Abstract
Introduction
Acute pain is common during the endotracheal extubation period, and is related to complications and adverse outcomes. Patients with delayed extubation after craniotomy are vulnerable to pain and complications of extubation. However, pain control during extubation is still inadequate. Remifentanil, a new opioid with rapid onset and short duration of action, provides adequate analgesia during procedures with minimal effect of respiratory depression.
Methods and analysis
The study is a prospective, randomised, double-blinded, controlled parallel-group design. Patients with delayed extubation after intracranial surgery are screened daily. Adult patients ready for extubation are enrolled and assigned randomly to one of the two treatment study groups, labelled as the ‘Remi group’ or ‘Saline group’. Patients in the Remi group receive an intravenous bolus dose of remifentanil 0.5 μg/kg over 60 s followed by a continuous infusion 0.05 μg/kg/min for 20 min. Patients in the Saline group receive an intravenous infusion of 0.9% sodium chloride at a volume and rate equal to that of remifentanil. Pain intensity is measured by the visual analogue scale (VAS) pain score. Adverse events during drug infusion are documented and reported. Patients will be followed up until hospital discharge, death or 60 days after the trial intervention on a first come, first served basis. Details of the incidence of reintubation and reoperation within 72 h after extubation, length of stay in the intensive care unit and hospital and mortality are collected. The primary end point is the incidence of severe pain (defined as a VAS pain score more than 5 cm) during the periextubation period (defined as the period of time from immediately before extubation to 20 min after extubation).
Ethics and dissemination
The study was approved by the Institutional Review Board (IRB) of the Beijing Tiantan Hospital, Capital Medical University. The study findings will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number
ClinicalTrials (NCT): ChiCTR-PRC-13003879.
Strengths and limitations of this study.
Pain is common during the extubation period, and is related to complications and adverse outcomes. Adequate analgesia is needed in this situation. The main strength of the study is that we will provide the evidence of a new opioid (remifentanil), with minimal respiratory depression effect and a rapid onset and short duration of action, for prophylactic analgesia during extubation in patients after craniotomy.
Pain management is a comprehensive algorithm, which includes pharmacological and non-pharmacological interventions. In the present study, only remifentanil is investigated for prophylactic analgesia during extubation. This is the main limitation of the study. Since there is no proven dose of remifentanil which can prevent sedation and respiratory depression in a neurosurgical patient being weaned off the ventilator, the dose of remifentanil used in the present study is arbitrary.
The evaluation of a visual analogue scale requires the patient's ability to self-report clearly, and this may limit the patient population eligible for the present study. So the results of this study will not be applied to all patients after craniotomy, especially for those with consciousness impairments.
Introduction
Clinical epidemiology studies have shown that critically ill patients in the intensive care unit (ICU) are at high risk of pain, and that inadequate treatment of pain is associated with adverse outcomes.1 2 Appropriate analgesia not only attenuates stress response resulting from pain but also reduces the incidence of iatrogenic complications, as well as shortening the duration of mechanical ventilation and length of stay (LOS) in the ICU.3–5 In addition to pain at rest, pain related to procedures is common in ICU patients, and inadequate treatment of procedural pain remains a significant problem for many patients.6 The revised clinical practice guidelines for management of pain, agitation and delirium from the Society of Critical Care Medicine (SCCM) recommend that prophylactic analgesia should be used to alleviate pain in adult ICU patients prior to chest tube removal and other types of potentially painful procedures, and also suggest that intravenous opioids should be considered as the first-line drug class of choice.7 Manipulation of the artificial airway, especially endotracheal extubation, is among the high pain-intensity procedures in ICU. By using the patient's self-report pain scale as the pain evaluation method, Gacouin et al8 found that 45% of patients experienced severe pain at the time of endotracheal extubation. Acute pain during extubation is associated with sympathetic nervous system activation, which could result in respiratory and circulatory instability.9 Therefore, it is reasonable to control pain and stress responses at the time of endotracheal extubation in some high-risk patients, such as those after intracranial surgery. It has been reported that the proportion of patients who underwent delayed extubation after craniotomy is about 10–50%.10 11 These patients are vulnerable to pain and complications of extubation.12 On the other hand, despite a greater awareness of pain during endotracheal extubation, clinicians remain reluctant to administer opioids in patients following craniotomy. The major concern is the side effects of respiratory depression and influence on consciousness of these drugs. To the best of our knowledge, up to now, no study has been published for adequate management of pain during extubation in patients with delayed extubation after craniotomy.
