Abstract
OBJECTIVE
To determine the safety and efficacy of an automated unified safety system (USS) in providing overnight closed-loop (OCL) control in children and adolescents with type 1 diabetes attending diabetes summer camps.
RESEARCH DESIGN AND METHODS
The Diabetes Assistant (DIAS) USS used the Dexcom G4 Platinum glucose sensor (Dexcom) and t:slim insulin pump (Tandem Diabetes Care). An initial inpatient study was completed for 12 participants to evaluate safety. For the main camp study, 20 participants with type 1 diabetes were randomized to either OCL or sensor-augmented therapy (control conditions) per night over the course of a 5- to 6-day diabetes camp.
RESULTS
Subjects completed 54 OCL nights and 52 control nights. On an intention-to-treat basis, with glucose data analyzed regardless of system status, the median percent time in range, from 70–150 mg/dL, was 62% (29, 87) for OCL nights versus 55% (25, 80) for sensor-augmented pump therapy (P = 0.233). A per-protocol analysis allowed for assessment of algorithm performance. The median percent time in range, from 70–150 mg/dL, was 73% (50, 89) for OCL nights (n = 41) versus 52% (24, 83) for control conditions (n = 39) (P = 0.037). There was less time spent in the hypoglycemic range <50, <60, and <70 mg/dL during OCL compared with the control period (P = 0.019, P = 0.009, and P = 0.023, respectively).
CONCLUSIONS
The DIAS USS algorithm is effective in improving time spent in range as well as reducing nocturnal hypoglycemia during the overnight period in children and adolescents with type 1 diabetes in a diabetes camp setting.
Introduction
There have been significant advances in automated, closed-loop systems designed for glucose control in patients with type 1 diabetes in recent years. Early studies demonstrated the feasibility of automated insulin modulation using subcutaneous insulin pumps and continuous glucose sensors (1–3). Further advances in both sensors and algorithms incrementally demonstrated improved protection against hypoglycemia, reduced variability, and decreased mean glucose levels in controlled, inpatient settings (4–6). Control to range strategies (7,8) are intended as an adjunct to standard insulin pump therapy and as such are designed to implement the patient’s predetermined insulin basal delivery if the current or predicted glucose levels are considered desirable. Such controllers achieved almost 80% time in the range of 80–140 mg/dL in the overnight period in a study of 12 adults with type 1 diabetes (7).
The recent development of portable, automated systems has facilitated the transition from controlled, research-center studies to larger, outpatient studies. The pediatric population presents additional challenges to the system due to differences in insulin sensitivity in children of varying ages (9), with younger children being more insulin sensitive and adolescents demonstrating insulin resistance (10).
The diabetes camp environment presents many challenges to optimizing glucose control. Children with type 1 diabetes are encouraged to participate in various physical activities that challenge their exercise endurance. Camp Conrad-Chinnock (Angelus Oaks, CA) is located at 6,800-feet altitude. The altitude, exercise, and dietary changes impact on glucose control, and severe, nocturnal hypoglycemia is a well-recognized complication at camps (11). The camp setting allows a number of studies to be conducted simultaneously with local remote monitoring and also allows testing of controller in a rugged, real-world setting.
The Diabetes Assistant (DIAS) (6) platform is a smartphone-based, modular, portable artificial pancreas device developed at the University of Virginia, in collaboration with the University of Montpellier. DIAS operates on a commercially available Android-based phone, enabling wireless communication with satellite devices such as an insulin pump, continuous glucose monitors, and any medical device using a standard wireless protocol including Bluetooth (BT), BT Low Energy (BLE), ANT+, and 802.11. Its modular architecture allows for different control modules to be swapped in for clinical trials. DIAS also integrates automated data transfer to a secured server, enabling remote-monitoring capabilities (12).
The objective of this study was to determine the safety and efficacy of an automated unified safety system (USS Virginia) in overnight closed-loop (OCL) control in children and adolescents with type 1 diabetes over multiple days in a diabetes camp setting. The primary outcome was defined as the percentage of time spent in range, from 70–150 mg/dL, during the overnight period.
