Abstract
A new vancomycin (VCM)-eluting mixed bilayer niosome formulation was evaluated for the control of staphylococcal colonization and biofilm formation on abiotic surfaces, a niosome application not explored to date. Cosurfactant niosomes were prepared using a Span 60/Tween 40/cholesterol blend (1: 1: 2). Tween 40, a polyethoxylated amphiphile, was included to enhance VCM entrapment and confer niosomal surface properties precluding bacterial adhesion. VCM-eluting niosomes showed good quality attributes including relatively high entrapment efficiency (∼50%), association of Tween 40 with vesicles in a constant proportion (∼87%), biphasic release profile suitable for inhibiting early bacterial colonization, and long-term stability at 4°C for a 12-month study period. Niosomes significantly enhanced VCM activity against planktonic bacteria of nine staphylococcal strains. Using microtiter plates as abiotic surface, VCM-eluting niosomes proved superior to VCM in inhibiting biofilm formation, eradicating surface-borne biofilms, inhibiting biofilm growth, and interfering with biofilm induction by VCM subminimal inhibitory concentrations. Data suggest dual functionality of cosurfactant VCM-eluting niosomes as passive colonization inhibiting barrier and active antimicrobial-controlled delivery system, two functions recognized in infection control of abiotic surfaces and medical devices.
Keywords: abiotic surfaces, biofilm, niosomes, staphylococcus aureus, vancomycin
INTRODUCTION
Biomedical devices such as intravascular catheters, prosthetic heart valves, and orthopedic implants are increasingly used in clinical practice to improve survival rates and the quality of life of millions of patients. Unfortunately, despite advances in surgical techniques and prophylactic systemic antibiotics, these abiotic surfaces are prone to bacterial infections mostly caused by Staphylococcus aureus and Staphylococcus epidermidis (1,2). Early bacterial colonization of the medical device surface may take place in the first 6-h postoperative "decisive period" with adherence of biofilm matrix (1). This impedes device integration into the surrounding tissue and protects bacteria from the host immune system and the action of systemic antibiotics. Thus, abiotic surface infections are recalcitrant to conventional systemic antimicrobial therapy based on susceptibility of planktonic cells (3,4). Development of potentially life-threatening systemic infections and device malfunction especially in critically-ill patients may necessitate risky and costly device removal or replacement procedures (5,6).
In this context, newer antimicrobial strategies were developed for combating abiotic surface infections (7). These are essentially based on a passive or active approach or a combination thereof. A passive approach involves inhibition of early microbial adhesion by modifying the device surface properties using antimicrobial-free coatings. As microbial surfaces are hydrophobic in nature (8), hydrophilic coatings made of polyethylene glycols and similar polymers confer antiadhesive properties, significantly reducing infection (9–11). An active approach is based on drug/medical device combination products providing a localized bacterial inhibition effect by functionalizing the device biomaterial and/or coating with antibiotics, antiseptics, and metals (12). Localized delivery of high doses of antibiotics enhances efficacy, prevents systemic toxicity, and reduces bacterial resistance (3,13).
More recently, drug delivery (14) and nanotechnology strategies (15) involving functionalization of biomaterials by surface coating (16,17), impregnation, or embedding (18,19) have emerged as a more effective approach for localized sustained antimicrobial control of medical biofilms. Lipid- and polymer-based drug carriers are the most exploited in this respect. Liposomes, phospholipid bilayer vesicles, confer antiadhesive properties to abiotic surfaces (7,14,15) and concentrate the antimicrobial pay load at the device surface and biofilm interfaces (15,20). In addition, liposomes can be designed to fuse with microbial cell membrane, enhancing antimicrobial efficacy (21) and to bind with the device-related infection site (22). Similar to liposomes, niosomes are bilayer hydrated vesicles of non-ionic surfactants and cholesterol (23). Being more physicochemically stable, easily handled, and less expensive, niosomes overcome the main limitations of liposomes. Niosomes are recognized to control the release and enhance the activity of antimicrobial agents including antibiotics (24,25), antibacterial, and antifungal agents (26–28) and to interact with phospholipid membranes (29). The use of hydrophilic polyethoxylated surfactants such as Tweens and Brijs either alone (30,31) or in combination with lipophilic surfactants (cosurfactant niosomes) (32) confers bilayer hydrophilicity which enhances entrapment of hydrophilic drugs and may prevent bacterial adhesion at niosome-treated surfaces. Accordingly, it could be hypothesized that cosurfactant antimicrobial-eluting niosomes may provide an alternative multifunctional approach to the control of abiotic surfaces infections, a niosome application not explored to date.