Remifentanil is a potent synthetic selective μ-opioid receptor agonist with a rapid onset and short duration of action, regardless of the duration of its administration.13 14 Remifentanil differs from other synthetic opioids in its metabolism by non-specific plasma and tissue esterases. A study in human volunteers has shown that the respiratory depression of remifentanil by bolus injection is mild and easily treated with requests to breathe or the administration of oxygen.15 These pharmacological properties suggest that remifentanil could be a potentially safe and effective analgesic in clinical situations requiring a brief period of intense control of pain, such as painful procedures in the ICU.16 There have been reports of remifentanil being used as a prophylactic analgesia during removal of a chest drain,17 insertion and removal of a long-term central venous access,18 dressing change19 and endotracheal suctioning.20 However, although plenty of studies have shown that remifentanil facilitates an emergency in the general anaesthesia and weaning process in mechanical ventilation,14 16 21 studies for prophylactic use of remifentanil in endotracheal extubation are limited.
There has been increased interest in the use of remifentanil in brain injured patients. In patients with traumatic brain injury, it has been demonstrated that remifentanil has no significant changes in systematic and cerebral haemodynamics, such as intracranial pressure, mean blood pressure (BP), cerebral perfusion pressure and cerebral blood flow velocity.22 Several studies also compared remifentanil with fentanyl or morphine as an analgesic in neurological ICU patients. A randomised multicentre study in patients with brain injury showed that mean neurological assessment times were significantly shorter with remifentanil than with fentanyl or with morphine, and that patients were extubated significantly faster after remifentanil than after morphine.23 Another retrospective study investigated patients with delayed extubation after brain tumour surgery and found that mean extubation times were significantly shorter after remifentanil/propofol than after fentanyl/midazolam, and that LOS in the ICU was significantly reduced.24 These results indicate that the rapid metabolism and lack of accumulation of remifentanil facilitate faster waking and neurological assessment, and also suggest that remifentanil might be a better choice of analgesic in patients with brain injury.
In the present study, remifentanil is used as prophylactic analgesics in patients with delayed extubation after craniotomy. The aim is to evaluate the efficacy and safety of remifentanil for control of pain and stress responses due to extubation. The primary hypothesis is that the prophylactic use of remifentanil will reduce the incidence of severe pain during endotracheal extubation.
Methods and analysis
Study design overview
The present study is a prospective, single-centre, randomised, double-blinded, placebo-controlled two-arm trial in patients with delayed extubation after craniotomy.
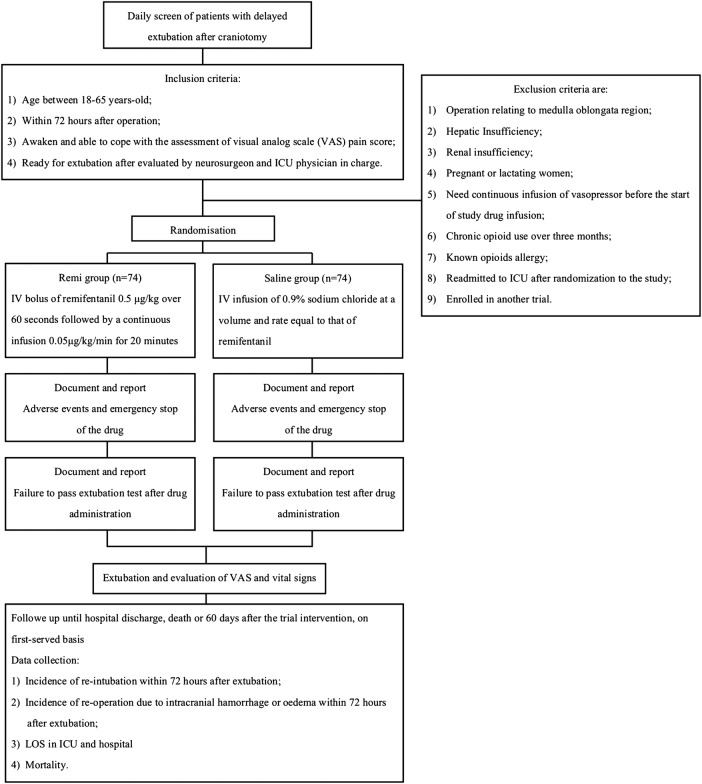
Trial schematic diagram is shown in figure 1.
Figure 1.
Trial schematic diagram (ICU, intensive care unit; IV, intravenous; LOS, length of stay).