Research Design and Methods
Closed-Loop System
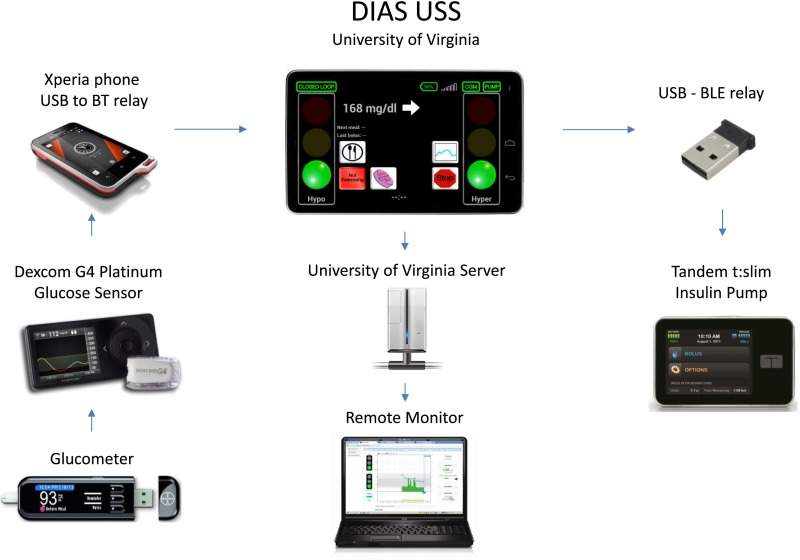
The components of the system, shown in Fig. 1, include a Dexcom G4 Platinum glucose sensor (Dexcom, San Diego, CA), a modified Tandem t:slim insulin pump (Tandem Diabetes Care, San Diego, CA), and a DIAS platform running a specifically modified Android operating system (Android, Inc.) and the USS. A micro–universal serial bus (USB) cable from the Dexcom receiver is connected to a separate cell phone (Xperia Active; Sony Ericsson) that functions as a BT relay. Communication between the DIAS and the pump is enabled by a USB BLE interface module. Data from the platform are uploaded automatically to a secured server running a specific Web application to enable real-time remote monitoring by the supervising clinical team (12).
Figure 1.
DIAS control to range system.
USS Virginia
Basal insulin delivery by the pump is stopped upon activation of the closed-loop and replaced by a specifically computed microbolus every 5 min. The microbolus is computed by the USS, an evolution of the previously published safety system module (13). USS first estimates the metabolic state of the patient by Kalman filtering of glucose values, insulin delivery, and meals. The internal model of the Kalman filter is a linear adaptation of the minimal model of glucose kinetics (14), using feed-forward models to predict the plasma insulin and glucose rate of appearance, both inputs of the minimal model of glucose kinetics (8). This technique enables the algorithm to estimate the plasma glucose and pending insulin action. This forms a representation of the current metabolic status of the patient. Based on this Kalman state estimate, the hypoglycemic module computes a 30-min prediction of hypoglycemic risk and downmodulates insulin delivery according to a patient-specific inverse proportionality (13).
