src inhibitors [10], this may be due to off-target effects of less well-characterized Src-inhibiting agents. Importantly, despite the differences in the individual assay results among the MB cells tested, which likely corresponds to underlying differences in the molecular makeup of the cells, each cell type was dose-dependently impaired in its growth following drug treatment and in direct correlation with the expression of the drug target, suggesting that target expression ultimately determines overall response. Further investigation utilizing specific genetic manipulation is necessary to confirm whether the responses to these treatments are indeed p53-dependent. While further studies are necessary to confirm in vivo efficacy of these agents in combination, our preclinical data suggest that dual blockade of Src and aurora kinases may be an effective therapeutic strategy to control tumor growth and migration of human medulloblastoma that express AurkA, AurkB and/or activated Src, irrespective of the molecular subgroup.
Supplementary Material
The highlights of this manuscript are:
Aurora kinase and activated Src protein expression is confirmed in medulloblastoma
Drug inhibition of aurora kinase and Src has synergistic anti-cancer activity
Dasatinib and AT9283 induce autophagy and p53 expression in medulloblastoma
Dasatinib has efficacy against medulloblastoma in vitro and in vivo
Acknowledgements
This study is supported in part by National Institute Health R01CA111835 (TJM), CURE Childhood Cancer (TJM), and the Georgia Research Alliance (TJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Conflict of Interest Statement The authors disclose no potential conflicts of interest
7. References
- [1].Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–45. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- [2].Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–7. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].von Hoff K, Rutkowski S. Medulloblastoma. Curr Treat Options Neurol. 2012;14:416–26. doi: 10.1007/s11940-012-0183-8. [DOI] [PubMed] [Google Scholar]
- [6].MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29:143–52. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- [7].Abouantoun TJ, MacDonald TJ. Imatinib blocks migration and invasion of medulloblastoma cells by concurrently inhibiting activation of platelet-derived growth factor receptor and transactivation of epidermal growth factor receptor. Mol Cancer Ther. 2009;8:1137–47. doi: 10.1158/1535-7163.MCT-08-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuan L, Zhang H, Liu J, Rubin JB, Cho YJ, Shu HK, et al. Growth factor receptor-Src-mediated suppression of GRK6 dysregulates CXCR4 signaling and promotes medulloblastoma migration. Mol Cancer. 2013;12:18. doi: 10.1186/1476-4598-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rossi A, Schenone S, Angelucci A, Cozzi M, Caracciolo V, Pentimalli F, et al. New pyrazolo-[3,4-d]-pyrimidine derivative Src kinase inhibitors lead to cell cycle arrest and tumor growth reduction of human medulloblastoma cells. FASEB J. 2010;24:2881–92. doi: 10.1096/fj.09-148593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schenone S, Brullo C, Musumeci F, Botta M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin Investig Drugs. 2010;19:931–45. doi: 10.1517/13543784.2010.499898. [DOI] [PubMed] [Google Scholar]
- [12].Kim LC, Rix U, Haura EB. Dasatinib in solid tumors. Expert Opin Investig Drugs. 2010;19:415–25. doi: 10.1517/13543781003592097. [DOI] [PubMed] [Google Scholar]
- [13].Du J, Bernasconi P, Clauser KR, Mani DR, Finn SP, Beroukhim R, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katayama H, Brinkley WR, Sen S. The aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–64. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- [15].Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–54. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- [16].Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, et al. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64:3103–11. doi: 10.1158/0008-5472.can-03-3968. [DOI] [PubMed] [Google Scholar]
- [17].El-Sheikh A, Fan R, Birks D, Donson A, Foreman NK, Vibhakar R. Inhibition of aurora kinase A enhances chemosensitivity of medulloblastoma cell lines. Pediatr Blood Cancer. 2010;55:35–41. doi: 10.1002/pbc.22465. [DOI] [PubMed] [Google Scholar]
