Abstract
Lipocalin 2 (Lcn2) has been previously characterized as an adipokine/cytokine and implicated in obesity and inflammation. Herein, we investigated the role and potential mechanism of Lcn2 in the regulation of macrophage polarization in obesity-associated inflammation. We observed that Lcn2−/− mice displayed an up-regulation of expression of M1 macrophage marker Cd11c but a down-regulation of M2 marker arginase 1 in adipose tissue and liver of mice upon a high-fat diet feeding. Lcn2-deficient bone marrow–derived macrophages (BMDMs) were more sensitive to lipopolysaccharide (LPS) stimulation, leading to a more profound up-regulation of expression of pro-inflammatory markers than wild-type (WT) BMDMs. Accordingly, LPS stimulation elicited an increase in the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), c-Jun, and STAT3 signaling pathways as well as an up-regualtion of expression of NF-κB and STAT3 target genes such as IL-1β, IL-6, iNOS, and MCP-1 in Lcn2−/− BMDMs compared with WT controls. Pre-treatment of recombinant Lcn2 attenuated LPS-stimulated degradation of IκBα and STAT3 phosphorylation as well as LPS-induced gene expression of IL-6 and iNOS in Lcn2−/− BMDMs. Moreover, the NFκB inhibitor markedly blocked LPS-stimulated STAT3 phosphorylation in Lcn2−/− BMDMs. These results together with the time course of Lcn2 secretion, NFκB and STAT3 phosphorylation in response to LPS stimulation, suggest that Lcn2 plays a role as an anti-inflammatory regulator in macrophage activation via modulating a feed-forward activation of NFκB-STAT3 loop.
Low-grade chronic inflammation in adipose tissue is one of the characteristics of obesity and plays an important role in the development of insulin resistance, diabetes, and metabolic complications (1). Among various types of cells in adipose tissue, macrophages are the major contributor to adipose tissue inflammation in obesity (2, 3). Adipose tissue macrophages (ATMs) can exhibit either classically (M1) or alternatively (M2) activated state depending on the inflammatory state in adipose tissue (4).
In obese subjects and rodents, ATMs are polarized toward an M1 phenotype, accompanied with the up-regulation of expression of proinflammatory cytokines. M1 macrophage-produced inflammatory cytokines including TNFα, IL-6, and IL-1β interfere with insulin-signaling transduction in adipocytes, resulting in insulin resistance (3). Studies have demonstrated that M1 macrophages are activated by proinflammatory stimuli such as lipopolysaccharide (LPS) and interferon-gamma via activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (5). In contrast, in lean or IL-4/IL-13-stimulated state, ATMs can be switched to a M2 subtype with the up-regulation of expression of anti-inflammatory cytokines/markers including IL-10, Ym1 (T-lymphocyte-derived eosinophil chemotactic factor), and Arginase 1 (Arg1), leading to the improvement of insulin sensitivity (6, 7).
Free fatty acids and adipokines that are released from adipocytes are important regulators of ATMs' recruitment and activation. It is well known that free fatty acids induce the activation of IkB kinase β and c-Jun N-terminal kinase (JNK) inflammatory pathways in adipose tissue, liver, and skeletal muscle, leading to cellular inflammation and insulin resistance (8–11). However, which adipokines regulate M1/M2 polarization and the mechanisms involved during obesity remain largely unknown.
Lipocalin 2 (Lcn2), also known as neutrophil gelatinase-associated lipocalin, is a recently identified adipokine (12, 13). Lcn2, as a member of the lipocalin subfamily, is a 25 kDa secreted protein and has binding capabilities for hydrophobic molecules including retinoids, fatty acids, and various steroids (14). Lcn2 promoter region contains the binding sites of several transcription factors such as NF-κB, STAT1, STAT3, CREB, and C/EBPβ (15–17), suggesting a potential role of Lcn2 in the control of inflammation and metabolism.
Emerging evidence has shown that Lcn2 is highly up-regulated when exposed to inflammatory conditions and plays a role in the modulation of macrophage activation in lung, liver, and brain (18–20) (21). However, little is known about the role and mechanism of Lcn2 in adipose tissue inflammation in obesity. We have previously shown that Lcn2 gene expression is induced by LPS in RAW264.7 macrophages. Recombinant Lcn2 attenuates LPS-stimulated gene expression of inflammatory cytokines (13). Our in vivo studies have demonstrated that Lcn2−/− mice on high-fat diet (HFD) displayed an up-regulation of expression of inflammatory cytokines such as MCP-1 and TNFα, but a down-regulation of anti-inflammatory Arg1 in adipose tissue (22). Lcn2−/− mice also exhibited decreased peroxisome proliferator-activated receptor gamma (PPARγ) expression in adipose tissues (22, 23). PPARγ has been known to regulate the recruitment and function of M2 macrophages in obesity (24).
In the current study, we investigated the role and potential mechanism for Lcn2 in the regulation of inflammation using bone marrow–derived macrophages (BMDM) and elicited peritoneal macrophages from Lcn2−/− mice. We observed that Lcn2−/− mice exhibited an up-regulation of expression of M1 marker Cd11c, but a down-regulation of M2 marker Arg1 in primary macrophages as well as adipose tissue and liver. Lcn2 deficiency led to the increased activation of LPS-induced inflammatory-signaling pathways including NF-κB, c-Jun, and STAT3 in BMDMs. recombinant Lcn2 exerted an anti-inflammatory effect in BMDM by reducing LPS-stimulated IκBα degradation and STAT3 phosphorylation. The NFκB inhibitor markedly blocked LPS-stimulated STAT3 phosphorylation in Lcn2−/− BMDMs. We also found that the effect of PPARγ agonist rosiglitazone on attenuating LPS-stimulated IκBα degradation and cytokine production was reduced in Lcn2−/− BMDMs. Our data suggest that Lcn2 plays a role as an anti-inflammatory factor in regulating M1/M2 polarization via modulating NF-κB-STAT3 loop activation.
Materials and Methods
Animals
As we described previously (22), wild type (WT) and Lcn2-null (Lcn2−/−) mice on a C57BL/6 background were used in this study. Mice were housed in a specific pathogen-free facility and were given free access to water and food. All experimental procedures were approved by the University of Minnesota animal care and use committee. Animal handling was performed according to National Institutes of Health guidelines.
From 4 weeks of age, male WT and Lcn2−/− mice were fed either a regular-chow diet (RCD) or a HFD (fat calories, 60%, F3282, Bio-Serv) for 12 weeks. These age-matched mice were used for tissue collection, macrophages isolation, and primary peritoneal macrophages harvest, respectively.
Isolation and culture of BMDM
BMDMs were isolated according to the protocol described previously (25, 26). In brief, bone marrows were aseptically collected from tibias and femurs of four to five mice on RCD at age 16 weeks. Bone marrows were flushed out from bone cavity by cold sterile RPMI 1640 (Life Technologies) and passed through a 70-μm nylon mesh (Cell strainer, Becton Dickinson) to remove cell clumps and bone fragments. After centrifuge, red blood cells were removed by red cell lysis buffer (Sigma-Aldrich) and fibroblasts in bone marrow were removed by plastic adherence. The remaining cells were then seeded into six-well plates and cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals), L-glutamine (2 mmol/L, Sigma-Aldrich), and 1% penicillin-streptomycin (Life Technologies). The bone marrow monocytes/macrophage progenitors were induced to proliferate and differentiate into mature BMDM by mouse recombinant macrophage colony-stimulating factor (CSF) (10 ng/ml, R&D Systems). The culture media were replaced by fresh ones every 2–3 days. On day 7, nonadherent cells were washed by PBS. Adhesive cells were used for in vitro experiments.
