Abstract
OBJECTIVE
This article examines the foundation of β-cell failure in type 2 diabetes (T2D) and suggests areas for future research on the underlying mechanisms that may lead to improved prevention and treatment.
RESEARCH DESIGN AND METHODS
A group of experts participated in a conference on 14–16 October 2013 cosponsored by the Endocrine Society and the American Diabetes Association. A writing group prepared this summary and recommendations.
RESULTS
The writing group based this article on conference presentations, discussion, and debate. Topics covered include genetic predisposition, foundations of β-cell failure, natural history of β-cell failure, and impact of therapeutic interventions.
CONCLUSIONS
β-Cell failure is central to the development and progression of T2D. It antedates and predicts diabetes onset and progression, is in part genetically determined, and often can be identified with accuracy even though current tests are cumbersome and not well standardized. Multiple pathways underlie decreased β-cell function and mass, some of which may be shared and may also be a consequence of processes that initially caused dysfunction. Goals for future research include to 1) impact the natural history of β-cell failure; 2) identify and characterize genetic loci for T2D; 3) target β-cell signaling, metabolic, and genetic pathways to improve function/mass; 4) develop alternative sources of β-cells for cell-based therapy; 5) focus on metabolic environment to provide indirect benefit to β-cells; 6) improve understanding of the physiology of responses to bypass surgery; and 7) identify circulating factors and neuronal circuits underlying the axis of communication between the brain and β-cells.
Introduction
Two major pathophysiologic abnormalities underlie most cases of type 2 diabetes (T2D): 1) insulin resistance and 2) defects in pancreatic β-cell function. The current consensus is that both are essential components in disease pathogenesis even if their relative importance, the precise temporal sequence of events, and underlying mechanisms vary considerably in different populations and individual patients.
In October 2013, the Global Partnership to Accelerate Diabetes Research, cosponsored by the Endocrine Society and the American Diabetes Association, assembled international experts (see appendix) to inform the global health research agenda by reviewing the state of the science and identifying pressing research needs related to β-cell dysfunction in T2D. The major issues addressed and outcomes of their discussion follow.
Genetics and Epigenetics
Powerful evidence for a genetic component to T2D (1) has driven extensive efforts to identify genetic variants contributing to risk. Monogenic forms of diabetes such as maturity-onset diabetes of the young (MODY) have proven to be natural models for understanding mechanisms underlying insulin secretion defects; genes discovered through family-based approaches are important regulators of insulin secretion and β-cell development. Findings that neonatal diabetes is most commonly due to activating mutations in genes encoding the ATP-sensitive potassium channel subunits Kir6.2 or SUR1 and can be treated with high-dose sulfonylureas despite being insulin dependent also provide a compelling case for genetic evaluation of monogenic diabetes with therapeutic and prognostic implications (2–6). Exome-wide and whole-genome sequencing approaches will expand current capacity to study these disorders.
Genome-wide association studies (GWAS) using high-density genotyping arrays have transformed understanding of the genetic architecture of T2D (7). At the time of writing, over 60 genetic loci have been convincingly associated with T2D, the great majority in some way involved in β-cell biology, underscoring the importance of β-cell dysfunction in T2D pathogenesis (8,9). GWAS data must be interpreted with great caution until the precise genes in the loci associated with T2D have been identified and the impact of specific variants on β-cell function is more clearly understood, as exemplified in a recent enigmatic study on the possible protective effects against T2D of loss-of-function mutations in SLC30A8 (encoding the islet zinc transporter ZnT8) (10). In any case, these variants explain only a small proportion of total genetic risk (11). Studies based on exome and whole-genome sequencing technology are under way to identify low-frequency, high-impact variants accounting for a greater component of risk (12). Other novel approaches have been proposed, but genetic discovery models to date have largely been simple case-control studies of this complex metabolic disorder (13). It is furthermore evident that other factors that influence gene expression contribute toward the complexity of T2D, specifically epigenetic mechanisms and microRNAs (miRNAs).
Epigenetic mechanisms refer to functional changes to the genome that do not involve any alteration in nucleotide sequence. Such mechanisms (e.g., DNA methylation and histone modifications) can be active during fetal as well as postnatal and adult life and impact the level of expression of select genes associated with T2D (14). While the epigenome may be dynamic and change due to environmental exposure, modifications may also be stable and inherited, making epigenetics a potentially important pathogenic mechanism. The possibility that the environment can alter the pancreatic islet epigenome and subsequently affect β-cell function and diabetes pathogenesis is specifically reflected in human and animal studies linking an impaired intrauterine environment and resulting low birth weight to an increased risk for postnatal metabolic disease, with decreases in β-cell proliferation, mass, and insulin secretion in the face of documented epigenetic modifications in key β-cell genes (15,16). In addition, a low-protein diet in utero alters the epigenetic profile of HNF4A in rodent islets, associated with impaired islet function (17,18)—findings supported by human studies.