- [18].Muscal JA, Scorsone KA, Zhang L, Ecsedy JA, Berg SL. Additive effects of vorinostat and MLN8237 in pediatric leukemia, medulloblastoma, and neuroblastoma cell lines. Invest New Drugs. 2013;31:39–45. doi: 10.1007/s10637-012-9831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Howard S, Berdini V, Boulstridge JA, Carr MG, Cross DM, Curry J, et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J Med Chem. 2009;52:379–88. doi: 10.1021/jm800984v. [DOI] [PubMed] [Google Scholar]
- [20].Lok W, Klein RQ, Saif MW. Aurora kinase inhibitors as anti-cancer therapy. Anticancer Drugs. 2010;21:339–50. doi: 10.1097/CAD.0b013e3283350dd1. [DOI] [PubMed] [Google Scholar]
- [21].Tanaka R, Squires MS, Kimura S, Yokota A, Nagao R, Yamauchi T, et al. Activity of the multitargeted kinase inhibitor, AT9283, in imatinib-resistant BCR-ABL-positive leukemic cells. Blood. 2010;116:2089–95. doi: 10.1182/blood-2009-03-211466. [DOI] [PubMed] [Google Scholar]
- [22].Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68:1768–76. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- [23].Abouantoun TJ, Castellino RC, MacDonald TJ. Sunitinib induces PTEN expression and inhibits PDGFR signaling and migration of medulloblastoma cells. J Neurooncol. 2011;101:215–26. doi: 10.1007/s11060-010-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- [25].Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–30. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–62. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- [27].Ris MD, Walsh K, Wallace D, Armstrong FD, Holmes E, Gajjar A, et al. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer. 2013;60:1350–7. doi: 10.1002/pbc.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mosse YP, Lipsitz E, Fox E, Teachey DT, Maris JM, Weigel B, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin Cancer Res. 2012;18:6058–64. doi: 10.1158/1078-0432.CCR-11-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Farrell P, Shi L, Matuszkiewicz J, Balakrishna D, Hoshino T, Zhang L, et al. Biological characterization of TAK-901, an investigational, novel, multi-targeted Aurora B kinase inhibitor. Mol Cancer Ther. 2013;12:460–70. doi: 10.1158/1535-7163.MCT-12-0657. [DOI] [PubMed] [Google Scholar]
- [31].Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C, et al. AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13:3682–8. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- [32].Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–40. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- [33].Boss DS, Witteveen PO, van der Sar J, Lolkema MP, Voest EE, Stockman PK, et al. Clinical evaluation of AZD1152, an i.v. inhibitor of Aurora B kinase, in patients with solid malignant tumors. Ann Oncol. 2011;22:431–7. doi: 10.1093/annonc/mdq344. [DOI] [PubMed] [Google Scholar]
- [34].Arkenau HT, Plummer R, Molife LR, Olmos D, Yap TA, Squires M, et al. A phase I dose escalation study of AT9283, a small molecule inhibitor of aurora kinases, in patients with advanced solid malignancies. Ann Oncol. 2012;23:1307–13. doi: 10.1093/annonc/mdr451. [DOI] [PubMed] [Google Scholar]
- [35].Sikkema AH, Diks SH, den Dunnen WF, ter Elst A, Scherpen FJ, Hoving EW, et al. Kinome profiling in pediatric brain tumors as a new approach for target discovery. Cancer Res. 2009;69:5987–95. doi: 10.1158/0008-5472.CAN-08-3660. [DOI] [PubMed] [Google Scholar]
- [36].Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005–12. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- [37].Aplenc R, Blaney SM, Strauss LC, Balis FM, Shusterman S, Ingle AM, et al. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children's oncology group phase I consortium. J Clin Oncol. 2011;29:839–44. doi: 10.1200/JCO.2010.30.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arai R, Tsuda M, Watanabe T, Ose T, Obuse C, Maenaka K, et al. Simultaneous inhibition of Src and Aurora kinases by SU6656 induces therapeutic synergy in human synovial sarcoma growth, invasion and angiogenesis in vivo. Eur J Cancer. 2012;48:2417–30. doi: 10.1016/j.ejca.2011.12.028. [DOI] [PubMed] [Google Scholar]
- [39].Ratushny V, Pathak HB, Beeharry N, Tikhmyanova N, Xiao F, Li T, et al. Dual inhibition of SRC and Aurora kinases induces postmitotic attachment defects and cell death. Oncogene. 2012;31:1217–27. doi: 10.1038/onc.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Glaser KB, Li J, Marcotte PA, Magoc TJ, Guo J, Reuter DR, et al. Preclinical characterization of ABT-348, a kinase inhibitor targeting the aurora, vascular endothelial growth factor receptor/platelet-derived growth factor receptor, and Src kinase families. J Pharmacol Exp Ther. 2012;343:617–27. doi: 10.1124/jpet.112.197087. [DOI] [PubMed] [Google Scholar]
- [41].Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.