Abstract
Hematopoiesis in vertebrates is sustained over the duration of an organism's lifetime due to strict regulation of the highly hierarchical hematopoietic system, where a few immature hematopoietic stem cells (HSCs) continuously regenerate the entire blood supply, which is constantly being replaced. Although HSCs self-regulate through cell-autonomous processes, they also receive a variety of signals from their microenvironment or niche. Within the microenvironment, HSCs are regulated through both cell-cell interactions and secreted signals, including hormones. HSCs at the apex of the blood supply integrate these signals to produce progeny to support hematopoiesis while simultaneously maintaining a stem cell pool. In the past 10 years, advances in genetic models and flow cytometry have provided the tools to test how the microenvironment regulates HSCs. This review is organized in 3 main parts and will focus on cellular components of the HSC niche that are potential targets for hormonal signals, then review critical regulatory signals in the HSC niche, and finally highlight the emerging role of hormonal and paracrine signals in the bone marrow.
The concept of a hematopoietic niche was first proposed in 1978, and the overall concept of a stem cell niche was first demonstrated in Drosophila gonads (1–3). Within mammalian bone marrow, hematopoietic stem and progenitor cells (HSPCs) interact with a variety of cells and signals, which constitute their niche or microenvironment. Cells of the microenvironment, through either direct contact or through secreted factors, can influence HSPC behavior in the marrow. These microenvironmentally imposed signals can regulate stem cell fate decisions, self-renewal, and residence in the marrow and are critical to maintaining the stem cell pool. Disruption of these signals in the microenvironment can lead to stem cell depletion, altered hematopoiesis, and malignancy. Over the past 10 years, numerous cell types and molecules of the HSPC niche have been identified and are discussed in several comprehensive reviews (4–6). Our review will focus on the cellular components of the hematopoietic stem cell (HSC) niche that are targets for hormonal signals (specifically, mesenchymal stem cells [MSCs] and the osteoblastic lineage as well as adipocytes) and how hormonal signals and signaling pathways are integrated in the bone marrow microenvironment.
Identifying the HSC
HSCs and their progeny can be defined 2 ways: functionally by their ability to reconstitute the hematopoietic system and prospectively by their expression of cell surface markers. Primitive murine hematopoietic cells can be identified immunophenotypically by their lack of committed lineage markers, and expression of both C-kit/CD117/ and Sca1/Ly6A/E (7). This population of lineage (Lin)-negative, Sca1-positive, and C-kit–positive (known as LSK) is heterogeneous with varying reconstitution capacity. The LSK population contains multipotent progenitors (MPP), short-term HSCs (ST-HSCs), and long-term HSCs (LT-HSCs). These cells within the LSK pool are identified using Fms-related tyrosine kinase 3 (Flt3) and signaling lymphocyte activation molecules (SLAM) markers CD48 and CD150 (8–12). MPPs by definition are multipotent but have very limited self-renewal capacity. ST-HSCs are defined as LSK, Flt3−, CD48−, and CD150− (8–12). LT-HSCs, which have the longest repopulating capacity in competitive transplants, are defined as LSK, Flt3−, CD48−, and CD150+ (8–12). Recent data have suggested that this long-term repopulating population can be fractionated further using 2 additional SLAM markers, CD229 and CD244 (13). Although the use of SLAM markers has been revolutionary for studies in mice, they are not beneficial for preclinical nonhuman primate or human studies because they do not enrich for HSCs (14). Agreement on human HSC markers remains controversial. However, it is clear that human HSCs also contain subsets of different quiescence and self-renewal, analogous to murine HSCs (15). Current strategies to enrich for human HSCs use Lin−, CD34+, CD38−, Thy1.1+, and CD45RA− (16). Human HSCs have also been reported to reside in a subpopulation of Lin−, CD34+, CD38−, Thy1.1+, CD45RA− cells that express CD49f and have high efflux capacity of rhodamine 123 (15). Although surface markers have been essential to finding HSC populations, competitive transplants with demonstration of multilineage reconstitution remain the gold standard for assessing true HSC potential and function.
Cellular Components of the HSC Niche That Are Potential Targets for Hormonal Signals
Osteoblastic cells
Cells of the osteoblastic lineage have been recognized as a key modulators of HSPC maintenance in the marrow (17). Two different studies from 2003 definitively showed using genetic models that activating osteoblasts could lead to in vivo expansion of the HSPC pool in the marrow and increase their long-term repopulating ability (18, 19). In our own study, osteoblasts were activated using a gain-of-function strategy by expressing a constitutively active (PTH receptor, driven by the mature osteoblast-specific, 2.3-kb fragment of the promoter for the mouse collagen 1α gene (18, 20). Zhang et al (19) used a loss-of-function strategy by conditionally deleting the bone morphogenic protein receptor IA using the myxovirus (influenza virus) resistance 1 (Mx1) inducible-Cre recombinase. In both studies, trabecular bone and osteoblasts were increased, and HSPCs were expanded.