Studies of pancreatic islets from nondiabetic donors (19,20) and patients with T2D (21) have identified epigenetic modifications in genes that potentially affect β-cell function. Such studies of human pancreatic islets together with in vitro studies of clonal β-cells further suggest that hyperglycemia alters DNA methylation of PDX1 and INS (22–25). DNA methylation mainly occurs on cytosines in CpG dinucleotides, and approximately 50% of single nucleotide polymorphisms (SNPs) associated with T2D introduce or remove a CpG site. These CpG-SNPs are associated with differential DNA methylation, gene expression, alternative splicing events, and hormone secretion in human pancreatic islets, suggesting strong genetic-epigenetic interactions (26).
It has also been suggested that histone modifications in human islets contribute to reprogramming α-cells to β-cells, possibly due to the large number of bivalent marks in α-cells (27). Lipid treatment also alters the activity of enzymes responsible for histone modifications in clonal β-cells, in parallel with decreased glucose-stimulated insulin secretion (28). Other recent studies indicate that histone deacetylases (HDACs) contribute to cytokine-mediated β-cell damage, suggesting HDAC inhibition as a possible diabetes treatment (29).
miRNAs are a class of small noncoding RNA molecules that modulate gene expression by binding to specific target messenger RNAs to prevent their translation and/or to promote degradation. It has been suggested that altered miRNA expression may contribute toward β-cell failure in T2D and that these molecules may serve as biomarkers for the disease (30).
Genetic and environmental stressors likely modulate miRNA expression, altering cellular phenotypes. Specific miRNAs are critical to pancreatic β-cell development, function, and adaptive turnover. Individual miRNAs are highly represented in β-cells (31), impacting function and mass both positively and negatively. For example, knockout of miR-375 promotes progressive hyperglycemia in mouse models due to decreased insulin content and progressive loss of β-cell mass (32). There is increasing interest in the modulation of miRNA expression, and a recent study has revealed an epigenetic mechanism in islets from patients with T2D (33).
Looking to the Future
Elucidating a full picture of genetic risk for diabetes is an increasingly daunting prospect. This will require insight and expertise from investigators in a wide range of fields that complement the specific skills of diabetes-focused researchers. Clearly, extensions of “conventional GWAS” are highly desirable, including evaluation of genetic models beyond single genes, coupled with more sophisticated quantitative measures of β-cell function. A major barrier is the lack of large-scale, population-based samples with high-quality metabolic measurements of β-cell function and the dearth of explanations for how discovered T2D genes actually mediate diabetes risk.
Studies on epigenetics and miRNAs are distinctive in going beyond statistical associations to integrate multiple pathways to identify function, but they are in their infancy. Understanding factors altering the expression of miRNAs and the epigenome in pancreatic islets and β-cells from prediabetic and T2D subjects and, further, developing selective small molecules that target epigenetic enzymes to improve β-cell function and/or treat diabetes is essential. Environmental modification could influence both miRNAs and epigenetics, and a provocative question is whether such modifications can be used to predict β-cell failure risk. Finally, combining epigenetic and genetic research to integrate the entire body of data will be necessary to explain the “missing” heritability in diabetes.
THE FoundationS of β-Cell Failure: Dysfunction, Dedifferentiation, or Death?
β-Cell loss in response to nutrient excess and stress was traditionally felt to occur exclusively via β-cell death. Although β-cell death might be a final common pathway in the natural history of T2D, more recent evidence indicates a more complex situation in which β-cells can initiate several alternative responses to avert irreversible loss, suggesting the potential for earlier intervention. Mouse studies led by Accili and colleagues (34) have shown that β-cell dysfunction due to FoxO1 deficiency during pregnancy and aging is primarily associated with β-cell dedifferentiation rather than death. This finding revisits an earlier one (35) suggesting β-cell dedifferentiation during disease development, although additional experimentation is needed to determine whether cells lose their defining β-cell characteristics temporarily, revert to an immature fetal or neonatal-like state with impaired glucose-stimulated insulin secretion that reinitiates expression of fetal hormones, or indeed revert to an undifferentiated progenitor state. Relevance to human diabetes remains to be established.
Oxidative stress can inactivate key islet transcription factors, producing “stunned” β-cells that temporarily stop responding to glucose and storing normal amounts of insulin (36,37). Emerging evidence in mice also shows considerable plasticity within islets, allowing intraislet cell conversions, but only in the face of extreme β-cell destruction (38). The similarity between α-cell and β-cell transcriptomes in mice and humans supports this model, as does the discovery that hormone gene promoters in different islet cell types present similar methylation patterns.