Collection and culture of peritoneal macrophages
Peritoneal macrophages were collected from four mice by peritoneal lavage 4 days after injection of 2 ml of 3% thioglycolate medium (Sigma-Aldrich). The cells were then cultured in RPMI 1640 containing 10% fetal bovine serum and 1% penicillin-streptomycin for 2 days in a 37°C and 5% CO2 incubator followed by LPS stimulation.
RNA isolation and relative quantitative RT-PCR
Total RNA was extracted from frozen tissues with TRIZOL reagent (Invitrogen). First-strand cDNA was synthesized from DNase-treated total RNA using a Superscript II reverse transcriptase kit (Invitrogen). Quantitative amplification by PCR was carried out using SYBR Green qPCR Master Mix (SABiosciences) by a StepOne Real-Time PCR System (Applied Biosystem). The ΔΔCt method was used to calculate the results. For quantification, β-actin mRNA served as an endogenous control. The primer sequences for amplifying the target genes and the GenBank accession number are summarized in Supplemental Table 1.
Western blot analysis
The BMDMs and peritoneal macrophages were homogenized in a lysis buffer before subjection to Western blot analysis with specific antibodies. The primary antibodies included goat polyclonal antibody against Lcn2 and neutralizing antibody against IL-6 (R&D Systems), mouse monoclonal antibodies to NF-κB and c-Jun, and rabbit monoclonal antibodies to phospho-NF-κB p65, phospho-c-Jun, IκBα, phospho-p38 MAPK, p38 MAPK, phospho-STAT3 (Tyr705), STAT3, and β-actin, (Cell Signaling Technology). Densitometric quantification was determined using an image analysis program (Alpha Innotech) and reported as a ratio to total protein or β-actin as suggested in the result sections.
Statistical analysis
Results are expressed as mean ± SEM. A two-tailed Student t test was used to test for differences between genotypes or treatment. P < .05 was considered significant.
Results
Lcn2 expression is induced by LPS in BMDMs and peritoneal macrophages
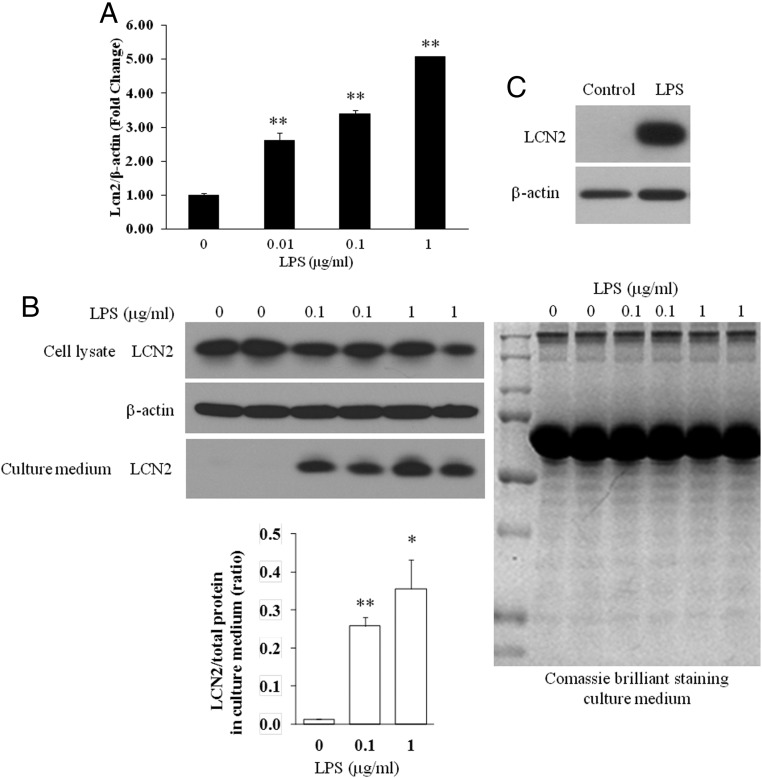
It has been known that most of macrophages in adipose tissue are derived from bone marrow and the macrophage content in adipose tissue is associated with the severity of obesity (2, 27). We have previously shown that LPS strongly induced Lcn2 gene expression in RAW264.97 macrophages (13). In this study, we examined the regulation of Lcn2 expression and secretion by LPS in BMDMs and peritoneal macrophages. We found that Lcn2 gene expression was up-regulated by LPS stimulation in BMDMs isolated from normal mice in a dose-dependent manner (Figure 1A). As shown in Figure 1B, LPS treatment for 3 hours led to a slight decrease or no change in intracellular Lcn2 protein, but a significant increase in secreted Lcn2 in the culture medium of BMDMs. Consistently, in peritoneal macrophages isolated from normal mice, the Lcn2 protein expression was markedly increased with 6-hour LPS treatment (Figure 1C).
Figure 1.
LPS induction of Lcn2 mRNA and protein expression in macrophages. A, Lcn2 mRNA expression in BMDMs treated with different doses of LPS for 6 h. B, Lcn2 protein expression (40 μg of total protein loaded) and secretion in the culture medium (40μl loaded) of BMDMs treated with different doses of LPS for 3 h (left panels). Comassie brilliant blue staining of the SDS-PAGE gel is shown as a loading control of culture medium (right panel). C, Lcn2 protein expression (20 μg of total protein loaded) in peritoneal macrophages treated with LPS (1μg/ml) for 6 h. All values were mean ± SEM (n = 3). *, P < .05; **, P < .01 vs control.