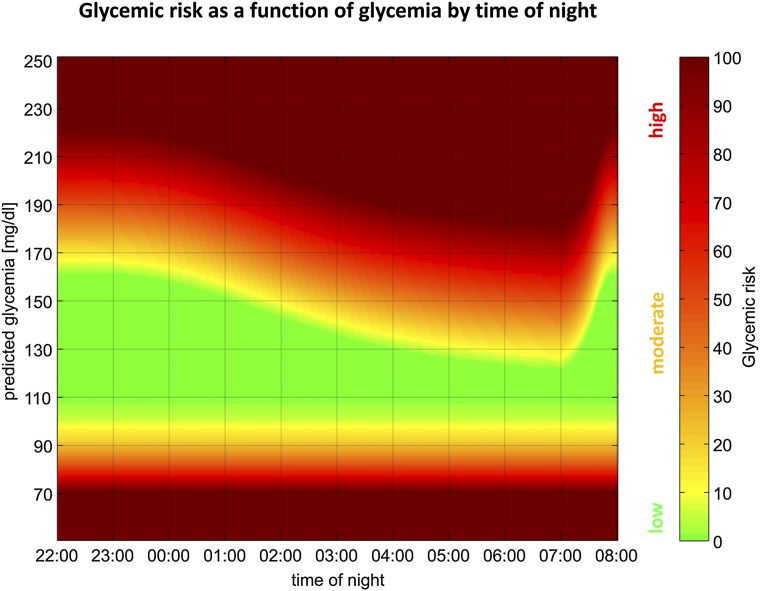
In addition, hyperglycemic safety is monitored by estimating insulin on board (IOB) from previous insulin delivery using a second-order linear model corresponding to 4-h insulin action curves (15). IOB represents the estimated amount of active insulin from delivered insulin. The system compares IOB to an internal estimate of the “insulin that should be on board.” This estimate is based on current glucose values, past-hour trends, and a time-dependent upper glycemic limit. The glycemic target is 160 mg/dL from 2300 to 0200 h and then gradually decreases to 120 mg/dL by 0600 h, as shown in Fig. 2. If the current IOB is insufficient or less than the “insulin that should be on board,” the system attempts to reduce the difference over the subsequent 30 min by increasing the basal rate up to a maximum of three times the usual basal rate. This calculation occurs every 5 min. Unless hypoglycemic risk or insufficient IOB is detected, the system remains silent (i.e., the patient’s basal rate will be commanded).
Figure 2.
USS. Glycemic risk as a function of glycemia by time of night. Glycemic risk is depicted to be high (red), moderate (amber), or low (green).
Study Procedures
An initial inpatient study was completed to evaluate the safety and efficacy of the system in 12 participants with type 1 diabetes. Further details are included in the Supplementary Data.
For the camp sessions, subjects were eligible to participate if they were 10–35 years, diagnosed with type 1 diabetes for ≥12 months, using an insulin pump for ≥3 months, and attending diabetes camp. The protocol was approved by the Stanford University institutional review board.
Exclusion criteria included diabetes ketoacidosis in the preceding 30 days, hypoglycemic seizure or coma in the preceding 3 months, pregnancy, history of a seizure disorder, and medical or psychiatric conditions considered to interfere with completion of the protocol. There were no A1C exclusion criteria. Written informed consent was obtained prior to enrollment.
Participants were randomized to either OCL or sensor-augmented therapy for the first night and then crossed over every other night to the other therapy over the course of the 5- to 6-day camp session.
A Dexcom G4 Platinum glucose sensor (Dexcom) was inserted on arrival at camp. All calibrations were performed using fingerstick meter blood glucose measured by the Bayer Contour Next USB glucometer (Bayer HealthCare, Leverkusen, Germany). Following the initial calibration at 2 h following insertion, patients calibrated a minimum of twice daily prior to breakfast and dinner.
Control Conditions
During the control conditions, patients continued on sensor-augmented pump therapy only. Low- and high-glucose alerts were set at 70 and 250 mg/dL, respectively. Patients were not remotely monitored and were under the care of camp medical staff. Overnight glucose monitoring included routine meter glucose testing at 0000 and 0300 h if clinically indicated.
Data from each closed-loop night were shared with the clinical team in order to facilitate changes to insulin therapy as required for subsequent nights. Pump settings were regularly modified during the course of the camp. Changes to overnight basal rates on control nights were incorporated into the closed-loop algorithm the following night.
Closed-Loop Initiation
OCL was commenced in the evening as participants prepared for sleep. Initiation parameters including patient height, weight, total daily dose, basal rates, and insulin sensitivity factor were entered into the DIAS USS system. Prior to the start of closed-loop, the Tandem t:slim insulin pump replaced the patient’s own pump, and a steel infusion set (Contact Detach; Unomedical AS, Lejre, Denmark) was inserted. OCL was initiated if sensor glucose values were between 80 and 250 mg/dL and within 20% of meter glucose values. Closed-loop control was stopped between 0700 and 0730 h the following morning. Patients resumed sensor-augmented pump therapy during the day.
Meter glucose values were obtained at 0000, 0300, and 0600 h. If the sensor glucose differed from the meter glucose by >20%, the meter glucose was entered as a calibration value, and a repeat meter glucose reading was obtained after 1 h. If the second reading differed from the sensor glucose by >20%, closed-loop control was stopped.