A challenge in understanding β-cell failure is elucidating key elements responsible for their function and survival, including their apparently unique vulnerability to environmental changes. Here, clonal cell lines selected for characteristics such as secretory defects after high-glucose exposure or susceptibility to cytokine-induced death have proved useful (39). Insights have also come from studying the unique substrate metabolism of β-cells, chiefly focusing on the link between pyruvate cycling and glucose-induced insulin secretion (40). Studying the transcriptional control of replication is yet another way in which basic models may provide valuable translatable information and ultimately generate hypotheses for evaluation in primary β-cells in vitro and in vivo (41).
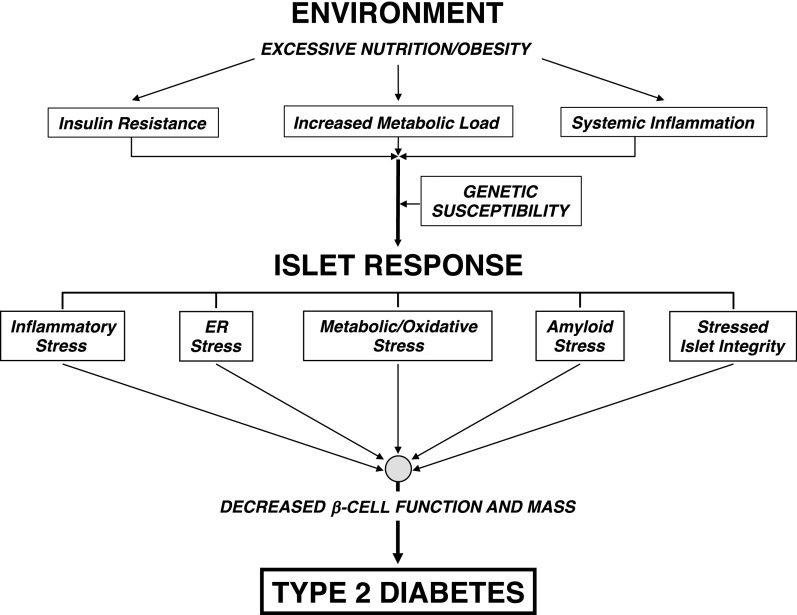
Many physiologic stressors impact β-cell function in the environment of metabolic overload and insulin resistance commonly found in human obesity-linked T2D (Fig. 1). While β-cells initially respond by activating compensatory pathways to improve the insulin secretory response, eventually they initiate several pathologic programs that synergistically promote β-cell dysfunction and, ultimately, death. To understand and intervene in disease progression, ongoing investigations are exploring which of the following pathologic conditions, or β-cell stressors, are initiated first and which might represent the most effective intervention points.
Figure 1.
Stressors on the β-cell in the pathogenesis of T2D. In the excessive nutritional state found in obesity, hyperglycemia and hyperlipidemia develop, increasing metabolic load coupled with concurrent inherent insulin resistance and chronic inflammation. The pancreatic islet response to this new environment is likely variable among individuals with differing genetic susceptibility but may include inflammatory stress, ER stress, metabolic and oxidative stress (e.g., glucotoxicity, lipotoxicity, and glucolipotoxicity), amyloid stress, and loss of islet cell integrity. If untreated, these interrelated stressors increase with time, promoting β-cell dysfunction (coupled with increased glucagon secretion) and ultimately loss of β-cell mass and possibly dedifferentiation that mark the onset of T2D.
Endoplasmic Reticulum Stress
Endoplasmic reticulum (ER) stress appears to arise when markedly increased insulin production to meet metabolic demand necessitates increasing flux through the rough ER, with stress evident in the unfolded protein response that chaperones newly synthesized proinsulin along the secretory pathway. Such changes may promote β-cell secretory dysfunction and, under chronic challenge, apoptosis. While ER stress may play a key role in the pathogenesis of certain forms of monogenic diabetes, its role in common T2D remains unclear.
Metabolic and Oxidative Stress
Metabolic and oxidative stress, primarily from obesity’s excessive nutritional state, leads to β-cell damage associated with glucotoxicity, lipotoxicity, and glucolipotoxicity (42). An emerging concept is a link between oxidative stress and observed DNA damage leading to altered transcription factor expression. Although β-cells are uniquely geared for efficient oxidative metabolism—both to provide energy via ATP production and to generate secondary signaling mechanisms—markedly increased glycolytic flux in hyperglycemia may underlie dysfunction. Moreover, because β-cells lack certain antioxidant enzymes that dispose of reactive oxygen species (ROS), increased ROS production may promote dysfunction and even apoptosis. Mishandling of excessive cholesterol commonly seen in T2D, with accumulation in β-cells, could perhaps impair secretion (43).