Since these 2 pioneer studies, many have further characterized the role of the osteolineage in HSPC support and maintenance. Genetic ablation of osteoblastic cells that express the 2.3-kb fragment of the promoter for the rat collagen 1α gene leads to bone marrow hypocellularity and extramedullary hematopoiesis in the spleen (21). This suggests that certain populations of osteoblastic cells are critical for HSPC support in the marrow. Interestingly, osteoblastic expansion using strontium leads to a bone-anabolic effect without increasing the number or frequency of HSPCs, suggesting osteoblastic expansion is not enough to promote HSPC support in the marrow (22). Another possibility is that strontium is expanding a subset of cells in the osteolineage, which is not involved in regulation of HSPCs. Cells within the osteolineage that have been shown to have a higher capacity for HSPC support are more immature (see also Mesenchymal stem cells below) (23–25). These immature osteoblasts express high levels of Runt-related transcription factor 2 (Runx2), a transcription factor critical in the differentiation of MSCs to the osteolineage (23). In addition to these, immature human osteoprogenitors expressing CD146 provide HSPC support through their interactions within bone marrow sinusoids (26). On the other hand, osteocytes, the terminally differentiated progeny of the osteoblastic lineage, appear to have an inhibitory effect on HSPC support (10). Osteoblasts have even been implicated as a necessary component in recovery from radiation injury, because their numbers double after total body injury (27). Together these studies highlight the heterogeneity and complexity of the osteolineage as supportive cells for hematopoiesis and maintenance of HSPCs in the marrow.
Recent studies have found that cells of the osteoblastic lineage may be biased toward support for lymphopoiesis and lymphoid tissue function (28). Depletion of the critical chemokine C-X-C motif chemokine 12 (CXCL12) from osteoblasts leads to a loss of lymphoid progenitors in the marrow (29). These mice did not have altered frequencies of the most primitive HSPCs, but did have significantly fewer common lymphoid progenitors (29). Despite this loss of common lymphoid progenitors, these mice have normal levels of committed B cell progenitors and early thymic progenitors. These studies raise the possibility that osteoblasts may be a specialized niche for lymphoid progenitors.
Disruption of homeostatic osteoblast function underscores their role in hematopoiesis, as this has been shown to cause malignant transformation of the marrow. Three different studies have identified different osteoblastic factors that are required to maintain a benign marrow (30–32). In the first study, mice with a global deletion of the retinoic acid receptor γ (RARγ) were irradiated and reconstituted with wild-type (WT) bone marrow (32). Although these RARγ−/− were reconstituted with WT marrow expressing RARγ, they still developed myeloproliferative syndromes (32). This conversion of the marrow is believed to be the result of increased expression of TNF-α from RARγ−/− cells at sites of hematopoiesis (32). The results of this study suggest that nonhematopoietic cells in the marrow require expression of RARγ to maintain the noninflammatory conditions necessary to support normal hematopoiesis. The next study characterized mice in which the Dicer1 gene was deleted from the osteoblastic lineage using the osterix-Cre (Osx-Cre), which targets immature osteoblasts (31). Mice with conditional deletion of the Dicer1 gene have impaired osteoblast differentiation and develop myelodysplasias (31). Of note, the conditional Dicer1-knockout mice have decreased expression of Shwachman-Bodian-Diamond syndrome (Sbds) gene in their osteoprogenitors (31). The Sbds gene is commonly inactivated in humans with increased incidence of bone marrow failure, myelodysplastic syndrome (MDS), and acute myelogenous leukemia (AML), suggesting that these results have implications for human diseases (31, 33). The most recent study showed that a gain of function for β-catenin solely in osteoblasts leads to AML in mice by 6 weeks of age (30). The lethality of the AML in the β-catenin gain-of-function mice was dependent on Notch signaling initiated by Jagged1 (30). This was demonstrated by genetic ablation of Jagged1 in osteoblasts or by γ-secretase inhibition, because both increased survival (30). The authors also saw active nuclear β-catenin in human bone marrow biopsies of patients with MDS or AML, but not in normal controls, again suggesting that this murine discovery may have relevance for human disease (30). This finding could provide a new clinical tool in the diagnosis of MDS and AML. Because hematopoiesis is defective in AML, we asked whether leukemia disrupts the osteolineage pool. Our data show that AML inhibits osteoblastic function. This osteoblastic inhibition is promoted by the expression of the chemokine CCL3 from leukemic cells (11). In a mouse model of chronic myelogenous leukemia, leukemic expression of CCL3 initiates fibrosis of the endosteum, which forms a leukemic niche (34). CCL3 has previously been implicated in a hematological malignancy: CCL3 is upregulated in multiple myeloma, where increased CCL3 results in decreased expression of osteocalcin and suppresses osteoblast function (35). Although CCL3 can act through either of its receptors, CCR1 or CCR5, the authors found that it is primarily signaling through CCR1 that maintains this malignant state (35). Overall, these studies underscore the importance of the osteoblastic niche both as a homeostatic component of HSC maintenance as well as a potential therapeutic target in the setting of malignancy.