Hypoglycemia
Closed-loop proceeded uninterrupted when sensor glucose values were between 70 and 250 mg/dL. When sensor glucose decreased <70 mg/dL via remote monitoring, a meter glucose was obtained. If the meter glucose was <70 mg/dL, hypoglycemia treatment was given in the form of 15 g of fast-acting carbohydrate—either juice or glucose tablets. A repeat meter glucose was obtained after 15 min, and if meter glucose values were >70 mg/dL, 15 g of complex carbohydrate was given and OCL continued. If meter glucose values were <50 mg/dL, oral glucose treatment was given, and OCL was suspended for the patient that night.
Hyperglycemia
If sensor glucose values were >250 mg/dL, a meter glucose was obtained, and, if verified, blood ketone levels were obtained. If blood ketones were ≤0.6 mmol/L, OCL continued. If ketone levels were >0.6 mmol/L, OCL was stopped, patients received a subcutaneous insulin correction dose, a new insulin infusion set was inserted, and OCL was suspended for a minimum of 2 h.
Closed-loop would be suspended for the individual patient if meter or plasma glucose values were <50 or >400 mg/dL. Ketones were measured daily at 0700 h.
Sample Size
The sample size for the camp study was calculated based upon the percentage of time in range, from 70–150 mg/dL. For a similar cohort (16) of subjects on sensor-augmented pump, the time spent in range was 51 ± 30%. We assumed a 20% absolute improvement in the time in range would be clinically significant. At a significance level of 0.05 and 90% power, we would require 48 nights of OCL and 48 control nights to detect a 20% improvement using a paired t test. Twenty subjects were recruited with the anticipation that each subject would have up to 6 nights and to allow for sensor error, which would prevent initiation of closed-loop control.
Data Analysis
For the intention-to-treat analysis, all sensor values from 2300 to 0700 h from both of the OCL and control groups were compared, regardless of system status.
For the per-protocol analysis, data from OCL nights during which there were technical problems such as infusion set failure, sensor error >20%, or pump failure resulting in a >60-min interruption to closed-loop control were removed to allow for analysis of algorithm performance. Only nights with a minimum of 5 h of OCL were included, and all glucose data were included in the analysis. For comparison, only data from nights during which sensor error was <20% with a minimum of 5 h were included in the control group.
Depending on distribution, data are expressed as mean ± SD or as median and interquartile range (25th–75th centile). Comparisons between OCL and control conditions were made using a Mann–Whitney U test. The number of nights during which there was ≥1 event <70 or >250 mg/dL under each condition was calculated for each subject, and the sum of the nights was compared using the Wilcoxon signed rank test. An event was described as having at least 10 min of sensor glucose within the described range. Analyses were performed using SigmaStat, version 11.0.
Results
Inpatient Studies
For the inpatient phase, the mean ± SD age of the 12 participants (6 male) was 15.3 ± 2.1 years (range 12.1–18.4 years), duration of diabetes was 7.6 ± 4.6 years, and A1C was 8.7 ± 0.7% (72 ± 8 mmol/mol). The median percent time spent between 70 and 150 mg/dL for plasma glucose was 89% (58, 100), and median time spent <70 mg/dL was 0% (0, 0) for the duration of closed-loop. Two patients were given oral glucose treatment for meter glucose values <70 mg/dL within the first 3 h of commencing OCL. The overall day 1 mean absolute relative accuracy (ARD) of the Dexcom G4 sensor (Dexcom) was 10.4 ± 9.1% with a median of 7.7% (4.7, 13.6) (n= 201). Further details are included in the Supplementary Data.
Camp Sessions
During the camp sessions, 20 participants (10 male) were enrolled and studied over 106 nights. The mean age of participants was 15.3 ± 2.9 years (range 10.2–20.7 years), diabetes duration was 5.6 ± 3.5 years, and A1C was 8.1 ± 1.1% (range 6.0–10.4%).