Study setting and population
The study setting is a neurosurgical ICU (30 beds) at the Beijing Tiantan Hospital (1100 beds), Capital Medical University, Beijing, China.
All patients after intracranial surgery with delayed extubation admitted to our ICU are screened daily for study eligibility.
Inclusion criteria are:
Age between 18–65 years;
Within 72 h after operation;
Awake and able to cope with the assessment of the visual analogue scale (VAS) pain score;
Ready for extubation after evaluation by the neurosurgeon and the ICU physician in charge.
Exclusion criteria are:
Operation relating to the medulla oblongata region;
Hepatic insufficiency;
Renal insufficiency;
Pregnant or lactating women;
Need continuous infusion of the vasopressor before the start of the study drug infusion;
Chronic opioid use over 3 months;
Known opioids allergy;
Readmitted to the ICU after randomisation to the study;
Enrolled in another trial.
The patient's endotracheal tube is extubated at the discretion of the neurosurgeon and the ICU physician in charge of the patient. The neurosurgeon and the ICU physician discuss the patient's status and evaluate the patient by a screen checklist (table 1).10 When the answers of all items in a checklist are “yes”, the patient is ready for extubation. At this time, a 24 h on-call study coordinator will be informed immediately, and the patient's eligibility for the study is evaluated and confirmed.
Table 1.
Screening checklist used to determine the patient's suitability for extubation
| Question | Answer |
|---|---|
| 1. Awake and alert with cerebral function adequate for patient co-operation or equivalent preoperative state of consciousness? | Yes/no |
| 2. Haemodynamic stability (lack of vasopressor support and mean arterial pressure within 10–15% of baseline)? | Yes/no |
| 3. Adequate recovery of muscle strength? | Yes/no |
| 4. Normal tidal volumes, normocapnia (end-tidal carbon dioxide 30–45 mm Hg), minimum pulse oximetry >95% with FiO2 0.5? | Yes/no |
| 5. Intact gag reflex and swallow function (presence of clearly audible cough during suctioning)? | Yes/no |
The answer to all questions must be “yes” in order for extubation to be approved.
FiO2, fraction of inspired oxygen.
Randomisation, double-blind and allocation concealment
The study has a prospective, randomised, double-blinded, controlled parallel-group design. Randomisation is based on the computer-generated random digits table. The allocation sequence is sealed in numbered and opaque envelopes to ensure that the sequence is concealed. Enrolled patients are randomly assigned 1:1 to receive remifentanil (labelled as the Remi group) or placebo (labelled as the Saline group) infusion. Patients may be randomised into this study only once, unless they were discharged from the hospital and were readmitted beyond 180 days of the first enrolment. The study does not allow a cross-over and, if any occur, they will be reported as protocol violations.
Experimental drugs and placebos with the same character are prepared by a pharmacist. Patients and all study personnel except the investigative pharmacist are blind to treatment assignment. The details of the series are unknown to any of the investigators and are contained in a set of opaque and sealed envelopes, each bearing on the outside only the number.
Data collected at study entry
At baseline, data on the demographics, history of past illness characteristics and diagnosis of the patients are obtained. The surgical site, operation time, use of sedatives and analgesics during anaesthesia and ICU stay, time of mechanical ventilation, formulation and dose of postoperative patient-controlled-analgesia (PCA) pump, and time between end of operation and study drug infusion are recorded. Acute Physiology and Chronic Health Evaluation II score (APACHE II) is calculated.
Trial interventions
All patients are randomised 1:1 to receive remifentanil (Remi group) or placebo (Saline group) infusion. Patients in the Remi group receive an intravenous bolus dose of remifentanil 0.5 μg/kg over 60 s followed by a continuous infusion of 0.05 μg/kg/min for 20 min. Patients in the Saline group receive an intravenous infusion of 0.9% sodium chloride at a volume and rate equal to that of remifentanil. Study drugs are administered by using a syringe pump.
Immediately after drug infusion, the ICU physician evaluates the patient by using an extubation screen checklist shown in table 1. If the patient passes the evaluation, endotracheal extubation will be carried out immediately by registered ICU nurses. The patient will be labelled as “failing to pass extubation test after drug administration” if he or she does not pass the evaluation. The reason for test failure will be documented. The patient will be re-evaluated every hour thereafter, and data about extubation will be documented.