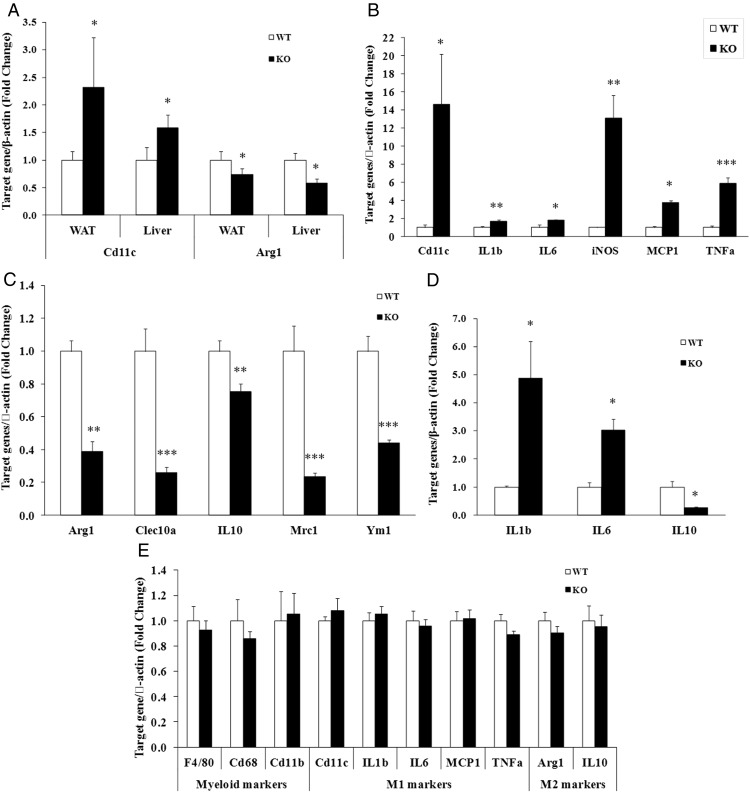
Lcn2 deficiency alters macrophage polarization in adipose tissue, liver, BMDMs, and peritoneal macrophages
We have previously reported that the gene expression of MCP1 and TNFα was up-regualted in Lcn2−/− mice fed a HFD (22). To explore further the role of Lcn2 in macrophage activation in adipose tissue during obesity, we first examined the gene expression of M1 and M2 macrophage markers to suggest the status of macrophage polarization in visceral adipose tissue and liver of Lcn2−/− mice upon a HFD feeding. The results showed that the gene expression of M1 marker Cd11c was increased whereas M2 marker Arg1 was decreased (Figure 2A) in epididymal adipose tissue and liver in Lcn2−/− mice compared with those in WT mice fed a HFD. We next assessed the direct effect of Lcn2 deficiency on the activation of BMDMs. BMDMs were isolated from WT and Lcn2−/− mice on a RCD and treated with LPS for M1 or IL4 for M2 polarization (28). Lcn2 deficiency resulted in the up-regulation of gene expression of M1 markers and proinfalmmatory cytokines such as Cd11c, IL-1β, IL-6, iNOS, MCP1, and TNFα in LPS-treated BMDMs (Figure 2B), whereas it led to the down-regulation of M2 markers including Arg1, Clec10a, IL-10, Mrc1, and Ym1 in IL4-treated BMDMs (Figure 2C). Consistent with BMDMs, peritoneal macrophages isolated from HFD-fed Lcn2−/− mice showed a similar change in gene expression of IL-1β, IL-6, and IL-10 in response to LPS stimulation (Figure 2D).
Figure 2.
Effect of Lcn2 deficiency on the expression of M1 and M2 macrophage markers in tissues and macrophages. A, Gene expression of macrophage M1 marker Cd11c and M2 marker Arg1 in epididymal white adipose tissue (WAT) and liver from mice on HFD (n = 8 in each group). B, Gene expression of M1 markers and proinflammatory molecules by LPS stimulation (1 μg/ml) for 6 h (n = 4). C, M2 markers by IL4 treatment (10 ng/ml) for 24 h in BMDMs (n = 5) isolated from RCD-fed mice. Cytokine mRNA expression in peritoneal macrophages treated with LPS (1 μg/ml) for 6 h. D, (n = 4, peritoneal macrophages were isolated from RCD-fed mice). The values were mean ± SEM. *, P < .05; **, P < .01; ***, P < .001 vs WT.
To test the possibility that Lcn2 deficiency affects macrophage differentiation, we examined the gene expression of myeloid markers as well as M1 and M2 macrophage markers in WT and Lcn2−/− bone marrow–derived cells after the incubation with macrophage CSF under nonstimulated conditions. As shown in Figure 2E, the basal levels of myeloid markers F4/80, CD68, and Cd11b as well as M1 and M2 markers including Cd11c, IL-1β, IL-6, MCP1, TNFα, Arg1, and IL-10 were not different between WT and Lcn2−/− BMDMs. This result suggests that Lcn2 does not influence the proliferation and differentiation of macrophages.
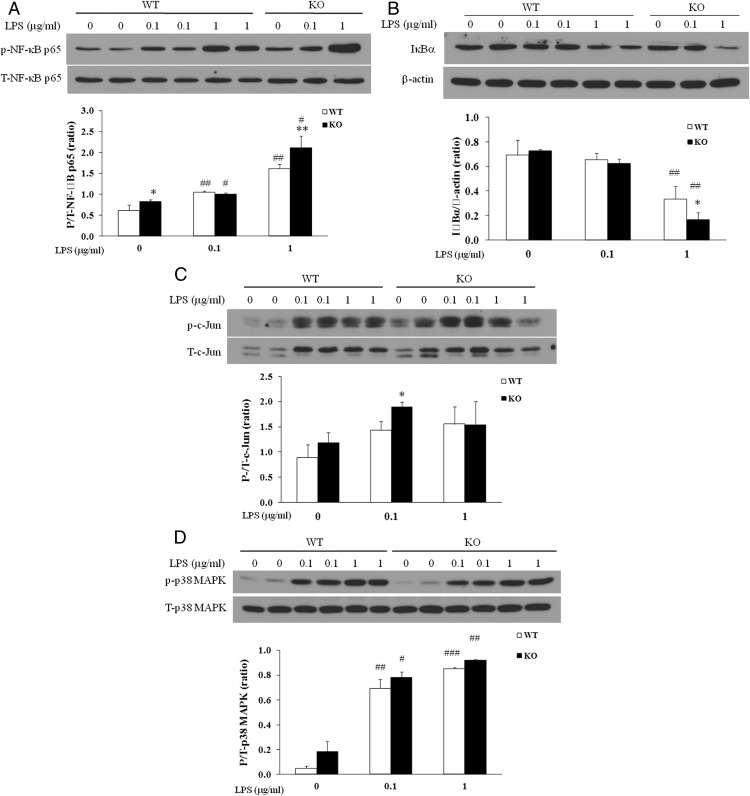
Lcn2 deficiency results in increased activation of LPS-stimulated NFκB signaling pathway
It has been known that LPS induces the activation of multiple proinflammatory transcription factors and signaling pathways, including NF-κB, the MAPK p38, and JNK, leading to the activation of M1 macrophages. Among these signaling pathways, NF-κB signaling pathway plays a central role in mediating inflammatory signals and controlling the production of proinflammatory mediators. We subsequently examined the activation of inflammatory signaling pathways in WT and Lcn2−/− BMDMs treated with the TLR4 ligand LPS. Our results showed that LPS treatment for 15 minutes led to an increase in NF-κB phosphorylation (Figure 3A) and an enhancement in IκBα degradation (Figure 3B) in Lcn2−/− BMDMs compared with WT cells. We also observed that the phosphorylation of c-Jun was increased after 3-hour treatment with LPS at the dose of 0.1 μg, but not 1.0 μg in Lcn2−/− BMDMs compared with WT cells (Figure 3C). However, Lcn2 deficiency did not affect LPS-stimulated p38MAPK phosphorylation; LPS treatment for 1 hour induced p38MAPK phosphorylation to a similar extent in WT and Lcn2−/− BMDMs (Figure 3D).
Figure 3.
Effect of Lcn2 deficiency on the activation of inflammatory signaling pathways in BMDMs in response to LPS stimulation. Representative Western blots for A, phosphorylated NFκB p65; B, IκBα degradation; C, phosphorylated c-Jun; and D, phosphorylated p38 MAPK in BMDMs stimulated with LPS at the indicated doses for 15 min, 3 h, and 1h, respectively. The experiments were repeated two to three times. The values were mean ± SEM (n = 3). *, P < .05; **, P < .01 vs WT; #, P < .05; ##, P < .01; ###, P < .001 vs no LPS.