Mesenchymal stem cells
Multipotent MSCs capable of generating the osteoblastic lineage have been identified as critical cell type capable of supporting HSPCs in the marrow and are currently being used as a novel therapeutic tool. Human MSCs have previously been shown to expand HSPCs ex vivo (36). Within the MSC pool, those that express a GFP reporter for the intermediate filament protein Nestin have the highest expression of genes that support HSPCs in the marrow (37). These Nestin-GFP–expressing cells are hormone-sensitive, because they express the PTH receptor and PTH administration expands their numbers 2-fold (37). Depletion of Nestin-expressing MSCs in vivo leads to a loss of the most immature HSPCs, suggesting these cells are a critical niche component (37). Human MSCs that express Nestin have been recently identified in fetal marrow and reside in a population of marrow cells that are CD45−, CD235a−, CD31−, CD51+, and PDGFRα+ (38). These cells are capable of forming mesenspheres in vitro, and support human hematopoiesis ex vivo (38). Mesenspheres support hematopoiesis through expression of various cytokines such as stem cell factor (38). Cells capable of mesensphere formation and HSPC support have also been isolated from human cord blood (39). These cells from cord blood are defined as CD45−, CD31−, CD71−, CD146+, CD105+, and nestin+ (39). The ability of MSCs to support HSPCs ex vivo highlights the therapeutic potential by studying the HSC niche. Cotransplantation of MSCs with HSCs in a stem cell transplant in primates has been shown to increases the HSCs' engraftment capacity (40). This concept has been translated into human therapies. Currently, there is a phase I trial using a defined MSC culture to expand human cord blood before transplantation (41). In this system, cord blood is grown on human MSCs from healthy donors, which are enriched for expression of STRO-3 (41). Cord blood in this system was expanded by an average factor of 12.2 and was able to engraft faster compared with transplants that were not grown on MSCs (41). This clinical trial highlights the important role of MSCs for HPSC maintenance in vivo, but also ex vivo for their use in stem cell transplants.
Adipocytes
Although many cell types have been identified as positive regulators of HSPCs in the marrow, adipocytes have been recognized as an inhibitory component. This was demonstrated using the A-ZIP mouse strain, which has virtually no adipose tissue, by forced expression of a dominant-negative CCAAT-Enhancer-Binding Protein (C/EBPα) (42). The A-ZIP strain has hematopoiesis in marrow cavities that are normally filled with adipose tissue. This strain is also able to reconstitute their marrow faster than WT littermates after myeloablative injury (43). Moreover, inhibiting adipogenesis in WT mice through peroxisome proliferator-activated receptor-γ antagonism leads to faster recovery of peripheral white blood cells after myeloablative injury and marrow transplant (43). Marrow cavities with higher adipose content contain fewer HSPCs compared with cavities without adipose, reinforcing the concept that adipocytes are an inhibitory component of the niche (43). However, the adipose-initiated signals resulting in HSPC inhibition remain unexplored. There is still a lot to learn about this cell type, although studies have suggested they have a transcriptome distinct from white adipose tissue (44, 45). More recent insights on bone marrow fat are reviewed elsewhere (46).
Genetic inhibition of adipogenesis remodels the marrow, with a dramatic increase of trabecular bone. This has been demonstrated by genetically knocking in Wnt10b, a known inhibitor of both white and brown adipogenesis (47, 48). One study expressed Wnt10b in adipocytes using the FABP4 promoter, which is adipose-specific (49). These mice exhibit a large expansion of trabecular bone, which infiltrates into the diaphysis (49). This phenotype does not result in depletion of MSCs, because this increase in trabecular bone is maintained during the course of the mouse's lifetime, suggesting enhanced osteoblastogenesis (49). Another possibility is that the increased bone is the result of decreased serum leptin, a known inhibitor of bone formation (50). The same group also saw increased trabecular bone when they expressed Wnt10b under the control of the mature osteoblastic-specific osteocalcin promoter without altering serum leptin (51). The ability of Wnt10b to inhibit adipogenesis and promote bone anabolism reinforces the concept that there is a balance between bone and adipose tissue in the marrow (50, 52, 53).
Critical Regulatory Signals in the HSC Niche
CXCL12 (Stromal Derived Factor-1, SDF-1)/C-X-C chemokine receptor type 4 (CXCR4) signaling axis
The chemokine CXCL12, although not a hormone, represents a fundamental signaling axis, which regulates HSPC self-renewal and marrow residence and which is often the common pathway mediating cellular and/or hormonal signals from the microenvironment to HSCs (54). Signaling by CXCL12 was first observed as a crucial component of hematopoiesis in the marrow, when it was genetically deleted globally in mice (54). CXCL12−/− mice die around embryonic day 18.5 and those that survive past that point survive only hours after birth (54). The bone marrow of CXCL12−/− mice is hypocellular with reduced numbers of lymphoid and myeloid progenitors (54). In vitro, marrow of CXCL12−/− mice had reduced colony-forming potential for both myeloid cells and pre-B cells (54). This study was the first to show the importance of CXCL12 for hematopoiesis in the marrow. CXCL12 acts through its receptor C-X-C chemokine receptor type 4, which has also been shown to be required for hematopoiesis in the marrow and engraftment of transplanted HSCs (55). The HSPC-mobilizing agent granulocyte colony-stimulating factor (GCSF) acts through this signaling axis by decreasing marrow CXCL12 while simultaneously upregulating CXCR4 (56). HSPC egress from the marrow could be blunted by blocking CXCR4 or by preventing CXCL12 degradation by neutrophil-derived elastase (56). Other groups have reported that in response to GCSF, CXCR4 is cleaved on HSPCs, which prevents them from responding to marrow CXCL12, facilitating mobilization from the marrow (57). The role of CXCR4 in HSPC homing has also been demonstrated by inhibiting Gαi with pertussis toxin (PTX) (58). PTX diminishes the ability of HSPCs to respond to CXCL12, reducing the engraftment potential of these cells (58). Many cells in the marrow express CXCL12, but the highest expression of CXCL12 is observed in a subset of mesenchymal cells called CXCL12-abundant reticular (CAR) cells (59, 60). CAR cells reside in the bone marrow at both vascular and endosteal sites associated with HSPC residence (59). The transcription factor Forkhead Box C1 (FoxC1) has recently been identified as an essential mediator of CAR cell fate determination and function (61). CXCL12 is also significant component to MSC support of HSPCs in the marrow (62). Genetic removal of CXCL12 in MSCs using a Paired Box1 (Prx1)-Cre leads to a loss of HSPC quiescence and depletion of lymphoid progenitors (62). Functionally, cells in the marrow that produce CXCL12 are able to influence stem cell fate. CXCL12 maintains the marrow stem cell compartment by inhibiting cell cycle entry in HSPCs (63). These studies show that nonhematopoietic cells are able to regulate the stem cell pool through production of CXCL12 and by regulating the HSPC cell cycle as well as egress from the marrow.