Amyloid Plaques
Amyloid plaques, which characterize islets in T2D, consist mainly of islet amyloid polypeptide (IAPP). In chronic hyperglycemia/hyperlipidemia, (pro)IAPP synthesis increases in β-cells, parallel to proinsulin, and can reach threshold levels that allow proapoptotic IAPP oligomers to form (44) that induce interleukin (IL)-1β release to recruit macrophages and enhance local islet inflammation (45).
Inflammation
Whether increased local islet inflammation, well established in T2D pathogenesis (46), results from a janitorial macrophage infiltration to clear damaged islet β-cells and/or an innate inflammatory response remains unresolved. What has become clear, however, is that anti-inflammatory therapies (i.e., IL-1β antagonism) can preserve some β-cell functional mass in T2D (47).
Islet Integrity/Organization
Islet integrity/organization is often disrupted in T2D pathogenesis, potentially perturbing cell–cell communication within islets. This may contribute to poorly regulated secretion of insulin and glucagon, perhaps contributing toward hyperglucagonemia that exacerbates hyperglycemia in T2D. In addition, loss of islet integrity could diminish the β-cell incretin response.
Looking to the Future
β-Cell demise is a multifactorial process involving many stressors. It remains unclear which pathway is disrupted first, and this may actually depend on the individual. Regardless of the initiation event, a feed-forward loop becomes induced that is difficult to stop. Given the likely molecular cross talk and convergence between the pathways, targeting a single molecule could have beneficial effects by blocking multiple stressor pathways. Recent studies have offered novel insight into pathways activated within β-cells to cope with stress. Novel therapies that exploit these natural defense mechanisms to prevent or reverse β-cell failure in T2D may be possible. It is postulated that if β-cell dysfunction is not ameliorated by effective therapy, with time there is loss of β-cell identity through dedifferentiation and ultimately death. This sequence suggests the need to intervene as early as possible in the course of disease.
Natural History of β-Cell Failure
Impaired insulin secretion assessed by oral or intravenous glucose tolerance testing and hyperglycemic clamp studies is seen in prediabetes as well as early in the disease, with reduced secretion negatively correlated with glycemia (48). Although declines in β-cell mass and function are not well correlated, presumably because function depends greatly on diabetes milieu, climbing glucose levels and deteriorating β-cell function are tightly correlated (49). While β-cell function appears to decline progressively, insulin secretion defects seem at least partially reversible, especially early in the disease.
Family history and obesity are major risk factors for both youth and adults. In youth, a family history of T2D is also associated with a high risk for decreased insulin sensitivity and response, and increased proinsulin-to-insulin ratio (50). Impaired glucose tolerance (IGT) in youth is characterized by β-cell dysfunction manifested in impaired first-phase, but preserved second-phase, insulin secretion relative to sensitivity. However, youth with IGT are no more insulin resistant than peers with normal glucose tolerance (NGT) if matched for body composition and fat topography (51). Once treated with metformin after progressing to T2D, youth show a greater rate of treatment failure than adults (52).
β-Cell mass increases during the first decade of life due to proliferation during the first 5 years (53,54), stabilizing during adolescence with considerable variation (three- to fivefold) unrelated to age or BMI. β-Cell mass in T2D likewise varies, overlapping with that of normal individuals (55–57), although current data suggest a 20–60% reduction (58). Currently, measurements of human β-cell mass rely entirely on postmortem, cross-sectional assessments; inability to assess mass noninvasively via imaging or biomarkers impedes determining temporal changes. Furthermore, histomorphometric measurement of β-cell area, volume, and mass is often imprecisely defined and complicated by technical inconsistencies (59). Importantly, too, because insulin sensitivity may vary as much as 10-fold in humans, variations in β-cell mass may be linked to individual insulin sensitivity. Thus, an individual with low insulin sensitivity and T2D may have a higher than “normal” β-cell mass that is functionally reduced, suggesting that de facto variation might be considerably reduced if corrected for insulin sensitivity.
Looking to the Future
Marked variation in degree of β-cell loss in T2D could potentially be reconciled by adopting standardized approaches to quantification using area, volume, and/or mass in available pancreases. It would be enormously helpful to be able to measure β-cell mass in humans with noninvasive techniques. Many approaches have been and are being examined, as has recently been reviewed (60). One promising approach is to use fluorescent exendin-4 derivatives, which should bind preferentially to β-cells (61,62). However, a major issue concerns how much sensitivity and precision can be achieved by any noninvasive approach. A desirable goal might be the ability to measure changes in β-cell mass as small as 5% because such changes are expected to be important for β-cell function.