The aim of the study was to evaluate a vancomycin (VCM)-eluting cosurfactant niosome formulation combining the potentials of Tween-hydrophilized vesicles as drug reservoir with bacterial adhesion impeding properties and those of VCM, recommended for the prophylaxis and treatment of implant-related staphylococcal infections (3,33). The attributes of the vesicles in terms of size, drug entrapment efficiency, Tween 40 content, VCM release characteristics, and physical stability were assessed with emphasis on vesicular retention of Tween 40 as a water soluble bilayer component, an aspect not clearly understood yet. Further, the in vitro antimicrobial activity of VCM-eluting niosomes, mainly the antibiofilm effectiveness was assessed against different staphylococcal strains.
MATERIALS
The materials used were vancomycin hydrochloride (MW 1449.3, courtesy of Julphar, Gulf Pharmaceutical Industries, UAE), sorbitan monostearate (Span 60, HLB 4.7), polyoxyethylene sorbitan monopalmitate (Tween 40, HLB 15.6), cholesterol (Sigma-Aldrich Chemie Gmbh, Munich, Germany), methanol (Adwic, El-Nasr Pharmaceutical Co., Egypt), crystal violet (Merck, Darmstadt, Germany), and Tryptone soya broth (Difco, Detroit, USA). Other reagents were of analytical grade. Visking dialysis tubing (size 3, 20/32 in.), MW cut off 12,000–14,000 Da, was purchased from Medicell Int. Ltd., London, UK. Staphylococcal strains and clinical isolates were obtained from the collection of the Department of Pharmaceutical Microbiology, Faculty of Pharmacy, and the Department of Microbiology, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
METHODS
Preparation of Niosomes
Niosomes were prepared by the film hydration method using a lipid mixture of 75 μM of each of Span 60 and Tween 40 with 150 μM of cholesterol (surfactant: cholesterol ratio 1:1). Surfactants and cholesterol were dissolved in 3 mL chloroform which was evaporated under reduced pressure at 60°C in a rotary evaporator (Buchi Rotavapor, Switzerland). For blank niosomes, the formed film was hydrated with 5 mL distilled water by shaking in a water bath (Gesellschaft Fur Labortechnik, Germany) for 1 h at 60°C. For VCM-eluting niosomes, the film was hydrated with 5 mL VCM solution (2% w/v) in distilled water at 60°C. VCM was completely soluble at the concentration used. Dispersions were allowed to cool to room temperature (∼25°C) and left overnight at 4–5°C for swelling of the vesicles. Niosomal dispersions were not sonicated. In some experiments, VCM-loaded niosomes were separated from the supernatant by ultracentrifugation at 28,621×g for 60 min at 4°C (Sigma laborzentrifugen refrigerated centrifuge 3K-30, GMBH, Germany). The niosome pellet was washed with distilled water and recentrifuged for 60 min.
Characterization of Niosomes
Transmission Electron Microscopy (TEM)
A drop of niosome dispersion was placed on a carbon-coated grid and allowed to be adsorbed for 2 min before excess liquid was drawn off with a filter paper. A drop of 2% uranyl acetate aqueous solution was added to niosomes for negative staining. Samples were examined after 3 min at room temperature using a transmission electron microscope (Jeol-100 CX, Japan) equipped with a digital camera at 80 KV accelerating voltage.
Niosome Size Analysis and Zeta Potential
The mean diameter of niosomes was determined using Cilas laser diffraction particle size analyzer (Model 1064, Cilas, France) at 780 nm and a scattering angle of 175o at 25°C. Niosome dispersions were diluted 100-folds with deionized water and sonicated for 60 s. The change in size of VCM niosomes stored in their original solution at 4°C was monitored at 3-month intervals for 12 months. Size measurements were done in duplicate. The zeta potential of niosomes was determined at 25°C using Malvern dynamic light scattering particle size and zeta potential analyzer (Zetasizer nano ZS90, Malvern instruments, Worcestershire, UK) in triplicate.
Determination of Niosome-Associated Tween 40
The amount of Tween 40 associated with niosomes was determined using a colorimetric method (34). Briefly, blank niosomes were separated by ultracentrifugation. The vesicles pellet was washed and recentrifuged. Vesicles were dissolved in 10 mL chloroform and treated with 10 mL of cobalt thiocyanate aqueous solution (30 g/L cobalt nitrate hexahydrate and 200 g/L of ammonium thiocyanate) in a separating funnel. The funnel was shaken for 5 min for complete extraction of the blue colored complex in the organic layer. Absorbance was determined at λ max 623 nm against chloroform as blank (Shimatzu UV-visible spectrophotometer, model UV-1601 PC Shimatzu, Japan). Supernatants were similarly treated to determine their Tween 40 content. The percent recovery of niosome-associated Tween 40 was calculated as follows:
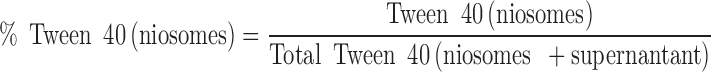 |
Tween 40, having high hydrophilicity (HLB 15.6) and hence, likely to leak out of vesicles, was selected as a niosome stability-monitoring parameter. The effect of storage of niosomes at 4°C on the retention of Tween 40 in blank niosomes was examined under two conditions: (a) niosomes kept in their original solution for 12 months and (b) niosomes separated by ultracentrifugation and redispersed in fresh dispersion medium (distilled water) three times at 2-week intervals (at 0, 2, and 4 weeks). Tween 40 in both vesicles and supernatant was determined (at 2, 4, and 6 weeks) 2 weeks after each niosome separation and redispersion as described above.