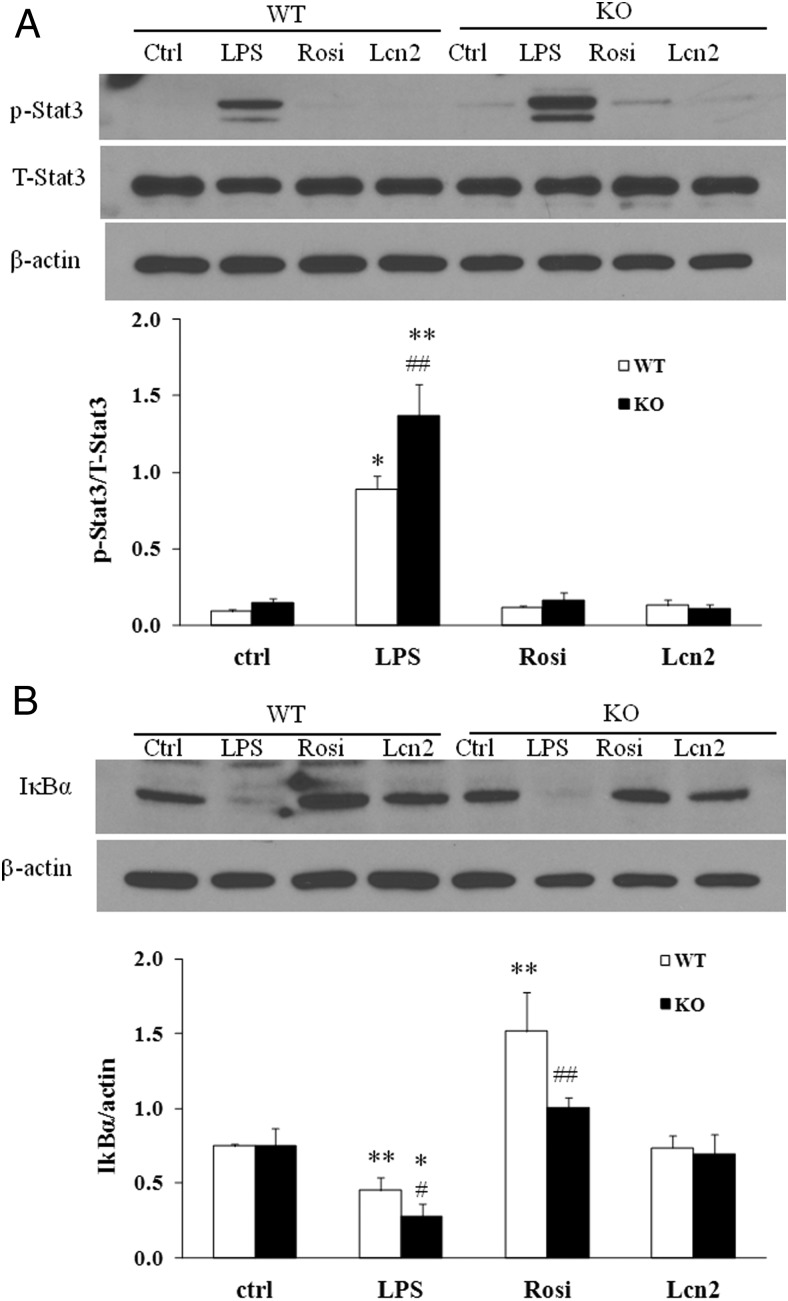
Lcn2 deficiency results in increased activation of LPS-stimulated STAT3 signaling pathway
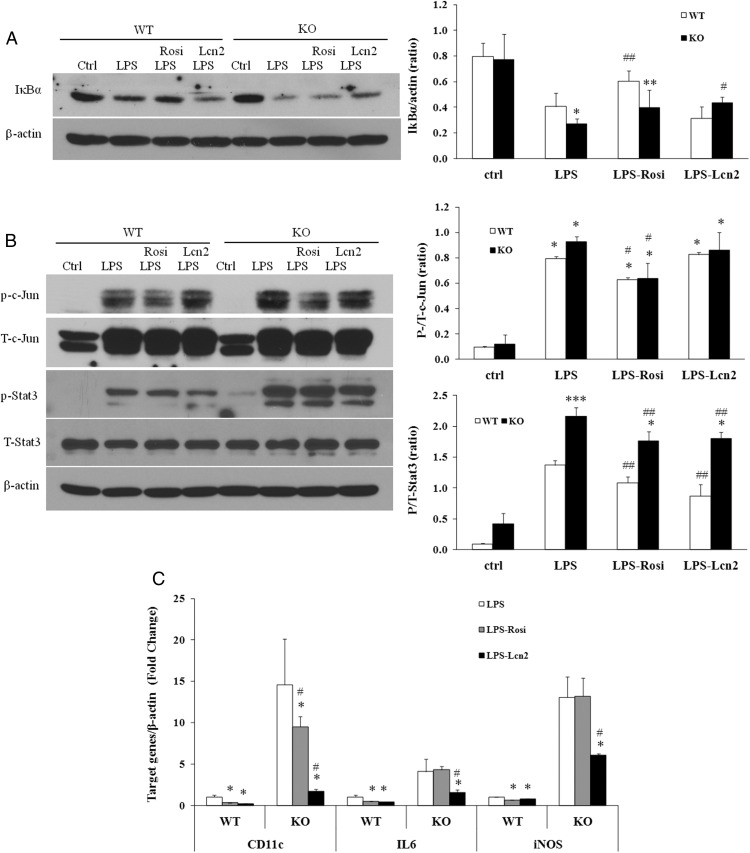
Signal transducer and activator of transcription (STAT) family proteins, especially STAT3 is highly interconnected with NF-κB signaling (29–31). Studies in cancer cells have demonstrated that there is a direct interaction between STAT3 and NF-κB, leading to a trap of NF-κB in the nucleus, thereby contributing to the constitutive activation of NF-κB and inflammation (32). As shown in Figure 2, B and C, STAT3 and NF-κB target genes such as IL-1β, IL-6, iNOS, MCP-1, and IL-10 were significantly altered in Lcn2−/− BMDMs, suggesting that Lcn2 plays a role in controlling the activation of NF-κB-STAT3 loop. Therefore, we examined whether Lcn2 deficiency alters STAT3 pathway activation and if Lcn2 directly regulates NF-κB and STAT3 activity. We treated BMDMs from WT and Lcn2−/− mice with recombinant Lcn2 and assessed the phosphorylation of STAT3 and the degradation of IκBα; LPS and rosiglitazone (Rosi) served as a positive and negative control, respectively, for the activation of these two inflammatory signaling pathways. Interestingly, LPS treatment for 2 hours induced much stronger phosphorylation of STAT3 in Lcn2−/− BMDMs compared with WT cells (Figure 4A). The treatment of recombinant Lcn2 alone or Rosi had no effect on STAT3 phosphorylation in WT and Lcn2−/− BMDMs (Figure 4A). As expected, LPS significantly stimulated IκBα degradation whereas Rosi inhibited IκBα degradation in WT BMDMs (Figure 4B), even under the nonstimulated condition; this inhibitory effect of Rosi was diminished in Lcn2−/− BMDMs (Figure 4B). However, recombinant Lcn2 alone showed no significant effect on IκBα degradation in WT and Lcn2−/− BMDMs under the nonstimulated condition (Figure 4B).
Figure 4.