Intention-to-Treat Analysis
There were 54 nights of OCL and 52 nights of sensor-augmented pump therapy. There were 7 nights during the control period in which the starting glucose levels were outside the 80–250 mg/dL range. On an intention-to-treat basis, with glucose data analyzed regardless of system status, the median percent time in range, from 70–150 mg/dL, was 62% (29, 87) for OCL versus 55% (25, 80) for sensor-augmented pump therapy (P = 0.233). The mean overnight glucose values were similar, with 147 ± 34 mg/dL for OCL and 146 ± 42 mg/dL for control nights (P = 0.887). There was no difference in the glucose variability as measured by SD of glucose values, with a median of 30 mg/dL (20, 42) for OCL and 26 mg/dL (18, 37) for control nights (P = 0.304).
Overall, the median duration for closed-loop for all 54 nights was 7.6 h (6.6, 8.3), and the system was active 95% (89, 100) of the time. No intervention was required for 15 nights (28%). On 9 nights (17%), OCL was stopped, and the subject reverted to sensor-augmented pump for the remainder of the night. On the remaining 45 nights, OCL proceeded until the morning. There were a number of connectivity issues that required staff intervention. The median duration of interruptions during active closed-loop control was 15 min (0, 39). Interventions included: failed sensor or loss of sensor signal (16 events), loss of BT connection between the controller and pump (15 events), closed-loop program stopped requiring system reset (10 events), infusion set failure (4 events), device low battery requiring replacement (3 events), and pump failure (1 event).
OCL was suspended once due to glucose level <50 mg/dL within 30 min of start-up. There were two sessions suspended due to an event >250 mg/dL with ketones of >0.6 mmol/L, both of which were attributed to infusion set failure. There were no events stopped due to values >400 mg/dL without ketones.
There were six episodes of hypoglycemia with confirmed meter glucose <70 mg/dL requiring treatment during the 54 OCL nights. Five of these episodes occurred within 3 h of starting closed-loop and were associated with insulin action from previous manual boluses and minimal insulin delivery by the closed-loop controller. One episode occurred at 0515 h. Hypoglycemia treatment was also given on one additional occasion when the meter glucose was 72 mg/dL, and the patient was symptomatic.
During sensor-augmented pump therapy, there were four episodes with confirmed meter glucose <70 mg/dL requiring treatment with oral glucose. There was one additional episode with meter glucose >70 mg/dL during which the patient was symptomatic and requested hypoglycemia treatment.
The number of nights with ≥1 event <70 mg/dL was 11 for OCL versus 21 for control conditions (P = 0.110). The number of nights with ≥1 event >250 mg/dL was 12 for OCL versus 7 for control conditions (P = 0.492).
Fasting meter glucose levels checked at 0700 h following OCL nights were lower but not significantly different than those following control nights, with a median of 132 mg/dL (122, 164) versus 150 mg/dL (124, 170), respectively (P = 0.239). Ketone levels were also similar, with a median of 0 mmol/L (0, 0.2) in each group (P = 0.845).
More glucose measurements were obtained during OCL compared with control nights, with a median of 3 (3, 4) versus 1 (0, 1) additional overnight measurement (P < 0.001). There was no difference in the number of calibrations required during OCL for sensor error >20% at 0 (0, 1) compared with those initiated by the patient during control nights being 0 (0, 1) (P = 0.065).