Vancomycin Entrapment Efficiency (EE %)
VCM-loaded niosomes were separated by ultracentrifugation, washed, recentrifuged, and disrupted using 10 mL methanol. VCM was assayed spectrophotometrically at λmax 281 nm. VCM concentration in the supernatant was also determined for mass balance calculation. The EE % was calculated with reference to total drug recovered as follows:
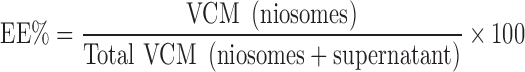 |
The procedure was used to monitor, at 3-month intervals, leakage of entrapped VCM from the drug-loaded vesicles stored in their original solution at 4°C for 12 months.
Vancomycin Release
A dialysis method was used (35). The dialysis tube was soaked in distilled water for 24 h. Niosome dispersion (0.5 mL, equivalent to 10 mg VCM) was placed in the dialysis bag. VCM aqueous solution (0.5 mL containing 10 mg VCM) was used as control. Dialysis bags were immersed in 75 mL distilled water in stoppered conical flasks and shaken in a water bath at 37°C. At time intervals (1, 2, 4, 6, 12, and 24 h), 5 mL of the recipient medium was removed and VCM assayed spectrophotometrically at λmax 281 nm. The removed samples were replaced with fresh medium at 37°C. The procedure was used to monitor VCM release stability at 3-month intervals for a 12-month storage period at 4°C. Experiments were run in duplicate.
In Vitro Antimicrobial Activity of Niosomal Versus Free Vancomycin
Activity of VCM After Niosome Preparation
Possible adverse effect of the niosome preparation procedure on VCM activity was checked by comparing antibacterial efficacy of niosome-eluted VCM with standard VCM. Elution was allowed by placing a niosome sample in a dialysis bag immersed in distilled water at 37°C in a shaking water bath for 6 h. The eluted VCM was assayed. The antibacterial efficacy of freshly prepared VCM solution and eluted VCM were compared at the same concentration by the cup-plate method against seven staphylococcal clinical isolates (S30, S32, S37, S75, MRSA, S. epidermidis ATCC 12228, and S. aureus ATCC 6538P). VCM niosomes were also included.
Screening for Biofilm-Forming Bacteria
A total of 33 staphylococcal strains (29 clinical isolates, 2 MRSA, standard S. aureus ATCC 6538p, and standard S. epidermidis ATCC 12228) were identified according to the Practical Medical Microbiology and Diagnostic Microbiology guidelines (36,37). Bacteria were screened for biofilm formation as reported (38). Sterile 96-well flat-bottom polystyrene microtiter plates with lids (Spektar, Cacak Yugoslavia) were filled with 200 μL of test bacterial cultures (106 CFU/mL) in tryptic soy broth (TSB) fortified with 0.25% glucose. Negative control wells contained 200 μL broth. Plates were covered and incubated aerobically for 48 h at 37°C. Following aspiration of the liquid content, wells were washed three times with 250 uL sterile saline. Plates were shaken to remove non-adherent bacteria. Attached bacteria were fixed with 200 μL of 99% methanol per well, and after 15 min, plates were emptied and left to dry. Plates were stained for 5 min with 0.2 mL 2% Hucker crystal violet per well. Excess stain was rinsed off. Stained biofilms were evaluated by measuring optical density at λmax 630 nm (OD630) using a microtiter plate reader (Biotek, USA). The cut off OD for biofilm formation was set at three standard deviations above the mean OD of the negative control (38,39). In the present study, OD higher than 0.1 were considered indicative of biofilm formation.
Blank niosomes were compared with VCM-loaded niosomes, a physical mixture of VCM and blank niosomes and a solution of free VCM to examine the antibacterial activity against seven staphylococcal isolates (S30, S32, S37, S75, MRSA, S.epidermidis ATCC 12228, and S.aureus ATCC 6538P) by the cup-plate method.
Determination of Minimum Inhibitory Concentrations (MICs)
The MICs of free and niosomal VCM were determined in nine staphylococcal isolates (S21, S23, S15, S5, S30, S35, MRSA, S.epidermidis ATCC 12228, and S. aureus ATCC 6538p) using standardized broth microdilution technique with final inoculums of 5 × 105 CFU/mL per well (40). Experiments were performed in duplicate with drug concentrations ranging from 0.03 to16 μg/mL.
Antibiofilm Activity
This was assessed using biofilm-forming staphylococcal isolates and sterile 96-well flat-bottom microtiter plates as abiotic surfaces.