Circadian rhythms
The action of circadian rhythms on the HSC niche highlights the fundamental role of CXCL12 in the regulation of HSCs and also aligns the marrow microenvironment with multiple hormonal systems. HSPCs are regulated in 3 dimensions not only by their niche but also through oscillation of circadian rhythms (64). This was first shown as circadian rhythms alter colony-forming potential in peripheral blood over the course of the day (65). Circadian rhythms also modulate catecholamine levels, which have been previously shown to influence HSPC self-renewal and exit from the marrow (65). A circadian clock is also present in mouse whole bone marrow, as classic clock genes like Per2 and Rev-erbα oscillate in expression over the course of the day, although only Per2 fluctuates in the most primitive marrow cells (66). The mechanism for circadian-induced HSPC exit from the marrow involves the fluctuation of CXCL12 by niche cells in the marrow (67). Mechanistically, stimulation of the suprachiasmatic nucleus stimulates norepinephrine (NE) release from the SNS into the marrow, acting on CAR cells expressing β3-adrenergic receptors facilitating destruction of the transcription factor Sp1 (67). Sp1 promotes expression of CXCL12, which is required for homing of HSPCs in the marrow (67). In this way, circadian rhythms initiated by the suprachiasmatic nucleus are able to stimulate catecholamine release into the marrow to regulate CXCL12 production by CAR cells (Figure 1A) (67). Neutrophils have also been shown to be a component of HSPC mobilization during circadian rhythms (68). Aged neutrophils defined as having decreased expression of L-selectin and high expression of CXCR4 home to the marrow at the end of a resting period (68). Aged neutrophils are then phagocytized by marrow macrophages, which stimulate endogenous LXR activity (68). This process inhibits CAR cells in a LXR-dependent process, leading to reduced CXCL12 in the marrow (68). Loss of CXCL12 in this process facilitates egress of HSPCs from the marrow into circulation (68). Thus, circadian rhythms are a constituent of the niche by regulating both catecholamine release and CXCL12 production to influence both HSPC self-renewal and egress.
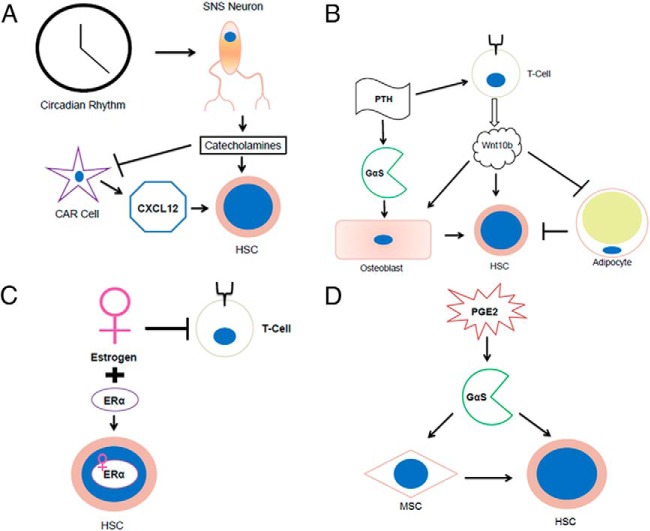
Figure 1.
HSCs can be regulated through activation of various signaling molecules and cell types. A, Circadian rhythms can activate SNS neurons to release catecholamines that can act directly on HSCs but also inhibit CAR cell production of CXCL12, facilitating HSC egress (18, 78). B, PTH stimulation activates T cells to produce Wnt10b, which stimulates HSC support directly and also through activating osteoblasts (53, 79). Wnt10b stimulation also inhibits adipogenesis, an effect which may decrease an inhibitory HSC niche component (B) (47–51). C, Estrogen has been shown to have opposing effects on the hematopoietic system, including inhibiting T lymphocytes, but can also expand HSCs expressing estrogen receptor-α (ERα) (85, 87). D, PGE2 can stimulate HSC support both autonomously and cell nonautonomously by activating GαS on MSCs (89, 92).