Per-Protocol Analysis
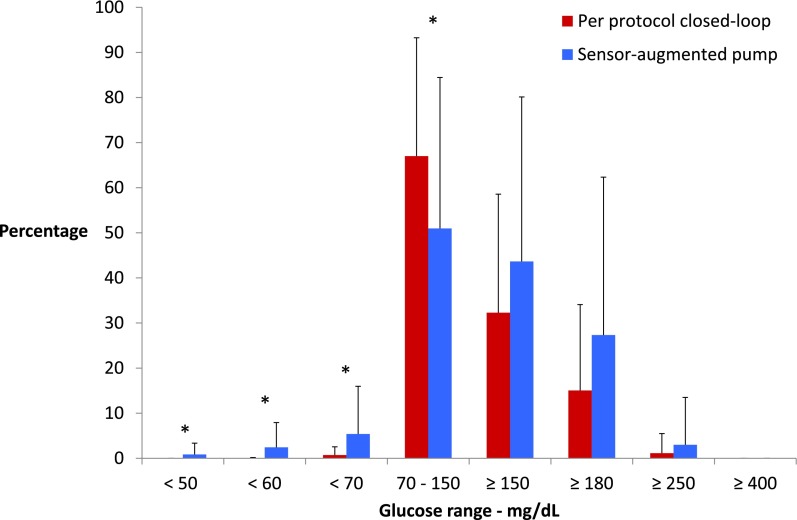
The per-protocol analysis included 41 OCL nights compared with 39 sensor-augmented pump nights. The median time spent in range from 70–150 mg/dL was 73% (50, 89) for OCL versus 52% (24, 83) for control nights (P = 0.037). There was less time spent in the hypoglycemic range (<50, <60, and <70 mg/dL) during OCL compared with the control period (P = 0.019, P = 0.009, and P = 0.023, respectively), as shown in Fig. 3. There was no difference between the groups for glucose ranges >150, ≥180, or ≥250 mg/dL. The time spent in range from 70–180 mg/dL was also greater for OCL at 92% (69, 100) versus 80% (48, 95) during the control period (P = 0.022).
Figure 3.
Per-protocol analysis of percent time spent in range for OCL nights (n = 41) versus sensor-augmented pump nights (n = 39). Results are mean ± SD. *P < 0.05.
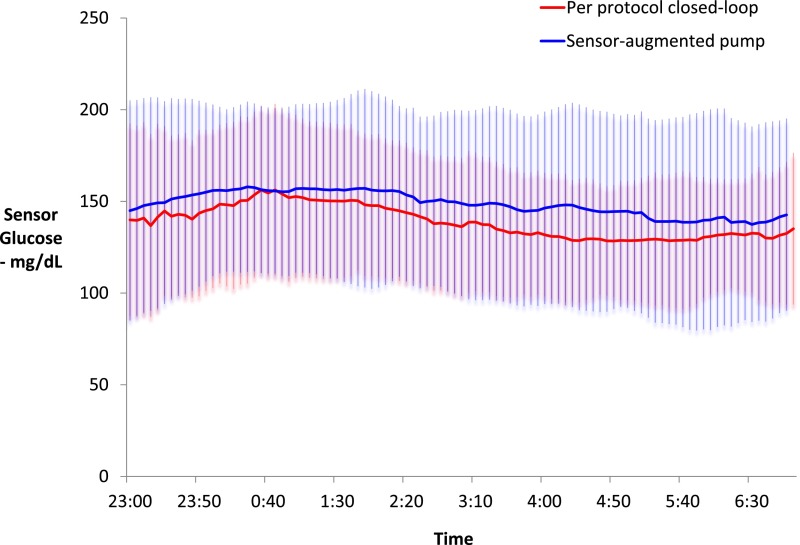
Fig. 4 demonstrates the mean ± SD glucose values per protocol over the course of the night for both OCL and control conditions. Mean glucose values were also calculated per night under both conditions for each patient. These values were similar for both conditions with a mean of 140 ± 18 mg/dL for OCL nights versus 147 ± 36 mg/dL for control nights (P = 0.340). Glucose variability was no different between the two conditions with a mean SD of 35 ± 11 mg/dL for OCL versus 36 ± 15 mg/dL for the control conditions (n = 18 subjects).
Figure 4.
Per-protocol analysis of sensor glucose values over 8 h during OCL (n = 41) versus sensor-augmented pump nights (n = 39). Results are mean ± SD.
The number of nights with ≥1 event <70 mg/dL was 7 for OCL versus 14 for control conditions (P = 0.034). The number of nights with ≥1 event >250 mg/dL was 4 for both conditions (P = 1.00).
In terms of sensor performance over the camp sessions, the mean ARD was 17.5%, and the median ARD was 13.5% (n = 740).