Given the difficulty of studying human β-cells, well-defined clinical phenotypes correlated with β-cell defects and/or noninvasive imaging or biomarkers defining proliferation and death in pancreatic samples could help elucidate the discordance between functional and morphometric assessments as well as help identify changes indicating early β-cell failure in high-risk individuals (63).
Standardizing methodologies measuring β-cell function and defining approaches for specific clinical questions may also facilitate cross-study comparisons. Currently, β-cell function is variably assessed using fasting indices derived from insulin and glucose values, dynamic testing with oral glucose or standardized meals, and responses following intravenous glucose infusions (64). Interpreting β-cell function in the context of glycemia and concurrent insulin resistance is critical in clinical studies evaluating therapeutic efficacy. Other features of potential interest include sustainability and durability of treatment effects during and after therapy.
Impact of Therapeutic Interventions
Interventions to prevent and treat diabetes by improving β-cell function are based on the premise that β-cell dysfunction can be reversed. This appears true for at least some portion of the pathogenesis spectrum, but limits of reversibility remain unexplored. Available data suggest that in established diabetes effects on β-cell function are not sustained following withdrawal of active therapies; whether this is true at earlier stages of diabetes or in prediabetes is unknown. In the Diabetes Prevention Program (DPP), greater baseline β-cell function and insulin sensitivity contributed independently to the restoration of NGT following lifestyle changes (65,66). Improvements in β-cell function also appear to play a role in pharmacologic approaches to prevention and treatment. Interventions in the Troglitazone in Prevention of Diabetes (TRIPOD) and Pioglitazone in Prevention of Diabetes (PIPOD) and Actos Now for Prevention of Diabetes (ACT NOW) (pioglitazone) studies, for example, significantly improved the oral disposition index. Moreover, changes such as regression to IGT, maintenance of NGT, or progression to diabetes were proportional to insulin secretion response (67). GLP-1–based therapies magnify glucose-stimulated insulin secretion, as seen with significantly improved β-cell function in T2D following short-term infusions of GLP-1 (68,69) and longer-term treatments with dipeptidyl peptidase-4 inhibitors (70) and GLP-1 receptor agonists (71,72). However, while augmented insulin production as assessed with glucose and arginine stimulation continues during active treatment and is of clinical value, none of these agents have produced a meaningful, persistent change following therapy withdrawal (71,73).
New evidence suggests that Roux-en-Y gastric bypass surgery (RYGB) exerts antidiabetes effects in part via β-cell functional improvements. RYGB is unique among weight-loss surgery approaches to diabetes for producing marked improvements in metabolic status, including disease remission in at least 80% of patients (74). The overall benefit clearly has a basis in the direct effects of weight loss, with attendant reduction in insulin resistance, and acute caloric restriction (75,76). However, many lines of evidence demonstrate glycemic benefits independent of weight (77), including changes in β-cell function (78). Although studies to date have not clearly dissected changes in insulin sensitivity from changes in β-cell function, augmented GLP-1 secretion amplifies RYGB’s antidiabetes effects on pancreatic islets (79), and post-RYGB hyperinsulinemic hypoglycemia may involve β-cell hypertrophy or neogenesis as well (80,81). The observation that the strongest predictor of diabetes remission is duration of diabetes and insulin use prior to surgery (82) rather than weight regain also suggests underlying β-cell health as a limiting factor for RYGB’s antidiabetes benefits.
Novel mechanisms of action and targets in β-cells, such as fatty acid receptor activation, glucokinase activators, fractalkine, betatrophin, and β-secretase 2 inhibitors, continue to emerge, adding to the potential approaches to alter the natural history of diabetes. For decades it has been known that relieving hyperglycemia can itself improve insulin secretion and restore metabolic control, at least temporarily. Recently, early interventions with insulin therapy in newly diagnosed T2D (83), with targeted anti-inflammatory therapy using an IL-1β antagonist (84), and with GLP-1 receptor agonists (85) have demonstrated that β-cell dysfunction can be reversed temporarily. However, durability of these effects following therapy withdrawal remains challenging. To date, these responses have been evaluated only in established diabetes; whether they can alter the natural history of β-cell failure earlier in disease progression is unknown.