Sympathetic nervous system signals
Signals from the sympathetic nervous system (SNS) have been recently identified as an unexpected component in the regulation of HSPCs in the marrow. It has been previously demonstrated that the SNS can regulate bone formation through β2-adrenergic signals that stimulate expression of Receptor activator of nuclear factor kappa-B ligand in osteoblasts (69). Genetic inhibition of myelination, resulting in decreased nerve conductance, prevents HSPC egress from the marrow (70). Egress of HSPCs can also be blocked by depleting catecholamine stores with 6-hydroxydopamine, suggesting that sympathetic signals are regulating this process (70). Human HSPCs express both dopamine and adrenergic receptors, suggesting they are able to respond to SNS-mediated signals (71). Treatment of HSPCs with dopamine agonists enhanced colony formation in vitro, but only in the presence of myeloid cytokine GCSF, or granulocyte-macrophage colony-stimulating factor (71). Pretreatment of human HSPCs with dopamine agonists enhanced their ability to engraft immunocompromised mice (71). This effect is dopamine-dependent because treatment of HSPCs with dopamine antagonists decreases their ability to engraft (71). NE treatment of HSPCs promotes migration through expression of Matrix Metalloproteinase-2 but also enhances engraftment capabilities in vivo and colony formation in vitro (71). Both neurotransmitters activate canonical Wnt signaling, which has been previously shown to activate gene programs necessary for HSPC survival and self-renewal in the marrow (71). The importance of SNS signals in HSPC maintenance and hematopoiesis starts during embryogenesis (72). The transcription factor Gata3 is expressed in the aorta-gonads-mesonephros region of the embryo, where the first HSC emerges (72). Gata3 promotes expression of genes required for catecholamine synthesis, such as tyrosine hydroxylase. Increasing endogenous catecholamines is a new therapeutic approach to increasing stem cell yield for transplants, because NE uptake inhibition increased GCSF-mediated mobilization of HSPCs (72). In addition to SNS neurons, nonmyelinating Schwann cells have also been shown to promote HSPC quiescence through production of TGF-β acting on Transforming Growth Factor, Beta Receptor II (73). Thus, the SNS acts through peripheral circuits to maintain a stem cell pool in the marrow while supporting normal hematopoiesis through HSPC egress (Figure 1A).
Hormones and Paracrine Factors That Regulate HSCs and the HSC Niche
Parathyroid hormone
PTH has become an instrumental tool in studying activation of the microenvironment and subsequent regulation of HSPCs. HSPCs do not express the PTH receptor, therefore PTH modulates HSPCs indirectly (18). Activation of the PTH receptor solely in osteoblasts is sufficient to expand HSPCs, whereas its activation in osteocytes is not (10, 18). Pharmacologic intermittent treatment with PTH (1–34) has rapid effects on the HSC pool (74). The ability of PTH (1–34) to promote HSPC support and maintenance is likely the result of its stimulatory effect on various cell types in the marrow, including osteoblasts and osteoclasts. This heterogeneity may stem from the ability of the PTH receptor to signal through multiple pathways, and even after internalization (75–77). PTH (1–34) is able to stimulate marrow support through osteoblasts, by activation of a cAMP/protein kinase A pathway, which leads to increased expression of Jagged1 (18, 78). It has also been shown that PTH (1–34) can act through T cells to promote Wnt10b secretion leading to activation of canonical Wnt signaling in both osteoblasts and HSCs (Figure 1B). This activation of Wnt signaling promotes a bone-anabolic effect on osteoblasts and self-renewal in HSPCs (53, 79). Wnt10b also stimulates expression of Jagged1 on osteoblasts, increasing their support capacity for the expanded ST-HSC population (79). PTH (1–34) also has therapeutic potential because it is able to stimulate murine HSPC mobilization and increases engraftment (80). Expression of a constitutively active PTH receptor in osteoblasts ameliorates tumor burden in a mouse model of chronic myelogenous leukemia (81). This finding emphasizes the importance of osteoblastic PTH type 1 receptor signaling as a homeostatic component of normal hematopoiesis and again highlights the regulatory role of osteolineage cells in the niche.
In humans, PTH may have similar effects, because patients with elevated serum PTH due to primary hyperparathyroidism have increased circulating HSPCs (82). Administration of PTH (1–34) to healthy individuals also stimulates HSPC mobilization from the marrow through a mechanism that requires GCSF (83). Of note, GCSF administration in humans depletes the marrow of HSPCs, whereas PTH (1–34) treatment does not (83). Due to these actions of PTH, and its safe formulation as teriparatide for the treatment of osteoporosis, PTH (1–34) is currently being tested as a possible therapeutic agent to enhance stem cell availability required for transplant because their numbers are often limiting.
Estrogen
As our understanding of the HSC niche has progressed, it has become apparent that physiologic signals (ie, circadian rhythms) target and alter HSC-niche interactions. One physiologic setting that is associated with expansion of the hematopoietic system is pregnancy, and based on this observation, research groups have identified effects of estrogen on both HSCs directly as well as important niche components. Administration of 17β-estradiol (5 mg/kg) to male mice leads to an increase of committed B-cell populations at the expense of HSPCs (84). Other studies have shown that estrogen treatments in mice deplete the number of T-cell–lineage progenitors (85). Removal of estrogen by ovariectomy (OVX) leads to an expansion of ST-HSCs (86). This expansion was dependent on T cells, because OVX of mice without T cells (TCRβ−/−) did not have expansion of ST-HSCs (86). The authors found that mice with OVX had increased expression of CD40 ligand, which stimulated T cells to secrete Wnt10b, a molecule previously shown to expand ST-HSCs (79). Although the effect of estrogen on erythroid, myeloid, and lymphoid differentiation was previously described, the Morrison laboratory focused on direct effects of estrogen on HSCs and reported an increase in cycling HSPCs in female compared with male mice, and this phenomenon was decreased with OVX (87). This group identified expression of estrogen receptors in HSCs and demonstrated that estrogen in pregnant females promotes HSPC self-renewal. This HSC response to estrogen is cell-autonomous and depends on estrogen receptor-α, because conditional inactivation of this receptor blocks the female-specific HSC cycling (Figure 1C) (87). These data demonstrate how hormones may engage HSCs both directly and through their niche. Because both effects are likely present during physiologic changes (such as pregnancy), it is likely that these effects occur in a dynamic fashion and that their disruption (using estrogen antagonism) may also initiate recovery mechanisms that return the system to homeostasis. However, these data point to unexpected effects of sex hormones on HSCs and their niche.