Conclusions
The diabetes camp setting provided a challenging environment to evaluate automated closed-loop control. Based on an intention-to-treat analysis, there was no difference in the percentage of time spent in range between 70 and 150 mg/dL for closed-loop versus sensor-augmented pump nights. In terms of per-protocol analysis, however, there was a significant reduction in both nocturnal hypoglycemia and increased time spent in range for closed-loop control. The system was tested over multiple days in a much less controlled environment than inpatient studies. We did not restrict recruitment by A1C, and recruited subjects were representative of a typical population of children and adolescents with type 1 diabetes.
The similar time spent in range between OCL and control nights is likely a reflection of the excellent medical care that is given at camp, with frequent monitoring of glucose levels overnight and active reduction of insulin delivery for all campers in general. We communicated any significant changes seen in the insulin requirements during OCL to camp medical staff, and this information was used to adjust basal rates on the subsequent control night. Likewise, any changes made to insulin delivery rates overnight by the camp medical staff were incorporated into our control algorithm during subsequent closed-loop nights. This shared communication between the research team and camp medical staff was important for patient safety and was mandated by the U.S. Food and Drug Administration. It did, however, cause convergence of insulin delivery rates between both groups. This, we speculate, contributed to less hypoglycemia and increased time in range for the control period. In addition, OCL was conducted with strict starting criteria, including sensor error <20% and treatment of meter confirmed values <70 mg/dL. The results, therefore, should be interpreted within this context of clinical supervision, which was available during closed-loop control. In addition, there were fewer nights with ≥1 event with sensor glucose <70 mg/dL prior to any intervention occurring during OCL. More data are required to demonstrate safety and robust performance of these systems before they can function without remote monitoring, and it will then be important to evaluate these systems without investigator intervention.
During the inpatient studies, the day 1 sensors performed well, with a mean ARD of 10.4%. This increased to 17.5% during the camp sessions when sensors were used by participants with minimal supervision at the time of calibration. All participants were instructed to wash their hands or use an alcohol swab before calibrating; however, research staff did not supervise all calibration events. This has important implications in terms of the safety and reliability in using automated closed-loop systems in the home environment.
The current system had several connectivity issues, namely, the absence of a native wireless communication between the receiver and DIAS as well as the t:slim pump BLE connectivity. These limitations necessitated intervention by staff, and although loss of connectivity was readily solved, this mitigation will not be available in the home setting. Future developments of the G4 Platinum system to obviate the need for a separate receiver as well as adding standard functions to the pump, such as programmed basal rate that can be resumed if there is loss of communication, are important considerations for future integrated systems.
A recent multicenter study also assessed the use of OCL control in a diabetes camp setting using the MD-Logic system (17). Both the MD-Logic and the DIAS USS systems were studied on 54 nights in a diabetes camp setting. There were several differences in study design. In the MD-Logic study, all participants completed a 5- to 10-day evaluation period of their pump and sensor data prior to commencing closed-loop, whereas with the DIAS USS system, only the current insulin profile and patient weight were required to initialize the system. The MD-Logic system was initialized before dinner, between 1600 to 1700 h, whereas our subjects were connected at bedtime, between 2200 and 2300 h. The DIAS system was tested on multiple nights on each subject with up to 3 nights per subject compared with 1 night per subject in the MD-Logic study. Phillip et al. (17) also did not show any difference in the mean overnight glucose values between the control and closed-loop conditions. With the MD-Logic system, there were 26 carbohydrate interventions given for a similar number of nights. The investigators intervened for hypoglycemia with a documented capillary glucose <60 mg/dL on 4 occasions and gave carbohydrates based on predictive alarms on a further 22 occasions. The mean capillary glucose at the time carbohydrates were given was 81 mg/dL. In our study, hypoglycemia treatment was only given if there was a sensor reading <70 mg/dL with a confirmed meter glucose value of <70 mg/dL. There were a total of seven hypoglycemia interventions with the DIAS USS system. There were six hypoglycemic events confirmed with a meter reading of <63 mg/dL in the MD logic study, whereas we had four confirmed meter glucose values <63 mg/dL.