Looking to the Future
Numerous pathophysiologic pathways contributing to progressive β-cell failure have been identified as viable targets for intervention. However, no study has established whether one pathogenic pathway dominates or can serve as a single major target. Moreover, different pathophysiologic processes may be active at different stages of progression, and optimal targets may shift accordingly. For example, early hyperglycemia may activate multiple pathophysiologic processes, including inflammation, amyloid accumulation, dedifferentiation, apoptosis, and genetic alterations; whether any one target is optimal or sufficient at this stage remains undetermined. Further, there may also be a stage of pathogenesis beyond which therapeutic interventions cannot sufficiently enhance β-cell function, making treatments targeting other aspects of metabolic dysregulation relatively more valuable. Interventions targeted by stage of pathogenesis or combinatorial approaches may be required to preserve or restore β-cell function.
Summary and Conclusions
Progressive loss of β-cell function is central to the development and progression of T2D. Deterioration of β-cell function antedates and predicts diabetes onset and progression, is genetically determined, and can be predicted with accuracy even though current tests are cumbersome and not well standardized. There is, however, continued debate surrounding the relative contributions of decreased function or mass to clinically manifest β-cell dysfunction. This leads to confusion that extends beyond semantics and that will not be resolved until there are precise noninvasive methods to relate changes in β-cell mass and function over time. Multiple pathways underlie decreased β-cell function and mass, some of which may be shared, with reduction in mass, perhaps a consequence of processes that initially caused dysfunction. In addition, the concept of β-cell dedifferentiation in T2D has regained favor. Even in the late stages of the disease, residual β-cells remain, and their number is possibly underestimated due to absence of markers of β-cell identity in dedifferentiated cells (86).
To date, most genes suggested by GWAS as associated with T2D are also associated with reduced β-cell function in the nondiabetic population and are known to be expressed in β-cells and implicated in their development, function, or survival. However, many of these genes are also expressed in other tissues where their dysfunction may disturb glucose homeostasis and thereby, indirectly, β-cell function. Other genetic variation associated with diabetes, both common and less common, will be identified as the power of genetic studies increases; the challenge will be to turn this information into new biological insights. The study of epigenetic changes in the β-cell in T2D is likely to provide important new insight as well, but these studies are severely limited by the small number of islets available from patients with T2D and the difficulties separating cause and effect in hyperglycemia.
A variety of interventions including weight loss, insulin, thiazolidinediones, and anti-inflammatory drugs can improve β-cell function temporarily with improved glucose control, and these outcomes are certainly of value to patients. However, the limited number of clinical studies with appropriate protocols indicates that existing therapy does not arrest progression of β-cell dysfunction in T2D let alone reverse it, with the possible exception of gastric bypass surgery.
The group of experts identified areas for future research that would improve our understanding of β-cell failure in T2D, hopefully leading to more effective prevention and the development of treatment with more durable beneficial effects on β-cell function than is possible today. The goals of future research are presented in Table 1.
Table 1.
Goals of future research

Article Information
Acknowledgments. The authors are grateful for the contributions of the speakers and participants in the 14–16 October 2013 meeting, listed, together with affiliations, in the appendix. In addition, the authors acknowledge the editorial assistance of Dr. Terra Ziporyn (freelance; Severna Park, MD) in the writing of the manuscript.
Duality of Interest. K.J.M. received study support from Novo Nordisk, Sanofi, and Merck & Co. C.J.R. received compensation for consulting work at Sanofi, Merck & Co., and enGene and also received compensation from Merck & Co. for a speaker role. G.C.W. received a consulting fee from Merck & Co., Novo Nordisk, and Eli Lilly & Co. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.A.H., K.S.P., D.W.B., M.A.H., C.L., K.J.M., A.C.P., C.J.R., L.S., and G.C.W. wrote the manuscript. P.A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Participants in the meeting “Beta Cell Failure in Type 2 Diabetes” held on 14–16 October 2013 are listed here.
Meeting Series Steering Committee: Robert H. Eckel, MD, University of Colorado; Ele Ferrannini, MD, University of Pisa, Italy; and Steven E. Kahn, MB, ChB, VA Puget Sound Health Care System and University of Washington.
Meeting Cochairs: P.A.H., PhD, University of Geneva, Switzerland, and K.S.P., MD, University of Chicago.
Session Cochairs: D.W.B., PhD, Wake Forest University; Robert L. Hanson, MD, MPH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH)*; L.S., PhD, Columbia University; C.J.R., PhD, University of Chicago; G.C.W., MD, Joslin Diabetes Center, Harvard Medical School; A.C.P., MD, Vanderbilt University School of Medicine; M.A.H., MD, Albert Einstein College of Medicine; and K.J.M., MD, Indiana University.