Prostaglandin E2
Prostaglandin E2 (PGE2), an inflammatory lipid produced by cyclooxygenase-2, was identified in a screen looking for prohematopoietic signals (88). PGE2 is able to act directly on HSCs because they express all receptors for PGE2 (89). The direct action of PGE2 on HSCs stimulates cAMP-initiated Wnt signaling, which leads to inhibition of apoptosis and HSPC self-renewal (90). PGE2 also enhances homing of HSPCs because treatment of HSPCs ex vivo with the PGE2 analog 16, 16-dimethyl PGE2 (dmPGE2) enhances their capacity to engraft lethally irradiated recipients (89). In our laboratory, we have shown that treatment of mice with dmPGE2 leads to an expansion of HSPCs, specifically ST-HSCs and MPPs without depleting LT-HSCs (91). Pretreatment of MSCs with dmPGE2 or an EP4 agonist leads to enhanced HSPC colony formation in vitro (92). This suggests that PGE2 is able to act directly on HSPCs while simultaneously stimulating the microenvironment (Figure 1D) (92). PGE2 rapidly increases in the marrow microenvironment after radiation, and treatment of mice with dmPGE2 24 hours after sublethal radiation injury suppresses apoptotic gene expression in LSK and stimulates cyclooxygenase-2 activity in the marrow, specifically in a population of αSmooth Muscle Actin+ macrophages (12, 93). These functions of PGE2 have even been shown in a nonhuman primate model, suggesting a conserved response (94). Macaques given transplants of CD34+ mobilized peripheral stem cells treated ex vivo with dmPGE2 had maintained multilineage engraftment 1 year after transplant (94). PGE2's prohematopoietic signal has therapeutic potential for both transplant recipients and donors, because it is able to enhance both HSC engraftment and self-renewal.
Signaling through GαS
The heterotrimeric GαS subunit is an integration point for many prohematopoietic signals that require activation of protein kinase A, and osteoblasts express many of these GαS-coupled receptors (PTH1R, PTGER2, PTGER4, and ADRB2) (18, 90, 95). Interestingly, many of the critical signals highlighted in this review signal through GαS (PTH, prostaglandin, and β-adrenergic signals,). Conditional deletion of GαS using the inducible Mx-1-Cre bypasses the lethality of a GαS global knockout (28). HSPCs from these mice have impaired homing capacity and do not respond to mobilizing agents such as GCSF (28). Constitutive activation of GαS in WT HSPCs by cholera treatment before transplant increased their engraftment potential 2-fold (28). Genetic ablation of GαS specifically in osteoprogenitors using Osx-Cre leads to a decrease in trabecular bone and B-lymphocyte precursors (96). Mechanistically, a loss of GαS leads to decreased IL-7 expression in osteoblasts, which is required for their ability to support normal B and T cell development (96). This suggests that GαS is required in osteoblasts to support normal lymphopoiesis in the marrow (96). Removing GαS from the terminally differentiated osteocyte using Dentin Matrix Acidic Phosphoprotein (Dmp1)-Cre leads to depletion of trabecular bone (97). These mice also have increased myelopoiesis, splenomegaly, and marrow cellularity (97). Loss of GαS in osteocytes is required for this phenotype. Transplantation of WT marrow into osteocyte GαS−/− mice leads to myeloproliferation, whereas this process is attenuated when osteocyte GαS−/− marrow is transplanted into WT recipients (97). Gain of function for GαS in osteoblasts was demonstrated by expressing a constitutively active engineered 5-Hydroxytryptamine receptor 4 receptor (98–100). These mice have dramatic bone remodeling with expansion of stromal populations (osteoblasts, MSCs, and endothelial cells) and fibrosis in the marrow (98, 100). Even though these HSPC-supportive populations are expanded in the setting of constitutive GαS signaling in osteoblasts, they suppress expression of genes required for HSPC maintenance (98). These studies provide evidence that GαS signaling has pleomorphic effects that are required both intrinsically and extrinsically in the marrow to support normal hematopoiesis and marrow structure. In addition to GαS signals, a role of orphan G protein-coupled receptors (GPRs) in the niche is beginning to emerge. Orphan receptor GPR56 has been characterized by Saito et al (101) as a component of both benign and malignant hematopoiesis. Expression of GPR56 by HSPCs facilitates matrix adhesion into the niche, which is transduced into the cell by activation of RhoA signaling (101). In leukemic cells, signals downstream of GPR56 mediate both chemotherapy resistance and cell survival (101). This study provides evidence that orphan GPCR signals are a component of the HSC niche and can be co-opted during malignant transformation.