Inhibition of Biofilm Formation
The Minimum Biofilm Inhibitory Concentrations (MBICs) of free and niosomal VCM were determined using four biofilm-forming S. aureus isolates (S21, S23, S15, S5) and standard S. aureus ATCC 6538P. Serial dilutions of VCM solution and VCM niosome dispersion (VCM concentration 0.03–16 μg/mL) were used. Blank niosomes similarly diluted were used for comparison. Plate wells were filled with 100 μL of the sample and 100 μL of the test bacterial cultures. Negative control wells contained 200 μL broth (TSB fortified with 0.25% glucose) while positive control wells contained 200 μL broth inoculated with the test bacterial culture. Plates were incubated aerobically for 48 h at 37°C and the procedure continued as described previously. The MBIC was determined as the lowest concentration giving a mean OD630 < 0.1. Experiments were run in duplicate.
Eradication of Surface-Borne Biofilms
The ability of VCM niosomes to eradicate surface-borne biofilms was investigated in comparison with free VCM solution by determining the minimum biofilm eradicating concentration (MBEC). Biofilm-forming staphylococcal isolates (S21, S23, S15, S5, and S. aureus ATCC6538P) were allowed to form biofilms. Wells containing biofilms were washed and filled with 200 μL serial dilutions of free VCM solution and niosome dispersions (0.03–16 μg/mL) in Muller Hinton broth (MHB). Wells filled with 200 μL MHB only were used as positive control. Plates were incubated aerobically for 24 h at 37°C. The well liquid content was aspirated and the well washed three times with 250 μL sterile saline. After antibiotic treatment, biofilms were fixed, stained, and evaluated by microplate reading at λmax 630 nm. Mean OD630 ≥ 0.1 indicated biofilm resistance.
Inhibition of Biofilm Growth
The biofilm growth inhibition effectiveness of VCM-eluting niosomes by adhesion to model biofilms (41,42) was assessed by measuring the reduction in OD630 of stained surface-borne biofilms subjected to free and niosomal vancomycin for 2 h. Two biofilm-forming isolates (S21, S23), and standard S. aureus ATCC 6538p were allowed to form biofilms as described before. Wells containing biofilms were treated with 200 μL of different concentrations (0.5, 0.75, 1, 1.5, and double the MIC for the respective strains) of niosomal VCM. Free VCM and a physical mixture of free VCM and blank niosomes acted as controls. After incubation for 2 h at 37°C, the liquid content of each well was aspirated and biofilms were washed. Biofilm regrowth was allowed by the addition of antibiotic-free growth medium for another 24 h. Liquid content was aspirated, and biofilms were fixed, washed, stained, and evaluated using the microplate reader at λmax 630 nm. The percent biofilm growth inhibition (BGI %) was calculated as follows:
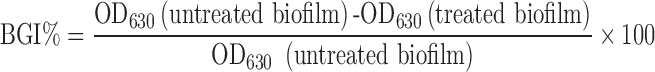 |
Induction of Biofilm Formation in Non-Biofilm-Forming Bacteria by Subminimum Inhibitory VCM Concentrations
The lowest concentrations of free and niosomal VCM that induced biofilm formation (Biofilm-Forming Concentration, BFC) were determined using three non-biofilm-forming clinical Staphylococcal isolates (S30, S35, and MRSA) and standard S. epidermidis ATCC 12228. Bacteria were incubated with serial dilutions below the corresponding MICs of free and niosomal VCM for 48 h at 37°C. Negative and positive controls were included. The biofilms developed were evaluated by microplate reading at λmax 630 nm.
RESULTS AND DISCUSSION
Niosomes can be made up of various hydrophobic and hydrophilic amphiphiles mixed with cholesterol as membrane stabilizer (30–32). A hydrophilic polyoxyethylated amphiphile either alone (30,31) or in combination with a lipophilic amphiphile (32,43) brings into play significant concentration and chain length dependent changes in niosome structure and properties. Less leaky vesicles characterized by greater EE % and reduced release rates of hydrophilic agents were reported (30–32). Thus, a blend of a Span 60 and Tween 40 and cholesterol was used. Span 60 and Tween 40, with a relatively long (C18 and C16, respectively) and saturated alkyl chain were selected to enhance VCM entrapment and niosome stability as reported for other drugs (31,32,44). A surfactant: cholesterol molar ratio of 1: 1 was used as equal molarity of non-ionic surfactant(s) and cholesterol enhances bilayer compactness, increasing entrapment efficiency (30,32,45). Regarding Span 60//Tween 40 M ratio, we demonstrated in an earlier study (27) that an equimolar blend of Span 60 and Tween 40 produced naftifine HCl-niosomes with good pharmaceutical attributes.
Characteristics of Niosomes
Cosurfactant niosomes were successfully prepared by the film hydration method using a VCM: Span 60: Tween 40: cholesterol molar ratio of 0.9: 1: 1: 2. The pH of the niosome dispersions in distilled water ranged from 2.9 to 3. 9. Attempts to use phosphate buffer saline pH 7.5 resulted in drug precipitation due to lower solubility of VCM in phosphate buffer (46).