A large body of work by Hovorka et al. (1,4), Elleri et al. (18), and Murphy et al. (19) has accelerated this field tremendously. In a series of inpatient randomized crossover studies in patients aged 5–18 years (1) using a manual system, OCL control improved the time spent in target glucose range and reduced hypoglycemia. The addition of daytime closed-loop and the use of an adaptive control algorithm has been shown to further improve overnight glucose control, at least in an inpatient setting. In a recent study (18) involving both day and night closed-loop over 32 h in a hospital in 12 adolescents, plasma glucose levels were in the range of 71–145 mg/dL for 76% on the first night and 95% on the second night. We achieved similar results in our inpatient studies with a median of 89% for time spent between 70 and 150 mg/dL on the first night.
A limitation of this study is the missing data from the sensor-augmented pumps during the control period, which prevented comparison of insulin delivery during this period.
In conclusion, on nights when the DIAS USS algorithm is functioning, it is effective in reducing both nocturnal hypoglycemia and increasing time spent in range during the overnight period compared with sensor-augmented pump therapy in children and adolescents with type 1 diabetes at a diabetes camp setting. These are encouraging results as we transition toward full day and night closed-loop with improved portable, automated closed-loop systems.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants and families for taking part in this study; the medical staff, directed by Dr. Kevin Kaiserman at Camp Conrad-Chinnock; and camp staff, directed by Dr. Rocky Wilson at Camp Conrad-Chinnock and Janet Kramschuster at Camp de Los Ninõs, for making this study possible. The authors also thank Tandem (San Diego, CA) and Dexcom (San Diego, CA) for providing access to the technology.
Funding. This work was supported by a grant from the Helmsley Foundation and by the Stanford Clinical and Translational Science Award (CTSA) to Spectrum (UL1 TR001085). T.T.L. is supported by the University of Western Australia F.A. Hadley Overseas Medical Fellowship.
Duality of Interest. M.D.B., P.K.-H., and B.P.K. hold patents or patent applications related to the study technology. M.D.B. and B.P.K. are consultants/advisors for Animas and Sanofi and have received research grant or study material support from Animas, BD Biosciences, Dexcom, Insulet Corporation, LifeScan, Inc., Sanofi, and Tandem Diabetes Care, Inc. B.A.B. is on medical advisory boards for Sanofi, Novo Nordisk, BD Biosciences, Unomedical, and Medtronic and has received research grant and/or material support from Medtronic, Dexcom, LifeScan, Inc., Insulet Corporation, Bayer, Unomedical, and Tandem Diabetes Care, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.T.L. contributed to study design, researched data, and wrote the manuscript. M.D.B., D.M.W., B.P.K. and B.A.B. contributed to study design and discussion and reviewed the manuscript. P.K.-H., D.D.S., P.C., K.B., B.M., D.C., and J.P. reviewed the manuscript. B.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01973413, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0147/-/DC1.
References
- 1.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 2.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 3.Kovatchev B, Cobelli C, Renard E, et al. Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Tech 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Grady MJ, Retterath AR, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breton M, Farret A, Bruttomesso D, et al. International Artificial Pancreas Study Group Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosman B, Dassau E, Zisser HC, Jovanovic L, Doyle FJ., 3rd Zone model predictive control: a strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Tech 2010;4:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cemeroglu AP, Thomas JP, Zande LT, et al. Basal and bolus insulin requirements in children, adolescents and young adults with type 1 diabetes mellitus on continuous subcutaneous insulin infusion (CSII): effect of age and puberty. Endocr Pract 2013;19:805–811 [DOI] [PubMed] [Google Scholar]
- 10.Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care 2008;31:44–46 [DOI] [PubMed] [Google Scholar]
- 11.Hasan KS, Kabbani M. Mini-dose glucagon is effective at diabetes camp. J Pediatr 2004;144:834. [PubMed] [Google Scholar]
- 12.Place J, Robert A, Ben Brahim N, et al. DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Tech 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes CS, Patek SD, Breton MD, Kovatchev BP. Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Tech 2010;4:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 15.Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care 2009;32:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desalvo DJ, Keith-Hynes P, Peyser T, et al. Remote glucose monitoring in camp setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther 2014;16:1–7 [DOI] [PubMed] [Google Scholar]
- 17.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 18.Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy HR, Kumareswaran K, Elleri D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care 2011;34:2527–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.