Conclusion
Various components in the marrow tightly regulate HSPC function to maintain the stem cell pool while allowing normal hematopoiesis to occur. Osteoblasts, their mesenchymal precursors, and SNS neurons have been shown to be important regulators of HSPCs not only through direct contact but also through secreted signals. The heterogeneity of these signals is a therapeutic advantage because it provides multiple targets in the investigation for new agents for hematopoietic recovery and to increase stem cell yields for transplantation. Furthermore, niche stimulation may also restore normal hematopoiesis as a possible adjuvant treatment for malignancies. Hormones such as PTH, estrogen, and catecholamines participate in the activation of the microenvironment and have been highlighted in this review. In the near future, the interaction between these signals is likely to be explored to continue to highlight the complex nature of the HSC niche. Moreover, whether there is identity between benign and malignant niches in the marrow will continue to be a critical question, not only for the understanding of the basic biology of this system but also to ensure the safety of translation of niche activation to the treatment of malignancies. Therefore, the identification and characterization of new niche components will not only enhance our knowledge of HSPC regulation but may also be exploited in the future for therapeutic ends.
Acknowledgments
We thank members of the Calvi Lab for helpful discussion.
This work was supported by the National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases (DK081843), National Cancer Institute (CA166280), National Institute of Aging (AG046293), and National Institute of Allergy and Infectious Diseases (AI107276 and AI091036).
C.M.H. contributed conception and design, manuscript writing, and final approval of manuscript; L.M.C. contributed conception and design, financial support, manuscript writing, and final approval of manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AML
- acute myelogenous leukemia
- CAR
- CXCL12-abundant reticular
- CXCL12
- C-X-C motif chemokine 12
- dmPGE2
- 16-dimethyl PGE2
- GCSF
- granulocyte colony-stimulating factor
- GPR
- G protein-coupled receptor
- HSC
- hematopoietic stem cell
- HSPC
- hematopoietic stem and progenitor cell
- LSK
- lineage-negative, Sca1-positive, and C-kit–positive
- LT-HSC
- long-term HSC
- MDS
- myelodysplastic syndrome
- MPP
- multipotent progenitor
- MSC
- mesenchymal stem cell
- NE
- norepinephrine
- OVX
- ovariectomy
- PGE2
- prostaglandin E2
- SLAM
- signaling lymphocyte activation molecule
- SNS
- sympathetic nervous system
- ST-HSC
- short-term HSC
- WT
- wild-type.
References
- 1. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25 [PubMed] [Google Scholar]
- 2. Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296(5574):1855–1857 [DOI] [PubMed] [Google Scholar]
- 3. Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100(8):4633–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JN, Calvi LM. Concise review: Current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calvi LM, Link DC. Cellular complexity of the bone marrow hematopoietic stem cell niche. Calcif Tissue Int. 2014;94(1):112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–245 [DOI] [PubMed] [Google Scholar]
- 8. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121 [DOI] [PubMed] [Google Scholar]
- 9. Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107(3):924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvi LM, Bromberg O, Rhee Y, et al. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119(11):2489–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119(2):540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Porter RL, Georger MA, Bromberg O, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31(2):372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larochelle A, Savona M, Wiggins M, et al. Human and rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood. 2011;117(5):1550–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221 [DOI] [PubMed] [Google Scholar]
- 16. Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–20017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998;16(1):7–15 [DOI] [PubMed] [Google Scholar]
- 18. Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846 [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841 [DOI] [PubMed] [Google Scholar]
- 20. Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224(2):245–251 [DOI] [PubMed] [Google Scholar]
- 21. Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264 [DOI] [PubMed] [Google Scholar]
- 22. Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111(3):1173–1181 [DOI] [PubMed] [Google Scholar]
- 23. Chitteti BR, Cheng YH, Streicher DA, et al. Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function. J Cell Biochem. 2010;111(2):284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng YH, Chitteti BR, Streicher DA, et al. Impact of maturational status on the ability of osteoblasts to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res. 2011;26(5):1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura Y, Arai F, Iwasaki H, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116(9):1422–1432 [DOI] [PubMed] [Google Scholar]
- 26. Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336 [DOI] [PubMed] [Google Scholar]
- 27. Dominici M, Rasini V, Bussolari R, et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114(11):2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams GB, Alley IR, Chung UI, et al. Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature. 2009;459(7243):103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712 [DOI] [PubMed] [Google Scholar]
- 30. Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rawls AS, Gregory AD, Woloszynek JR, Liu F, Link DC. Lentiviral-mediated RNAi inhibition of Sbds in murine hematopoietic progenitors impairs their hematopoietic potential. Blood. 2007;110(7):2414–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vallet S, Pozzi S, Patel K, et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25(7):1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6(4):311–317 [DOI] [PubMed] [Google Scholar]
- 37. Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pinho S, Lacombe J, Hanoun M, et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Isern J, Martín-Antonio B, Ghazanfari R, et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3(5):1714–1724 [DOI] [PubMed] [Google Scholar]
- 40. Masuda S, Ageyama N, Shibata H, et al. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37(10):1250–1257.e1251 [DOI] [PubMed] [Google Scholar]