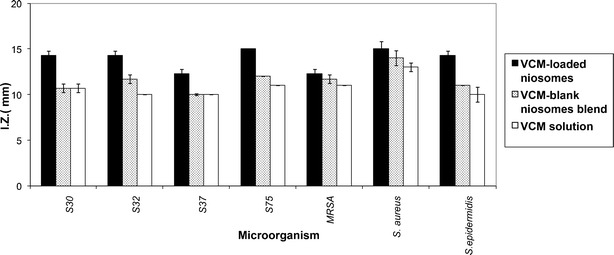
The antibacterial activity of VCM was not affected by the conditions of niosomes preparation. This was verified by identical inhibition zone diameters (mm), determined using the cup-plate method, of VCM fresh solution and VCM eluted from niosomes, tested against seven staphylococcal clinical isolates (Fig. 1). The figure also shows the comparatively larger inhibition zones of VCM-loaded niosomes in all tested strains.
Fig. 1.
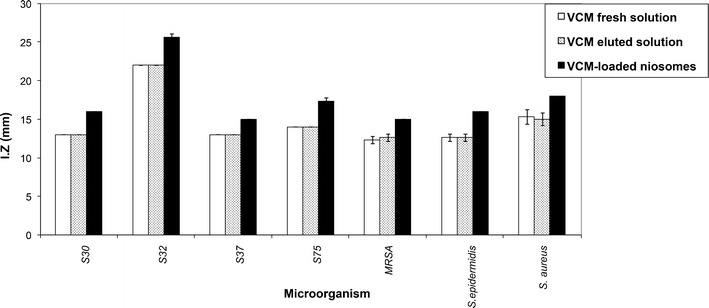
Inhibition zones, determined by the cup-plate method, of vcm eluted from niosomes, vcm fresh solution, and vcm-loaded niosomes (5 μg total vcm/ml in all cases) tested against seven staphylococcal isolates
Niosome Morphology, Size, and Zeta Potential
Figure 2a, b shows TEM micrographs of blank and drug-loaded niosomes. Discrete spherical vesicles were obtained. As niosomes prepared with lipophilic/hydrophilic surfactant blend may show elongated vesicles (32,47), morphology of VCM niosomes indicated adequate bilayer rigidity.
Fig. 2.
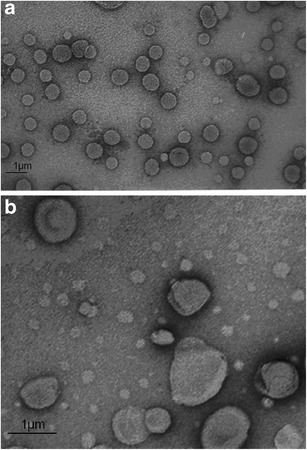
Transmission electron microscope photomicrographs of a blank niosomes and b vancomycin-loaded niosomes
The mean size of blank and VCM-loaded vesicles was 750 ± 40 nm and 820 ± 40 nm, respectively (Table I). This vesicle size was considered suited to the encapsulation of the large molecular weight VCM and no attempt was made to reduce the size by sonication or extrusion. A relatively large size is expected with cosurfactant niosomes as a result of hydration of the polyoxyethylene polar head groups and swelling of the niosome bilayer (31,32,47), though implication of the relatively long alkyl chains cannot be ruled out (30,45).
Table I.
Collective Stability Data Of Vancomycin Niosomes During Storage In Their Original Solution at 4°C for 12 months
| Time (month) | Vesicle mean diameter, nma | Vancomycin EE (%)b | Vesicle-associated Tween 40b (mg/5 mL) | Percentage of vancomycin release at 2 ha | Percentage of vancomycin release at 24 ha |
|---|---|---|---|---|---|
| Zero time | 820 ± 60 | 49.8 ± 1.73 | 83.5 ± 2.71 | 55 ± 2.04 | 72 ± 1.96 |
| 3 | 720 ± 30 | 49.4 ± 0.85 | 84.3 ± 4.89 | NDc | ND |
| 6 | 750 ± 40 | 48.9 ± 0.32 | 80.1 ± 1.40 | 50 ± 2.40 | 74 ± 2.23 |
| 9 | 640 ± 40 | 50.4 ± 1.14 | ND | ND | ND |
| 12 | 400 ± 40 | 51.7 ± 1.12 | 82.6 ± 1.80 | 50 ± 2.54 | 72 ± 1.79 |
aValues represent mean ± SD (n = 2)
bValues represent mean ± SD (n = 3)
c ND not determined
The change in size of VCM niosomes stored in their original solution at 4°C for 12 months indicated significant size reduction which was more evident in the last 6 months. The mean ± SD of vesicle diameter determined at 3, 6, 9, and 12 months was 720 ± 30, 750 ± 40, 640 ± 40, and 400 ± 40 nm, respectively (Table I). Vesicle shrinkage has been attributed to water evacuation until maximum compaction of vesicles with possible implication of difference in osmotic forces on both sides of the bilayer (48). In the present study, movement of VCM from the bilayer to the outer niosome surface during storage possibly created an osmotic force without affecting the EE equilibrium. Drug associated with the outer surface was not apparently removed during washing of the vesicle pellet as indicated by the unchanged EE % values during storage. Vesicle size data suggested lack of vesicle fusion and aggregation upon storage, probably as a result of the zeta potential of the niosome surface.