Vital signs, which include heart rate (HR), respiratory rate (RR), non-invasive BP and pulse oxygen saturation (SpO2), are monitored continuously. VAS pain score is used to measure the pain intensity by the study investigator.25 Each patient is instructed and asked to point to a mark on a 10 cm horizontal line labelled with descriptors of pain intensity (‘No pain’ at the 0 cm point and ‘Extreme pain’ at the 10 cm point). VAS and vital signs (HR, RR, BP and SpO2) are documented at four time points: before drug infusion (baseline), immediately before extubation, immediately to 3 min after extubation and 20 min after extubation.
Patients will be followed up until hospital discharge, death or 60 days after the trial intervention on a first come, first served basis. The following data are collected: incidence of reintubation within 72 h after extubation, incidence of reoperation due to intracranial haemorrhage or oedema within 72 h after extubation, LOS in the ICU and hospital, and mortality.
Adverse events management and emergency stop of the study drug
Patients are closely monitored during study drug infusion. Taking into account the potential adverse effects of remifentanil, experimental drugs must be terminated immediately when the following occur:
Unresponsive to calling and patting on the shoulder;
RR less than 8 respirations per minute and SpO2 less than 92%;
HR less than 50 bpm after atropine in a 0.25 mg bolus;
Systolic BP less than 90 mm Hg;
Serious allergic reactions such as throat swelling, bronchospasm or skin rash.
These data will be documented and reported as adverse events.
Study end points
The primary end point of the present study is the incidence of severe pain during the periextubation period. Periextubation is defined as the period of time from immediately before extubation to 20 min after extubation. Severe pain is defined as one of the VAS pain scores is more than 5 cm.
Secondary end points include:
VAS pain score and vital signs (HR, RR, BP and SpO2) during the periextubation period;
Incidence of failing to pass extubation evaluation after experimental drug infusion;
Incidence of reintubation within 72 h after extubation;
Incidence of reoperation due to intracranial haemorrhage or oedema within 72 h after extubation;
Incidence of adverse events during experimental drug infusion;
LOS in the ICU and hospital;
Mortality.
Current sample size justification
Primarily, we expect the incidence of severe pain during the periextubation period to decrease after remifentanil infusion in delayed extubation patients after craniotomy. Previous investigation showed that severe periextubation pain occurred in 45% of patients.8 It is expected that the incidence of severe pain would decrease to 30% after remifentanil infusion. Using the Power and Sample Size Calculation program, we will need to study 74 experimental participants and 74 control participants to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with a probability (power) of 0.8. The type I error probability with testing this null hypothesis is 0.05.
Statistical analysis
All analyses will be according to the intention-to-treat principle, that is, all randomised patients will be analysed in the groups to which they were originally allocated and will be blinded to treatment assignment.
Baseline characteristics will be summarised by univariate analyses. Categorical variables will be presented as numbers and percentages, and analysed by the χ2 test. Continuous variables will be checked for normal distribution and presented as the mean and SD or median and IQR as appropriate. Comparison of continuous variables will be performed by using Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables.
We use repeated measures of analysis of variance for comparing VAS pain score and vital signs (HR, RR, BP and SpO2) across different time points (before drug infusion and during the periextubation period) between the two groups.
All tests of significance will be at the 5% significance level and two-sided. Analyses are conducted by using SPSS V.17.0.
Ethics and dissemination
Ethical aspects and informed consent
Patients with delayed extubation after craniotomy are often unable to provide consent before extubation. After the patient's eligibility for the study is confirmed, the ICU physician will introduce the family to the study coordinator. The physician will make sure the family knows the credentials of the study coordinator, and says that this person is going to discuss a research programme that is being conducted, and that this person is qualified to do so. The study coordinator will take the family to a place where they can talk confidentially. Every relevant aspect of the project will be described. The study coordinator will stop frequently, ask if there are any questions, and request that the family repeat back in their own words what is being discussed, to make sure they understand.
The study coordinator will explain that the patient may experience pain during extubation and, if so, the patient could become worse. The coordinator will say that a new opioid drug, remifentanil, provides adequate analgesia with minimal respiratory depression. He (or she) will explain that in a small percentage of patients, remifentanil could cause bradycardia, hypotension and respiratory depression. The potential advantages of using or not using remifentanil will be described. The study coordinator will be especially careful to assure the family that they are free to decline consent without consequences and that they can withdraw consent at any time without impact on treatment. Family members will be provided with contact information for the study coordinator, local coinvestigator and the local ethical committee. Written consent will be obtained in the presence of a witness.