Effect of recombinant Lcn2 on the activation of inflammatory signaling pathways in BMDMs under the nonstimulated condition. Representative Western blots for A, phosphorylated STAT3; and B, IκBα degradation in BMDMs. The BMDMs were treated with Rosi (1 μM) or recombinant Lcn2 (500 ng/ml) for 24 h. The control and LPS groups were treated without or with LPS (1 μg/ml) for 2 h. The experiments were repeated two to three times. The values were mean ± SEM (n = 3). *, P < .05; **, P < .01; ***, P < .001 vs WT; #, P < .05; ##, P < .01 vs LPS.
Recombinant Lcn2 attenuates LPS-stimulated NF-κB and STAT3 pathway activation and the M1 polarization in Lcn2−/− BMDMs
In this experiment, we further examined whether Lcn2 exerts an anti-inflammatory effect as suggested by breaking the NF-κB-STAT3 loop. Mouse recombinant Lcn2 and Rosi were added to LPS-treated WT and Lcn2−/− BMDMs and their effect on NF-κB and STAT3 phosphorylation as well as the expression of cytokines IL-6, iNOS, and Cd11c were evaluated. First, we showed that the pretreatment of recombinant Lcn2 reduced LPS-stimulated IκBα degradation (Figure 5A) in Lcn2−/− but not WT BMDMs. Secondly, LPS treatment consistently caused a more profound increase in STAT3 phosphorylation in Lcn2−/− BMDMs compared with WT cells (Figure 5B). Rosi had no significant effect on LPS induction of STAT3 phosphorylation in both WT and Lcn2−/− BMDMs (Figure 5B). However, Lcn2 was able to reduce LPS-induced STAT3 phosphorylation in WT and Lcn2−/− BMDMs (Figure 5B) although the STAT3 phosphorylation still remained at a higher level in Lcn2−/− BMDMs than WT cells with Lcn2 treatment (Figure 5B). Third, we examined the effect of recombinant Lcn2 on the gene expression of M1 marker Cd11c and inflammatory cytokines IL6 and iNOS. As shown in Figure 5C, the gene expression of Cd11c, IL6, and iNOS was more profoundly up-regulated by LPS stimulation in Lcn2−/− BMDMs compared with WT cells. In WT BMDMs, both Rosi and recombinant Lcn2 treatment was able to reduce the LPS-stimulated expression of Cd11c, IL6, and iNOS. Interestingly, in Lcn2−/− BMDMs, the LPS-induced expression of Cd11c, IL6, and iNOS was significantly suppressed by recombinant Lcn2 treatment. However, Rosi failed to efficiently inhibit the LPS stimulation of Cd11c, IL6, and iNOS expression, which is in accordance with no effect of Rosi on STAT3 pathway activation in Lcn2−/− BMDMs.
Figure 5.
Effect of recombinant Lcn2 on the activation of inflammatory signaling pathways and the expression of macrophage markers under the LPS-stimulated condition in BMDMs. Representative Western blots for A, IκBα degradation; and B, phosphorylated c-Jun and STAT3 in BMDMs with the pretreatment of Rosi (1 μM) or recombinant Lcn2 (500 ng/ml) for 24 h, followed by LPS (1 μg/ml) stimulation for 15 min for IκBα degradation and 3 h for phosphorylation of c-Jun and STAT3. C, Gene expression of M1 macrophage markers in LPS-stimulated BMDMs. The isolated BMDMs were pretreated with Rosi (1 μM) or recombinant Lcn2 (500 ng/ml) for 24 h, followed by the stimulation of LPS (1 μg/ml) for 6 h. The experiments were repeated two to three times. The values were mean ± SEM (n = 3). *, P < .05; **, P < .01; ***, P < .001 vs WT; #, P < .05; ##, P < .01 vs LPS.
Inhibiting NFκB blocks the overactivation of STAT3 signaling pathway in Lcn2−/− BMDMs
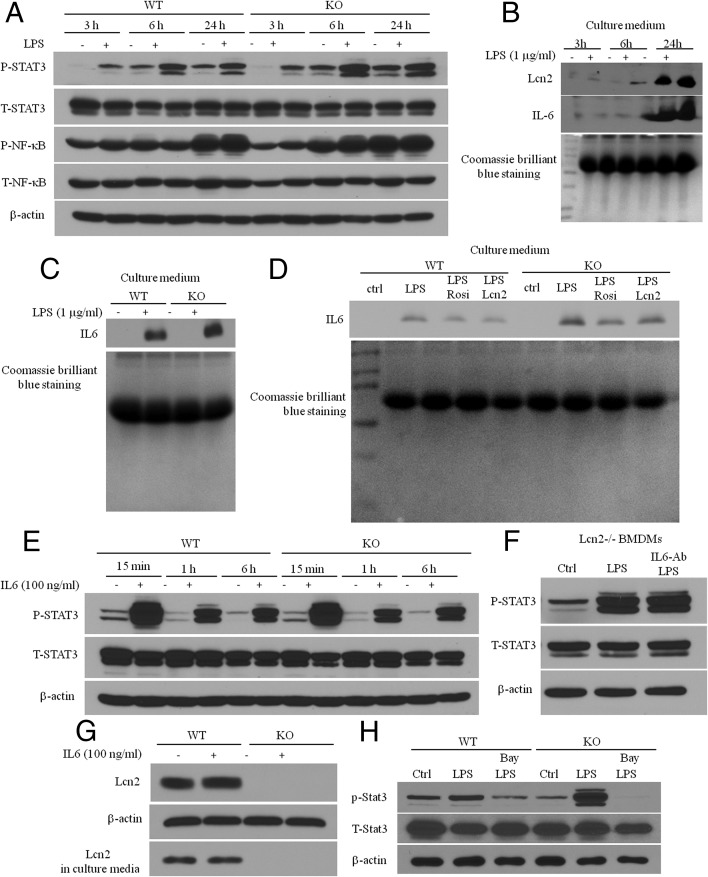
To demonstrate the hypothesis that Lcn2 exerts its anti-inflammatory effect by controlling NF-κB-STAT3 loop activation, we performed the time course of Lcn2 secretion, NFκB phosphorylation, and STAT3 phoshorylation in response to LPS stimulation and correlated Lcn2 levels to NFκB and STAT3 phosphorylation. We have already shown in Figure 3A that NFκB phosphorylation was rapidly induced after 15 minutes of LPS treatment. As shown in Figure 6A, LPS-stimulated NFκB phosphorylation was significantly blunted after 3-hour treatment and disappeared after 6-hour and 24-hour treatment in WT BMDMs. However, the response of NFκB phosphorylation to LPS stimulation remained longer (after 6-h treatment) and stronger in Lcn2−/− BMDMs compared with WT cells. Interestingly, compared with NFκB, STAT3 phosphorylation in response to LPS stimulation was delayed. We were unable to detect significant STAT3 phosphorylation with 15 minutes of LPS treatment (data not shown). After 3-hour treatment, LPS-stimulated STAT3 phosphorylation was increased and reached the maximal levels at the time point of 6-hour treatment, and then declined after 24-hour treatment (Figure 6A). These results demonstrate that LPS-induced NFκB activation occurs prior to STAT3 phosphorylation. In addition, the time-course study showed that Lcn2 secretion was markedly increased over the 24 hours of LPS treatment; Lcn2 accumulated in the culture medium reached the peak level after 24-hour treatment of LPS (Figure 6B). Our results clearly demonstrate that NFκB activation occurs first, followed by STAT3 activation and Lcn2 secretion in response to LPS stimulation. When Lcn2 secretion reaches the maximal level after 24-hour LPS treatment, STAT3 phosphorylation declines, suggesting the role of Lcn2 as an autocrine/paracrine factor in inhibiting NF-κB-STAT3 loop activation.