Zeta potential values indicated negatively charged surfaces of blank and VCM-loaded niosome (−36.7 mV to −36.9 mV). Similar results have been reported (26,49,50). Manosroi et al (49) attributed negative zeta potentials of blank and gallidermin loaded niosomes to the hydroxyl group in cholesterol molecule with uneven distribution of polarity. Dukhin and Göetz (50) suggested ionic dissociation of “non-ionic” Span surfactants with the formation of ionic impurities conferring highly negative charge as confirmed by conductivity measurements. Contribution of VCM which contains two basic and four acidic groups (46) to niosome surface charge may be obscured by multifactorial protonation-deprotonation equilibria (51).
Niosome-Associated Tween 40
Results indicated that approximately 87.7% of Tween 40 was associated with the vesicles following ultracentrifugation (calculated from Table I). Given that Tween 40 is highly hydrophilic, retention of a relatively large percentage in niosomes indicated integration into the mixed bilayer structure probably via molecular interactions between Tween 40 and Span 60 molecules. These involve mainly hydrophobic interaction of alkyl chain tails and hydrogen bonding between the closely packed polar head groups (43). Lower retention values were reported for Tween 20 and Tween 21 (44.3 and 55.1%, respectively) used as single surfactants in niosomes (52), pointing out the role of Span in enhancing niosomal retention of Tween molecules. It is worth noting that a water miscible additive, propylene glycol, was shown in an earlier study (53) to be similarly retained by liposomes at a constant 45% level.
Monitoring Tween 40 retention by blank niosomes upon storage at 4°C for 12 months (Table I) revealed no change. Constancy of percentage of Tween 40 retained (87.7%) indicated a state of equilibrium between Tween molecules in the supernatant and those associated with niosomes in favor of the vesicles.
Another important aspect of cosurfactant niosomes not investigated previously is the retention stability of the hydrophilic surfactant component under hydrodynamic conditions that may prevail in biomedical applications. The percentage of Tween 40 retained in niosomes stored at 4°C upon repeated dispersion in fresh medium at 2-week intervals for three consecutive times (Fig. 3) indicated movement of a proportion of Tween molecules out of the vesicles into the fresh medium to reestablish equilibrium. Although the amount of niosome-associated Tween 40 decreased by exposure to a fresh medium, its percentage retained in the vesicles, with respect to total Tween remaining was the same. Equilibrium was reestablished according to the same percentage retention (87.7%) throughout the study. The findings suggested that Tween release did not compromise vesicle integrity and also suggested potential resistance of cosurfactant niosomes to in-use hydrodynamic conditions, allowing for maintained performance.
Fig. 3.
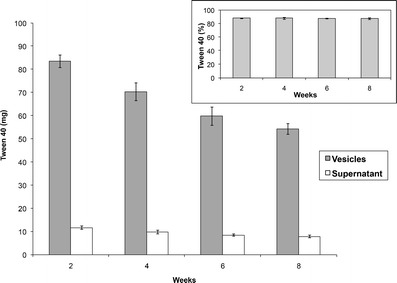
Tween 40 recovery from vesicles and supernatant 2 weeks after preparation (analyzed at 2 weeks) and 2 weeks after each repeated dispersion of separated vesicles in fresh medium (analyzed at 4, 6, 8 weeks). The inset shows percent Tween 40 in vesicles
Vancomycin Percent Entrapment Efficiency (EE %)
Despite VCM hydrophilicity, the classical film hydration method yielded cosurfactant niosomes with a relatively high mean EE % (49.8%, Table I). Relatively high EE % values were reported for hydrophilic drugs such as gentamicin sulfate (31) and diclofenac sodium (32) in cosurfactant niosome formulations. The EE % value obtained for VCM suggested that the drug was associated with the bilayer and partly entrapped as solution in the vesicle core. Hydration of the bilayer as a result of a surface charge may promote association of hydrophilic drugs to and into the bilayer structure (54).
Tween was also reported to modulate the hydrophobic bilayer environment in favor of larger less leaky vesicles and more efficient drug entrapment (30–32). VCM permeation was probably hindered by rigidity of the mixed bilayer conferred by closer packing of the polar head groups of Span and Tween (43,55). Apart from structural modulation, hydrogen bonding of Tween 40 (20 polyoxyethylene units) and VCM may also contribute to enhanced drug entrapment. Likewise, increased niosomal entrapment of ellagic acid (43) and para-hydroxybenzoic acid (56) was attributed to intermolecular hydrogen bonding.