- 41. de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reitman ML, Gavrilova O. A-ZIP/F-1 mice lacking white fat: a model for understanding lipoatrophic diabetes. Int J Obes Metab Disord. 2000;24(Suppl 4):S11–S14 [DOI] [PubMed] [Google Scholar]
- 43. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu LF, Shen WJ, Ueno M, Patel S, Azhar S, Kraemer FB. Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PloS One. 2013;8(8):e72367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone–new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–35509 [DOI] [PubMed] [Google Scholar]
- 48. Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953 [DOI] [PubMed] [Google Scholar]
- 49. Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207 [DOI] [PubMed] [Google Scholar]
- 51. Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–1932 [DOI] [PubMed] [Google Scholar]
- 52. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317 [DOI] [PubMed] [Google Scholar]
- 53. Terauchi M, Li JY, Bedi B, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638 [DOI] [PubMed] [Google Scholar]
- 55. Kawabata K, Ujikawa M, Egawa T, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96(10):5663–5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694 [DOI] [PubMed] [Google Scholar]
- 57. Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonig H, Priestley GV, Nilsson LM, Jiang Y, Papayannopoulou T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104(8):2299–2306 [DOI] [PubMed] [Google Scholar]
- 59. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988 [DOI] [PubMed] [Google Scholar]
- 60. Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–399 [DOI] [PubMed] [Google Scholar]
- 61. Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508(7497):536–540 [DOI] [PubMed] [Google Scholar]
- 62. Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205(4):777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Verma DS, Fisher R, Spitzer G, Zander AR, McCredie KB, Dicke KA. Diurnal changes in circulating myeloid progenitor cells in man. Am J Hematol. 1980;9(2):185–192 [DOI] [PubMed] [Google Scholar]
- 66. Tsinkalovsky O, Filipski E, Rosenlund B, et al. Circadian expression of clock genes in purified hematopoietic stem cells is developmentally regulated in mouse bone marrow. Exp Hematol. 2006;34(9):1249–1261 [DOI] [PubMed] [Google Scholar]
- 67. Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447 [DOI] [PubMed] [Google Scholar]
- 68. Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–520 [DOI] [PubMed] [Google Scholar]
- 70. Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421 [DOI] [PubMed] [Google Scholar]
- 71. Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8(10):1123–1131 [DOI] [PubMed] [Google Scholar]
- 72. Fitch SR, Kimber GM, Wilson NK, et al. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell. 2012;11(4):554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158 [DOI] [PubMed] [Google Scholar]
- 74. Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120(2):303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jüppner H, Abou-Samra AB, Freeman M, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026 [DOI] [PubMed] [Google Scholar]
- 76. Mahon MJ, Bonacci TM, Divieti P, Smrcka AV. A docking site for G protein βγ subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol Endocrinol. 2006;20(1):136–146 [DOI] [PubMed] [Google Scholar]
- 77. Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, Vilardaga JP. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc Natl Acad Sci U S A. 2013;110(4):1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Weber JM, Forsythe SR, Christianson CA, et al. Parathyroid hormone stimulates expression of the Notch ligand Jagged1 in osteoblastic cells. Bone. 2006;39(3):485–493 [DOI] [PubMed] [Google Scholar]
- 79. Li JY, Adams J, Calvi LM, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012;120(22):4352–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25(2):238–243 [DOI] [PubMed] [Google Scholar]
- 81. Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19(11):1513–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brunner S, Theiss HD, Murr A, Negele T, Franz WM. Primary hyperparathyroidism is associated with increased circulating bone marrow-derived progenitor cells. Am J Physiol Endocrinol Metab. 2007;293(6):E1670–1675 [DOI] [PubMed] [Google Scholar]
- 83. Ballen KK, Shpall EJ, Avigan D, et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biol Blood Marrow Transplant. 2007;13(7):838–843 [DOI] [PubMed] [Google Scholar]
- 84. Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA. Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology. 2000;141(7):2309–2318 [DOI] [PubMed] [Google Scholar]
- 85. Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 2006;176(12):7371–7378 [DOI] [PubMed] [Google Scholar]
- 86. Li JY, Adams J, Calvi LM, Lane TF, Weitzmann MN, Pacifici R. Ovariectomy expands murine short-term hemopoietic stem cell function through T cell expressed CD40L and Wnt10B. Blood. 2013;122(14):2346–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frisch BJ, Porter RL, Gigliotti BJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114(19):4054–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ikushima YM, Arai F, Hosokawa K, et al. Prostaglandin E(2) regulates murine hematopoietic stem/progenitor cells directly via EP4 receptor and indirectly through mesenchymal progenitor cells. Blood. 2013;121(11):1995–2007 [DOI] [PubMed] [Google Scholar]
- 93. Ludin A, Itkin T, Gur-Cohen S, et al. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13(11):1072–1082 [DOI] [PubMed] [Google Scholar]
- 94. Goessling W, Allen RS, Guan X, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hanyu R, Wehbi VL, Hayata T, et al. Anabolic action of parathyroid hormone regulated by the β2-adrenergic receptor. Proc Natl Acad Sci U S A. 2012;109(19):7433–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fulzele K, Krause DS, Panaroni C, et al. Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood. 2013;121(6):930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schepers K, Hsiao EC, Garg T, Scott MJ, Passegué E. Activated Gs signaling in osteoblastic cells alters the hematopoietic stem cell niche in mice. Blood. 2012;120(17):3425–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chang WC, Ng JK, Nguyen T, et al. Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PloS One. 2007;2(12):e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hsiao EC, Boudignon BM, Chang WC, et al. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A. 2008;105(4):1209–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saito Y, Kaneda K, Suekane A, et al. Maintenance of the hematopoietic stem cell pool in bone marrow niches by EVI1-regulated GPR56. Leukemia. 2013;27(8):1637–1649 [DOI] [PubMed] [Google Scholar]