Figure 6.
Mechanism for Lcn2 effect on NFκB-STAT3 loop activation. The time-course of A, NFκB and STAT3 phosphorylation; B, and Lcn2 and IL-6 secretion in the culture medium (15μl loaded) in response to LPS stimulation in BMDMs isolated from RCD-fed mice. C, IL-6 secretion in BMDMs treated with LPS (1 μg/ml) for 24 h. D, The effect of recombinant Lcn2 on 24-h LPS stimulation of IL-6 secretion. E, The time-course of IL-6-stimulated STAT3 phosphorylation in BMDMs. F, The effect of anti-IL-6 antibody on LPS stimulation of STAT3 phosphorylation; BMDMs were preincubated with anti-IL6 antibody (1 μg/ml) for 1 h, followed by the stimulation of LPS (1 μg/ml) for 6 h. G, IL-6 stimulation of Lcn2 expression and secretion in BMDMs. H, Representative Western blots for phosphorylated STAT3 in BMDMs with pretreatment of Bay 11–7082 for 1 h followed by 2-h LPS treatment. The experiments were repeated two to three times.
IL-6 has been known to play a role as a STAT3 activator in potentially linking NF-κB to STAT3 activation and maintaining the persistent activation of NF-κB-STAT3 loop in cancer cells (33–35). Next, we explored whether IL-6 contributes to Lcn2 deficiency–caused overactivation of NFκB-STAT3 loop. First, we detected IL-6 secretion from WT and Lcn2−/− BMDMs treated with LPS for 24 hours using Western blotting. As expected, LPS-induced IL-6 secretion was increased in Lcn2−/− BMDMs compared with WT cells (Figure 6C). We then determined whether Lcn2 can reduce the increase in LPS-induced IL-6 secretion in Lcn2−/− BMDMs. WT and Lcn2−/− BMDMs were treated with LPS, Rosi and LPS plus recombinant Lcn2 for 24 hours. Our results showed that cotreatment with recombinant Lcn2 significantly reduced LPS-induced IL-6 secretion in both WT and Lcn2−/− BMDMs (Figure 6D). These data support that Lcn2 does regulate LPS-stimulated IL-6 secretion. Second, we treated BMDMs with IL6 for 15 minutes, 1 hour, and 6 hours to assess whether Lcn2 deficiency affects IL-6-stimualted STAT3 phosphorylation. As illustrated in Figure 6E, IL-6 is a potent stimulus of STAT3 phosphorylation, and IL-6 alone can rapidly and strongly stimulate STAT3 phosphorylation (Figure 6E). However, unlike LPS, IL-6 stimulation of STAT3 phosphorylation was not altered in Lcn2−/− BMDMs (Figure 6E), suggesting that Lcn2 deficiency affects IL-6 secretion, but not IL-6 action on STAT3 activation. In attempt to more completely understand whether and how much IL-6 at its physiological concentration contributes to LPS-induced STAT3 activation in Lcn2−/− BMDMs, we determined whether blocking IL-6 action by anti-IL6 antibody could reduce LPS-induced STAT3 phosphorylation in Lcn2−/− BMDMs. However, the results showed that blocking IL-6 failed to reduce LPS-stimulated STAT3 phosphorylation in Lcn2−/− BMDMs (Figure 6F), suggesting that IL-6 may not be the major contributor of LPS-induced STAT3 phosphorylation in BMDMs. In addition, we showed that IL-6 did not significantly stimulate Lcn2 expression and secretion in BMDMs (Figure 6G), further suggesting that Lcn2 is unlikely a negative regulator of IL-6-stimulated STAT3 phosphorylation.
Because LPS can stimulate the secretion of a range of cytokines and other inflammatory mediators in macrophages, it is likely that many cytokines/inflammatory mediators rather than IL-6 alone may be involved in mediating LPS effect on NFκB-STAT3 loop activation. To test this hypothesis, we determined whether the overactivation of NF-κB-STAT3 loop can be blocked by inhibiting NF-κB activation, thereby cytokine production in Lcn2−/− BMDMs. We treated BMDMs from WT and Lcn2−/− mice with or without LPS in the presence or absence of Bay 11–7082, the NF-κB inhibitor. Figure 6H showed that the pretreatment with Bay11–7082 completely blocked LPS-induced STAT3 phosphorylation in WT and Lcn2−/− BMDMs. These results suggest the proposed role of a range of cytokines controlled by NFκB in stimulating STAT3 activation and the anti-inflammatory role of Lcn2 in regulating M1 polarization in BMDMs through controlling the NF-κB-STAT3 loop activation.
Discussion
We have previously shown that Lcn2 plays an important role in energy metabolism, obesity, and insulin resistance (22). In the present study, we explored the role and mechanism for Lcn2 in macrophage activation to gain a better understanding of the Lcn2 role in obesity-associated adipose tissue inflammation. Our results suggest that Lcn2 plays an anti-inflammatory role in regulating macrophage polarization by controlling the activation of NF-κB-STAT3 loop. Lcn2 deficiency leads to the macrophages toward M1 polarization as evidenced by the increased expression of M1 macrophage markers and the decreased expression of M2 makers in BMDMs. In vitro studies demonstrate that Lcn2−/− BMDMs are more sensitive to LPS stimulation in the expression of proinflammatory markers and the activation of inflammatory signaling pathways including NF-κB, c-Jun, and STAT3 pathways compared with WT BMDMs. recombinant Lcn2 suppressed LPS-induced expression of proinflammatory markers and attenuated LPS stimulation of NF-κB and STAT3 pathway activation and IL-6 secretion in Lcn2−/− BMDMs. Our data suggest that Lcn2 may play an anti-inflammatory role in macrophage polarization potentially through modulating the activation of NF-κB-STAT3 loop.
Based on the fact that ATMs reside in a milieu surrounded by adipocytes, ATMs have the potential to directly respond to the locally released adipokines/cytokines and lipid mediators as a result of metabolic perturbations in adipose tissue. To date, the regulatory role adipokines play in macrophage activation in obesity is far from understood. Previous studies from our group and others have implicated a role of Lcn2, as a new adipokine, in HFD-induced obesity and insulin resistance in mice although the results are conflicting among three groups (12, 22, 36). The discrepancy could be due to the differences in HFD used by three groups, ambient temperature and environment of mouse housing, and sources of Lcn2−/− mice. In addition, we recently reported that Lcn2 has a significant role in the regulation of brown fat activation through a nonadrenergic activation mechanism (37). However, the precise regulatory role and mechanism of Lcn2 in ATMs in the pathogenesis of obesity has not been clearly addressed. In this study, we showed that LPS significantly stimulates Lcn2 secretion in both BMDMs and peritoneal macrophages. Our results from Lcn2−/− mice confirmed that there was an up-regulation of expression of M1 markers, but a down-regulation of M2 markers in adipose tissue and liver in Lcn2−/− mice when challenged with HFD.