Further, stability of VCM retention by vesicles was reassessed at 3-month intervals for niosomes stored in their original solution at 4°C for 12 months. As the niosome dispersion was already in equilibrium, EE % was not expected to change provided that the vesicles remained intact during storage. Vesicle disruption and drug leakage during storage in the mother liquor would cause the EE % value to decrease depending on the extent of leakage.
Despite significant reduction in niosome size upon storage, the initial mean EE % (49.8 ± 1.73) did not significantly change (p < 0.05), the EE % after 12 months being 51.7 ± 1.12 (Table I). Almost unchanged VCM content in both of the vesicles and supernatant indicated lack of disruption of vesicles. This can be possibly attributed to rigidity of the mixed bilayer and VCM-bilayer interaction. Poor leakage of VCM could be related, at least in part, to Tween 40 retention in niosomes and interaction with the polyoxyethylene chain via hydrogen bonding. Similar interactions were reported between VCM and polyethylene glycol (57,58). Apart from Tween 40-mediated interactions, cholesterol is known to stabilize bilayers, increasing cohesion among the apolar portion (52) and preventing leakage of entrapped drugs (23,30). A compact well-organized niosome membrane is additionally promoted by equal molarity of non-ionic surfactants and cholesterol (45,52).
Vancomycin Release
Release data obtained at 37°C by a dialysis method for VCM niosomes versus free drug under sink conditions are shown in Fig. 4. Transport of free VCM to the receiver compartment was completed in ≈ 6 h, verifying dialyzability. Release of VCM from freshly prepared niosomes was sustained for the 24-h study period indicating vesicle structural integrity throughout the study. Slower release compared to free drug has been claimed in studies involving lipid vesicles (54). Present data generated a biphasic profile, characteristic of vesicular systems (27,31). A faster initial phase (0 to 2 h) during which free drug molecules diffused into the release medium was followed by a slower phase (2 to 24 h) of progressive diffusion of entrapped drug molecules out of the vesicles. Drug release reached approximately 70% in 24 h. The release characteristics of niosomal VCM potentially meet the in-use requirements of an antimicrobial-eluting delivery system (14,15). Relatively fast initial antimicrobial release in effective concentrations is needed to inhibit early colonization by intruding bacteria while slower release allows maintenance of antimicrobial effect for a period depending on the delivery system characteristics and the application.
Fig. 4.
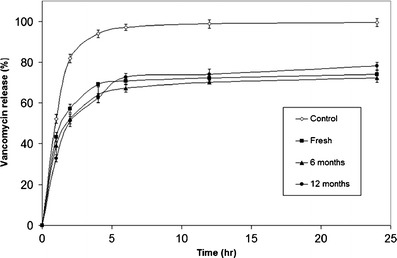
Release profiles at 37°C of vancomycin niosomes, freshly prepared and stored in their original solution at 4°C for 6 and 12 months. The control is vancomycin solution. Bars denote standard deviation
Linear regression analysis of the release data (2–12 h) indicated a diffusion-controlled mechanism. Higuchi parameters (slope, intercept, and correlation coefficient) are listed in Table II. Also included in the Table are percent unentrapped drug determined from EE values for comparison with Higuchi plot intercept. Similarity between the two columns of data indicated that the initial fast release was due to unentrapped drug.
Table II.
Higuchi Release Parameters Of Vancomycin Niosomes Before And After Storage In Their Original Solution at 4°C for 12 months
| Higuchi parameter | Correlation coefficient (r) | Slope | Intercept | Unentrapped VCMa (%) |
|---|---|---|---|---|
| Before storage | 0.9929 | 5.02 | 49.86 | 50.2 ± 1.73 |
| After storage | 0.9852 | 4.84 | 51.64 | 48.3 ± 1.12 |
Values represent mean values (n = 2). Coefficient of variation was below 6% for mean data shown
aEstimated from EE %
Release stability of VCM niosomes was assessed by monitoring reproducibility of release profiles at 3-month intervals during storage of niosomes in their original solution at 4°C for 12 months. Despite significant reduction in niosomes size upon storage, VCM release did not increase over the whole study period (Fig. 4). Coupling of VCM release stability data with those of VCM and Tween 40 leakage stability data (Fig. 4 and Table I) provided evidence for maintenance of niosome structural integrity for at least 12 months at 4°C.
The collective niosome physical stability results obtained at 4°C for 12 months (Table I) suggested that drug and Tween 40 leakage were interrelated and the reduction in niosome size during storage was a shrinkage process induced by osmotic effects. To this end, VCM niosomes appear to meet the pharmaceutical attributes of an antimicrobial delivery system that could be modulated for selected applications.