A register is kept of all patients evaluated for inclusion and of patients who withdraw from the study. The latter are clinically followed up without their data being analysed in the study.
The study protocol and consent forms were approved on 1 November 2013 by the Institutional Review Board of the Beijing Tiantan Hospital Affiliated to Capital Medical University (approval number KY2013-002-01). The study was registered on 8 November 2013 at the Chinese Clinical Trial Registry (ChiCTR-PRC-13003879).
Dissemination plan
Results of the trial will be submitted to an international peer-reviewed journal. Results will also be presented at national and international conferences relevant to the subject fields.
Trial status
The patient recruitment began on 6 January 2014, and the first patient was enrolled on the same day. The study will be completed in December 2014.
Summary
Endotracheal extubation is a critical step during postoperative care in neurosurgical patients.9 Pain is common during the extubation period, and is related to complications and adverse outcomes.8 Adequate analgesia is needed in this situation. Although the revised Clinical Practice Guidelines from SCCM have recommended that prophylactic analgesia should be used to alleviate pain in adult ICU patients prior to potentially painful procedures,7 studies for analgesia during endotracheal extubation are still limited. As a new opioid with minimal respiratory depression effect and a rapid onset and short duration of action, remifentanil seems suitable for prophylactic analgesia during extubation in patients after craniotomy.13–16 We hope to provide such evidence in the present study. Even a neutral result will provide an important insight, as this would mean that more studies are needed to explore a safe and effective way of pain management during endotracheal extubation. This is the main strength of the present study.
It should be emphasised the comprehensive characteristics of pain management. Apart from opioids, other analgesics (such as steroidal or non-steroidal anti-inflammatory drugs) and/or non-pharmacological interventions (such as relaxation) have been shown to alleviate pain in adult ICU patients.7 Although the main purpose of the present study is not to clarify the effect of comprehensive approach on manipulation of pain during extubation, we will collect data on the use of analgesics during anaesthesia and ICU stay, and the formulation and dose of the postoperative PCA pump. The use of non-opioids and non-pharmacological interventions during extubation needs to be investigated further.
Currently, vital signs are not recommended to be used alone for pain assessment.7 However, we still incorporated changes of vital signs during the periextubation period as a secondary end point for two reasons. First, nociceptive stimulus during endotracheal extubation may result in adverse events in patients after craniotomy, such as brain swelling and haemorrhage. These data will help to clarify whether remifentanil could diminish this stress response. Second, since large doses of opioid agents usually result in respiratory and circulatory depression, this secondary end point will provide safe consideration about the use of these agents.
There are some limitations to our study protocol. First, since there is no proven dose of remifentanil which can prevent sedation and respiratory depression in a neurosurgical patient being weaned off the ventilator, the dose of remifentanyl used in the present study is arbitrary. Second, we assumed a 15% reduction in severe pain after the administration of remifentanil in the calculation of the sample size. There are no previous studies to support this. However, an effectivity of 15% is generally adopted in clinical studies. Third, opioids may result in a change of consciousness. We only observe the response to calling and patting on the shoulder during the infusion of study agents. Documentation of sedation scales (such as the Vancouver interaction scale or sedation–agitation scale) will add value to the study. Finally, evaluation of VAS requires the patient's ability to self-report clearly, and this may limit the patient population eligible for the present study. So the results of this study will not be applied to all patients after craniotomy, especially for those with consciousness impairments.
Supplementary Material
Footnotes
Contributors: Y-XW and J-XZ participated in the design of the study and drafted the manuscript. HC participated in the design of the study. All authors edited the manuscript and read and approved the final manuscript.