In diet-induced obesity, the excessive lipids released by adipocytes have been known to promote the recruitment of M1 polarized macrophages in adipose tissue, which is mediated by TLR4 and inflammatory signaling pathway activation (38–42). In addition, the elevated circulating LPS and up-regulation of macrophage TLR4 expression was observed in obese subjects (43, 44), suggesting that LPS plays a role in mediating HFD-induced macrophage activation and inflammation in obesity. Hence, investigating the role of Lcn2 in LPS-stimulated macrophage activation in vitro is of physiological relevance. Our findings from in vitro studies demonstrated that BMDMs lacking Lcn2 are more sensitive to LPS induction in the expression of M1 macrophage markers than WT controls, suggesting a potential role of Lcn2 in the inhibition of M1 polarization. This result is in accordance with the evidence from a recent study supporting that Lcn2 is a deactivator of macrophages and plays a role as an anti-inflammatory factor in controlling the magnitude of immune responses in BMDMs (18). Moreover, our results are supported by the reports from others that Lcn2 deficiency exacerbates LPS-induced tissue damage and potentiates LPS-induced proinflammatory gene expression in spleen and liver (20). However, there are also some conflicting data for the role of Lcn2 in inflammation. Suk and colleagues reported that Lcn2-deficient mice exhibit a reduced M1 activation in response to LPS stimulation in microglia and astrocytes (45, 46) whereas others showed no difference between WT and Lcn2-deficient mice in host response to LPS in brain (47). These discrepancies could result from the differences in the doses of LPS used in the experiments or in the types of cells and tissues examined.
Among various proinflammatory signaling pathways, NF-κB-dependent pathways have been known to be the most important mediator of LPS-induced inflammation and proinflammatory cytokine production (48–51). TLR4 ligation by LPS promotes the phosphorylation of NF-κB and IκB degradation to free NF-κB, leading to the nuclear translocation and activation of NF-κB transcription. In addition, the activation of TLRs stimulates NF-κB-independent signaling pathways such as MAPK family, including p38MAPK and JNK; the set of genes that are activated by p38 MAPK and JNK signaling pathways substantially overlaps with that transactivated by NF-κB (52–54). More importantly, there exists the interaction between NF-κB and STAT3 inflammatory signaling pathways (29–32). Studies in cancer cells suggest that this interaction is connected primarily by IL-6 forming a NF-κB-IL-6-STAT3 loop that prolongs the NF-kB activation (33–35). This loop drives the constitutive polarization to M1 macrophages and plays an important pathological role in the development of chronic inflammation in cancer cells. In the present study, we observed the marked enhancement in the LPS-induced activation of NF-κB and STAT3 signaling pathways in Lcn2−/− BMDMs, the increased expression of NF-κB and STAT3 target genes such as IL-6, IL-1β, and MCP-1, and the increased IL-6 secretion in Lcn2−/− BMDMs isolated from RCD-fed mice. However, we did not observe the effect of Lcn2 deficiency on LPS-stimulated p38MAPK phosphorylation in Lcn2−/− BMDMs. These results suggest that the activation of NF-κB-STAT3 loop is increased, which may contribute to M1 macrophage activation in BMDMs in the absence of Lcn2. It has been proposed that M1 polarization is normally self limited and resolved by negative feedback loops (55). Because NF-κB is necessary for Lcn2 production (56), we speculate that Lcn2 acts as a negative feedback factor, which promotes the resolving of inflammation. In Lcn2 deficient BMDMs, this negative feedback loop of NF-κB-STAT3 is lost, leading to the increased magnitude of inflammatory responses.
To provide more evidence supporting the hypothesis described above, we assessed the effect of recombinant Lcn2 on the activation of inflammatory signaling pathways and the expression of proinflammatory markers in WT and Lcn2−/− BMDMs under the nonstimulated and LPS-stimulated conditions. Our results showed that the pretreatment of recombinant Lcn2 significantly prevented LPS-stimulated expression of proinflammatory markers Cd11c, IL6, and iNOS in both WT and Lcn2−/− BMDMs. Adding recombinant Lcn2 to Lcn2−/− BMDMs attenuated LPS-induced IκBα degradation and STAT3 phosphorylation. Nevertheless, when we treated the BMDMs with recombinant Lcn2 in the absence of LPS stimulation, Lcn2 had no effect on the activation of NF-κB-STAT3 signaling pathways. This suggests that Lcn2 per se is not proinflammatory, but exerts anti-inflammatory effect when the proinflammatory stimulation is present. Given that Lcn2 production is stimulated by NF-κB activation, the induction and secretion of Lcn2 by LPS could be a mechanism required for the resolution of inflammatory response in ATMs by inactivating a feed-forward loop of NF-κB-STAT3 signaling pathways. Our results from the time course of Lcn2 secretion, NFκB and STAT3 phosphorylation in response to LPS stimulation support the sequential event of NFκB activation followed by Lcn2 secretion and the suppression of STAT3 phosphorylation. In addition, IL6 is the predominant cytokine required for LPS-induced STAT3 activation (57, 58). Hence, it is conceivable that the higher level of IL-6 expression and secretion in LPS-treated Lcn2−/− BMDMs may contribute to the increased STAT3 phosphorylation and the sustained activation of NF-κB-STAT3 loop. However, our data do not seem to support the role of IL-6 as a primary cytokine in linking NF-κB-STAT3 loop in BMDMs. First, Lcn2 deficiency does not alter IL-6 stimulation of STAT3 phosphorylation. Second, LPS-stimulated STAT3 phosphorylation was not significantly affected by blocking IL-6 action, but completely suppressed by inhibiting NFκB activity. All the data together led us to propose a model that a range of NFκB-controlled cytokines (rather than just IL-6) mediate the NF-κB-STAT3 loop activation and Lcn2 breaks this loop by inhibiting NFκB activity (Figure 7). Interestingly, Rosi treatment significantly reduced LPS-induced expression of inflammatory genes as well as IκB degradation in WT, but not in Lcn2−/− BMDMs. Consistently, the inhibitory effect of Rosi on inflammatory markers including Cd11c, IL-6, and iNOS was diminished in Lcn2−/− BMDMs. However, Rosi did not affect LPS induction of STAT3 and p38MAPK (data not shown) activation. Our findings are in line with other studies that the down-regulation of PPARγ in LPS-stimulated macrophages is mediated by NF-κB other than p38MAPK, JNK, and AP-1 pathways (59), suggesting that Lcn2 is required for anti-inflammatory effect of Rosi, and Lcn2 deficiency impairs the interaction of PPARγ-NF-κB signalings in macrophages. The loss of Lcn2 negative feedback control for NF-κB activation may further inhibit PPARγ activity and potentiate LPS-induced proinflammatory gene expression.