Speakers: Domenico Accili, MD, Columbia University; Silva A. Arslanian, MD, University of Pittsburgh; David E. Cummings, MD, University of Washington; Stefano Del Prato, MD, University of Pisa, Italy; Michaela Diamant, MD, PhD, VU University Medical Center, the Netherlands; Marc Y. Donath, MD, University of Zurich, Switzerland; Yuval Dor, PhD, Hebrew University of Jerusalem, Israel; Judith E. Fradkin, MD, NIDDK, NIH*; Andrew T. Hattersley, FRS, FRCP, University of Exeter, U.K.; Ele Ferrannini, MD, University of Pisa, Italy; Steven E. Kahn, MB, ChB, VA Puget Sound Health Care System and University of Washington; Jack L. Leahy, MD, University of Vermont College of Medicine and Vermont Regional Diabetes Center; Barbara L. Linder, MD, NIDDK, NIH*; Charlotte Ling, PhD, Lund University, Sweden; Christopher B. Newgard, PhD, Duke University; Robert E. Ratner, MD, American Diabetes Association; Rebecca A. Simmons, MD, University of Pennsylvania; Markus Stoffel, MD, PhD, Swiss Federal Institute of Technology (ETH), Switzerland; and C. Bruce Verchere, PhD, University of British Columbia, Canada.
*Unable to attend due to U.S. government shutdown.
Fellows: Claudia Cavelti-Weder, MD, University Hospital of Zurich, Switzerland; Andrea Giuseppe Daniele, MD, PhD, University of Texas Health Science Center; Daniel T. Meier, PhD, University of Washington; Sara Michaliszyn, PhD, Children’s Hospital of Pittsburgh; Marcel H.A. Muskiet, MD, VU University Medical Center, the Netherlands; Anders Olsson, PhD, Lund University Diabetes Centre, Sweden; Richard Oram, MD, University of Exeter, U.K.; Lane Jaeckle Santos, PhD, University of Pennsylvania; Marta Seghieri, MD, University of Pisa, Italy; Sam Stephens, PhD, Duke University; and Clara Westwell-Roper, MD/PhD candidate, University of British Columbia, Canada.
Footnotes
This article is based on a conference jointly sponsored by the Endocrine Society and the American Diabetes Association and supported by an educational grant from Novo Nordisk, Sanofi, and Lilly USA.
This article has been copublished in the Journal of Clinical Endocrinology & Metabolism.
References
- 1.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000;49:2201–2207 [DOI] [PubMed] [Google Scholar]
- 2.Ellard S, Flanagan SE, Girard CA, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 2007;81:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–1849 [DOI] [PubMed] [Google Scholar]
- 4.Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med 2000;17:543–545 [DOI] [PubMed] [Google Scholar]
- 5.Babenko AP, Polak M, Cavé H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006;355:456–466 [DOI] [PubMed] [Google Scholar]
- 6.Pearson ER, Flechtner I, Njølstad PR, et al. ; Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006;355:467–477 [DOI] [PubMed] [Google Scholar]
- 7.Pal A, McCarthy MI. The genetics of type 2 diabetes and its clinical relevance. Clin Genet 2013;83:297–306 [DOI] [PubMed] [Google Scholar]
- 8.Voight BF, Scott LJ, Steinthorsdottir V, et al. ; MAGIC Investigators; GIANT Consortium. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosengren AH, Braun M, Mahdi T, et al. Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes 2012;61:1726–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flannick J, Thorleifsson G, Beer NL, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 2014;46:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature 2009;461:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrechtsen A, Grarup N, Li Y, et al. ; D.E.S.I.R. Study Group; DIAGRAM Consortium. Exome sequencing-driven discovery of coding polymorphisms associated with common metabolic phenotypes. Diabetologia 2013;56:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 14.Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 2012;92:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RF, Fazzari MJ, Niu H, Barzilai N, Simmons RA, Greally JM. Experimental intrauterine growth restriction induces alterations in DNA methylation and gene expression in pancreatic islets of rats. J Biol Chem 2010;285:15111–15118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 2008;118:2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandovici I, Smith NH, Nitert MD, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA 2011;108:5449–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res 2001;2:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhandare R, Schug J, Le Lay J, et al. Genome-wide analysis of histone modifications in human pancreatic islets. Genome Res 2010;20:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stitzel ML, Sethupathy P, Pearson DS, et al. ; NISC Comparative Sequencing Program. Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab 2010;12:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayeh T, Volkov P, Salö S, et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet 2014;10:e1004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang BT, Dayeh TA, Volkov PA, et al. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 2012;26:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang BT, Dayeh TA, Kirkpatrick CL, et al. Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia 2011;54:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling C, Del Guerra S, Lupi R, et al. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 2008;51:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkmar M, Dedeurwaerder S, Cunha DA, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J 2012;31:1405–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayeh TA, Olsson AH, Volkov P, Almgren P, Rönn T, Ling C. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia 2013;56:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramswig NC, Everett LJ, Schug J, et al. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 2013;123:1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malmgren S, Spégel P, Danielsson AP, et al. Coordinate changes in histone modifications, mRNA levels, and metabolite profiles in clonal INS-1 832/13 β-cells accompany functional adaptations to lipotoxicity. J Biol Chem 2013;288:11973–11987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen DP, Dahllöf M, Lundh M, et al. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med 2011;17:378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 2013;9:513–521 [DOI] [PubMed] [Google Scholar]