Funding: The study was funded by the Beijing Health Bureau (No: 2009-3-28).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The IRB of the Beijing Tiantan Hospital, Capital Medical University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Payen JF, Chanques G, Mantz J, et al. . Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 2007;106:687–95 [DOI] [PubMed] [Google Scholar]
- 2.Chanques G, Sebbane M, Barbotte E, et al. . A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 2007;107:858–60 [DOI] [PubMed] [Google Scholar]
- 3.Robinson BR, Mueller EW, Henson K, et al. . An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma 2008;65:517–26 [DOI] [PubMed] [Google Scholar]
- 4.Payen JF, Bosson JL, Chanques G, et al. . DOLOREA Investigators. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology 2009;111:1308–16 [DOI] [PubMed] [Google Scholar]
- 5.Chanques G, Jaber S, Barbotte E, et al. . Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med 2006;34:1691–9 [DOI] [PubMed] [Google Scholar]
- 6.Puntillo KA, White C, Morris AB, et al. . Patients’ perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care 2001;10:238–51 [PubMed] [Google Scholar]
- 7.Barr J, Fraser GL, Puntillo K, et al. . American College of Critical Care Medicine: clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306 [DOI] [PubMed] [Google Scholar]
- 8.Gacouin A, Camus C, Le Tulzo Y, et al. . Assessment of peri-extubation pain by visual analogue scale in the adult intensive care unit: a prospective observational study. Intensive Care Med 2004;30:1340–7 [DOI] [PubMed] [Google Scholar]
- 9.Popat M, Mitchell V, Dravid R, et al. . Difficult Airway Society Extubation Guidelines Group. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012;67:318–40 [DOI] [PubMed] [Google Scholar]
- 10.Cai YH, Zeng HY, Shi ZH, et al. . Factors influencing delayed extubation after infratentorial craniotomy for tumour resection: a prospective cohort study of 800 patients in a Chinese neurosurgical centre. J Int Med Res 2013;41:208–17 [DOI] [PubMed] [Google Scholar]
- 11.Cata JP, Saager L, Kurz A, et al. . Successful extubation in the operating room after infratentorial craniotomy: the Cleveland Clinic experience. J Neurosurg Anesthesiol 2011;23:25–9 [DOI] [PubMed] [Google Scholar]
- 12.Bruder N, Ravussin P. Recovery from anesthesia and postoperative extubation of neurosurgical patients: a review. J Neurosurg Anesthesiol 1999;11:282–93 [DOI] [PubMed] [Google Scholar]
- 13.Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 1999;89(Suppl 4):S7–S14 [DOI] [PubMed] [Google Scholar]
- 14.Beers R, Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs 2004;18:1085–104 [DOI] [PubMed] [Google Scholar]
- 15.Egan TD, Kern SE, Muir KT, et al. . Remifentanil by bolus injection: a safety, pharmacokinetic, pharmacodynamic, and age effect investigation in human volunteers. Br J Anaesth 2004;92:335–43 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm W, Kreuer S. The place for short-acting opioids: special emphasis on remifentanil. Crit Care 2008;12(Suppl 3):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey E, Lane A, Kuriakose D, et al. . Bolus remifentanil for chest drain removal in ICU: a randomized double-blind comparison of three modes of analgesia in post-cardiac surgical patients. Intensive Care Med 2010;36:1380–5 [DOI] [PubMed] [Google Scholar]
- 18.Burlacu CL, McKeating K, McShane AJ. Remifentanil for the insertion and removal of long-term central venous access during monitored anesthesia care. J Clin Anesth 2011;23:286–91 [DOI] [PubMed] [Google Scholar]
- 19.Le Floch R, Naux E, Pilorget A, et al. . Use of remifentanil for analgesia during dressing change in spontaneously breathing non-intubated burn patients. Ann Burns Fire Disasters 2006;19:136–9 [PMC free article] [PubMed] [Google Scholar]
- 20.Leone M, Albanese J, Viviand X, et al. . The effects of remifentanil on endotracheal suctioning-induced increases in intracranial pressure in head-injured patients. Anesth Analg 2004;99:1193–8 [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Yang SY, Na SW, et al. . Low-dose remifentanil infusion during ventilator weaning and tracheal extubation in postoperative intensive care unit patients sedated with propofol-remifentanil: a randomised clinical trial. Anaesth Intensive Care 2012;40:656–62 [DOI] [PubMed] [Google Scholar]
- 22.Engelhard K, Reeker W, Kochs E, et al. . Effect of remifentanil on intracranial pressure and cerebral blood flow velocity in patients with head trauma. Acta Anaesthesiol Scand 2004;48:396–9 [DOI] [PubMed] [Google Scholar]
- 23.Karabinis A, Mandragos K, Stergiopoulos S, et al. . Safety and efficacy of analgesia-based sedation using remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial [ISRCTN50308308]. Crit Care 2004;8:R268–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer C, Kreuer S, Ketter R, et al. . Remifentanil-propofol versus fentanyl-midazolam combinations for intracranial surgery: influence of anaesthesia technique and intensive sedation on ventilation times and duration of stay in the ICU. Anaesthesist 2007;56:128–32 [DOI] [PubMed] [Google Scholar]
- 25.Ho K, Spence J, Murphy MF. Review of pain-measurement tools. Ann Emerg Med 1996;27:427–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.