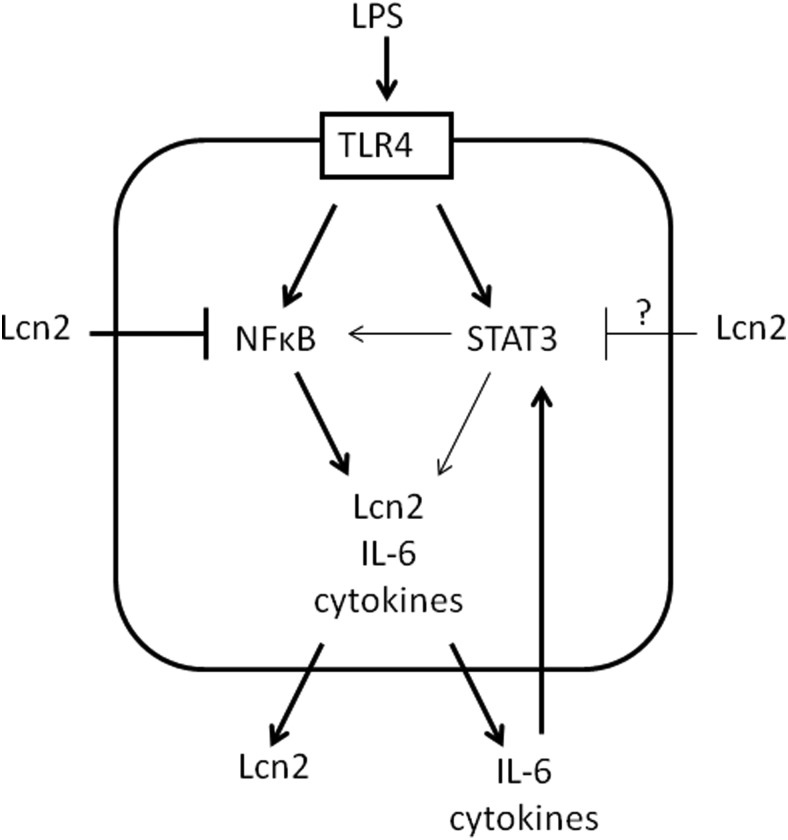
Figure 7.
A model for Lcn2 role as a negative regulator in NFκB-STAT3 loop activation in macrophages. Upon LPS stimulation, NFκB is rapidly activated, leading to the secretion of a range of proand anti-inflammatory cytokines. Proinflammatory cytokines such as IL-6 and other molecules subsequently stimulate STAT3 phosphorylation which further activates NFκB, forming a feed-forward loop. During the inflammatory response, Lcn2 is also increasingly secreted and acts as a fine-tuning regulator of inflammation in an autocrin/paracrine fashion. Lcn2 breaks the NFκB-STAT3 loop to limit the magnitude of inflammatory response by inhibiting NFκB activation. In the absence of Lcn2, NFκB-STAT3 loop activation and inflammation persist stronger and longer.
In conclusion, our data demonstrate that Lcn2 deficiency skews macrophages toward the M1 activation in BMDMs in response to LPS stimulation. We also showed that Lcn2 deficiency potentiates the activation of NF-κB-STAT3 pathways by LPS stimulation in BMDMs. recombinant Lcn2 reduces the LPS stimulation of M1 activation and NFκB-STAT3 phosphorylation, and inhibiting NFκB blocks LPS stimulation of STAT3 activation in Lcn2−/− BMDMs. Our results suggest that Lcn2 plays an anti-inflammatory role in controlling the activation of NF-κB-STAT3 loop, thereby limiting inflammatory responses. The detailed mechanisms or signaling pathways that mediate Lcn2 function in inflammation warrant further investigations.
Additional material
Supplementary data supplied by authors.
Acknowledgments
This work was supported by Grant No. R01DK080743 (to X.C.) from the National Institute of Diabetes and Digestive and Kidney Diseases and Minnesota Obesity Center (Grant No. 2P30DK050456 from the National Institute of Diabetes and Digestive and Kidney Diseases).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATM
- Adipose tissue macrophages
- BMDM
- bone marrow-derived macrophage
- CSF
- colony-stimulating factor
- HFD
- high-fat diet
- JNK
- c-Jun N-terminal kinase
- Lcn2
- lipocalin 2
- LPS
- lipopolysaccharide
- M1
- classically activated adipose tissue macrophages
- M2
- alternatively activated adipose tissue macrophages
- NF-κB
- nuclear factor kappa-light-chain-enhancer of activated B cells
- PPARγ
- proxisome proliferator-activated receptor gamma
- RCD
- regular-chow diet
- Rosi
- rosiglitazone
- STAT
- signal transducer and activator of transcription
- WT
- wild type.
References
- 1. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 2. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. [DOI] [PubMed] [Google Scholar]
- 6. Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. [DOI] [PubMed] [Google Scholar]
- 9. Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. [DOI] [PubMed] [Google Scholar]
- 10. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3–L1 adipocytes. J Biol Chem. 2005;280:35361–35371. [DOI] [PubMed] [Google Scholar]
- 12. Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akerstrom B, Flower DR, Salier JP. Lipocalins: Unity in diversity. Biochim Biophys Acta. 2000;1482:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. [DOI] [PubMed] [Google Scholar]
- 16. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. [DOI] [PubMed] [Google Scholar]
- 17. Zhao P, Stephens JM. STAT1, NF-κB and ERKs play a role in the induction of lipocalin-2 expression in adipocytes. Mol Metab. 2013;2:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warszawska JM, Gawish R, Sharif O, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borkham-Kamphorst E, van de Leur E, Zimmermann HW, et al. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim Biophys Acta. 2013;1832:660–673. [DOI] [PubMed] [Google Scholar]
- 20. Srinivasan G, Aitken JD, Zhang B, et al. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J Immunol. 2012;189:1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Foncea R, Deis JA, Guo H, Bernlohr DA, Chen X. Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes. PLoS One. 2014;9:e96997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo H, Jin D, Zhang Y, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin D, Guo H, Bu SY, et al. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure. FASEB J. 2011;25:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. [DOI] [PubMed] [Google Scholar]
- 26. Linde N, Gutschalk CM, Hoffmann C, Yilmaz D, Mueller MM. Integrating macrophages into organotypic co-cultures: A 3D in vitro model to study tumor-associated macrophages. PLoS One. 2012;7:e40058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell. 2009;15:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong Z, Wen Z, Darnell JE., Jr Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. [DOI] [PubMed] [Google Scholar]
- 34. Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334 (Pt 2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Lam KS, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Guo H, Deis JA, et al. Lipocalin 2 regulates brown fat activation via a non-adrenergic activation mechanism. J Biol Chem. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schilling JD, Machkovech HM, He L, Diwan A, Schaffer JE. TLR4 activation under lipotoxic conditions leads to synergistic macrophage cell death through a TRIF-dependent pathway. J Immunol. 2013;190:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. [DOI] [PubMed] [Google Scholar]
- 42. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. [DOI] [PubMed] [Google Scholar]
- 43. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. [DOI] [PubMed] [Google Scholar]
- 45. Jang E, Lee S, Kim JH, et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013;27:1176–1190. [DOI] [PubMed] [Google Scholar]
- 46. Lee S, Kim JH, Kim JH, et al. Lipocalin-2 Is a chemokine inducer in the central nervous system: Role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011;286:43855–43870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ip JP, Nocon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. J Neuroinflammation. 2011;8:124–2094–8–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan ED, Riches DW. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochem Biophys Res Commun. 1998;253:790–796. [DOI] [PubMed] [Google Scholar]
- 50. Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–944. [DOI] [PubMed] [Google Scholar]
- 51. Solinas G, Vilcu C, Neels JG, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. [DOI] [PubMed] [Google Scholar]
- 52. Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. [DOI] [PubMed] [Google Scholar]
- 53. Ogawa S, Lozach J, Jepsen K, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101:14461–14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell. 2009;35:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. [DOI] [PubMed] [Google Scholar]
- 56. Borkham-Kamphorst E, Drews F, Weiskirchen R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κB activation. Liver Int. 2011;31:656–665. [DOI] [PubMed] [Google Scholar]
- 57. Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Greenhill CJ, Rose-John S, Lissilaa R, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186:1199–1208. [DOI] [PubMed] [Google Scholar]
- 59. Necela BM, Su W, Thompson EA. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor gamma and nuclear factor-κB in macrophages. Immunology. 2008;125:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.