- 31.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004;432:226–230 [DOI] [PubMed] [Google Scholar]
- 32.Poy MN, Hausser J, Trajkovski M, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci USA 2009;106:5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kameswaran V, Bramswig NC, McKenna LB, et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab 2014;19:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004;53(Suppl. 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 36.Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 38.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schisler JC, Jensen PB, Taylor DG, et al. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci USA 2005;102:7297–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen MV, Haldeman JM, Zhang H, et al. Control of voltage-gated potassium channel Kv2.2 expression by pyruvate-isocitrate cycling regulates glucose-stimulated insulin secretion. J Biol Chem 2013;288:23128–23140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schisler JC, Fueger PT, Babu DA, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 2008;28:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta 2010;1801:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruit JK, Wijesekara N, Fox JE, et al. Islet cholesterol accumulation due to loss of ABCA1 leads to impaired exocytosis of insulin granules. Diabetes 2011;60:3186–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Bruce Verchere C. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab 2012;14(Suppl. 3):68–77 [DOI] [PubMed] [Google Scholar]
- 45.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010;11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 47.Donath MY. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab 2013;15(Suppl. 3):193–196 [DOI] [PubMed] [Google Scholar]
- 48.Ferrannini E, Natali A, Muscelli E, et al. ; RISC Investigators. Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 2011;54:1507–1516 [DOI] [PubMed] [Google Scholar]
- 49.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arslanian SA, Bacha F, Saad R, Gungor N. Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth. Diabetes Care 2005;28:115–119 [DOI] [PubMed] [Google Scholar]
- 51.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012;97:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 56.Bonner-Weir S, O’Brien TD. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner. Diabetes 2008;57:2899–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 58.Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci 2013;1281:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonner-Weir S, In’t Veld PA, Weir GC. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab. 8 January 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Gialleonardo V, de Vries EF, Di Girolamo M, Quintero AM, Dierckx RA, Signore A. Imaging of β-cell mass and insulitis in insulin-dependent (type 1) diabetes mellitus. Endocr Rev 2012;33:892–919 [DOI] [PubMed] [Google Scholar]
- 61.Reiner T, Thurber G, Gaglia J, et al. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc Natl Acad Sci USA 2011;108:12815–12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clardy SM, Keliher EJ, Mohan JF, et al. Fluorescent exendin-4 derivatives for pancreatic β-cell analysis. Bioconjug Chem 2013;25:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neutzsky-Wulff AV, Andreassen KV, Hjuler ST, et al. Future detection and monitoring of diabetes may entail analysis of both β-cell function and volume: how markers of β-cell loss may assist. J Transl Med 2012;10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mari A, Pacini G. Methods for the assessment of β-cell function in vivo. In Clinical Diabetes Research: Methods and Techniques. Roden M, Ed. Hoboken, New Jersey, Wiley & Sons, 2007, p. 7–26 [Google Scholar]
- 65.Kitabchi AE, Temprosa M, Knowler WC, et al. ; Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFronzo RA, Tripathy D, Schwenke DC, et al. ; ACT NOW Study. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 2013;62:3920–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
- 69.Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–386 [DOI] [PubMed] [Google Scholar]
- 70.Foley JE, Bunck MC, Möller-Goede DL, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia 2011;54:1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005;90:5991–5997 [DOI] [PubMed] [Google Scholar]
- 72.Vilsbøll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med 2008;25:152–156 [DOI] [PubMed] [Google Scholar]
- 73.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009;150:2518–2525 [DOI] [PubMed] [Google Scholar]
- 75.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 77.Cummings DE. Metabolic surgery for type 2 diabetes. Nat Med 2012;18:656–658 [DOI] [PubMed] [Google Scholar]
- 78.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rhee NA, Vilsbøll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab 2012;14:291–298 [DOI] [PubMed] [Google Scholar]
- 80.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005;353:249–254 [DOI] [PubMed] [Google Scholar]
- 81.Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia 2010;53:2307–2311 [DOI] [PubMed] [Google Scholar]
- 82.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 84.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 85.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 2014;57:362–365 [DOI] [PubMed] [Google Scholar]