In Vitro Antimicrobial Activity of Niosomal Versus Free Vancomycin
Using the cup-plate method, blank niosomes proved devoid of antibacterial activity as revealed by the lack of inhibition zones. Results for VCM solution, VCM-loaded niosomes, and VCM/blank niosomes blend are shown in Fig. 5. Larger inhibition zones were observed for VCM-loaded niosomes, in the same species tested in a separate experiment (Fig. 1).
Fig. 5.
Inhibition zones of VCM-loaded niosomes, VCM/blank niosomes blend, VCM solution (5 μg total VCM/mL in all cases), and blank niosomes tested against seven staphylococcal isolates. Blank liposomes gave no inhibition zones
Minimal Inhibitory Concentrations (MICs)
The MIC for niosomal VCM versus free VCM determined by the broth dilution method against nine staphylococcal strains are shown in Table III. MICs for free VCM (0.5 to 8 μg/mL) were in agreement with literature values (59). For all test organisms, presentation of VCM in a niosomal form resulted in significant lowering of MIC, a two- to eight-fold reduction being observed. Results indicated potentials of VCM-eluting niosomes as an effective antimicrobial delivery system. Enhancement of antimicrobial activity by niosomal entrapment has been attributed to interaction between niosomes and microorganisms (24,26). As a vesicular system, niosomes may undergo fusion with the outer membrane of organisms, eliciting increased fluidity (24,60) and enhancing permeability of the drug released in the vicinity. Niosome encapsulation was also reported to enhance the antimicrobial activity of other antibiotics such as fluoroquinolones (24,25,61).
Table III.
Antimicrobial (MIC), Antibiofilm (MBIC & MBEC), and Biofilm-Forming (BFC) Activity of Free and Niosomal Vancomycin Determined in Different Staphylococcal Isolates
| Organism | MICs (μg/mL)a | MBICs (μg/mL)b | MBEC (μg/mL)c | BFCs (μg/mL)d | |||||
|---|---|---|---|---|---|---|---|---|---|
| Free VCM | Niosomal VCM | Free VCM | Niosomal VCM | Free VCM | Niosomal VCM | Free VCM | Niosomal VCM | ||
| Biofilm-forming strains | S21 | 4 | 1 | 4 | 1 | 8 | 2 | NDe | ND |
| S23 | 2 | 1 | 2 | 1 | 16 | 4 | ND | ND | |
| S15 | 4 | 1 | 4 | 1 | 8 | 8 | ND | ND | |
| S5 | 8 | 1 | 8 | 1 | 16 | 8 | ND | ND | |
| S. aureus | 4 | 1 | 4 | 1 | 8 | 2 | ND | ND | |
| Non-biofilm-forming strains | S30 | 0.5 | 0.25 | ND | ND | ND | ND | 0.25 | 0.015 |
| S35 | 2 | 0.50 | ND | ND | ND | ND | 0.25 | 0.015 | |
| MRSA | 8 | 1 | ND | ND | ND | ND | 4 | 0.125 | |
| S. epid. | 8 | 1 | ND | ND | ND | ND | 4 | 0.250 | |
a MIC minimum inhibitory concentration
b MBIC minimum biofilm inhibitory concentration
c MBEC minimal biofilm eradication concentration
d BFC biofilm-forming concentration; threshold concentration at which the antibiotic initiates biofilm formation in non-biofilm forming staphylococcal strains
e ND not determined
Antibiofilm Activity Against Biofilm-Forming Staphylococcal Strains
Control of abiotic surfaces infection depends on inhibition of early bacterial adherence and formation of biofilms which are tightly packed assemblies of bacteria showing greater tolerance to antibiotics (38,62). The effect of niosomal antibiotics on biofilms has not been documented to date. In the present study, antibiofilm activity in terms of inhibition of biofilm formation, eradication of a surface-borne biofilm, and inhibition of biofilm growth following exposure to niosomes was assessed. Further, induction of biofilm formation in non-biofilm-forming strains by subminimum inhibitory concentrations of VCM was evaluated.
Inhibition of Biofilm Formation
Among the 33 bacterial strains tested, only four S. aureus isolates (S21, S23, S5, and S15) and the standard S. aureus ATCC 6538p could form well defined biofilms as reported (38). Inhibition of biofilm formation was assessed by determining the Minimum Biofilm Inhibitory Concentrations (MBICs) using a plate reader to measure optical density at 630 nm (OD630). MBICs values were significantly lower for niosomal VCM compared to free VCM (Table III). Reduction in MBICs and MICs was matching for all test bacteria. Niosomal VCM inhibited biofilm formation at 1/2 the concentration of free VCM for S23, 1/4 that of free VCM for S21, S15 and standard S. aureus while for S5 biofilms, 1/8 the concentration of the free drug was inhibitory to biofilm. In each staphylococcal strain tested, MBIC coincided with the respective MIC. Similar values for VCM MIC (median 2 mg/L) and MBIC (median 2 mg/L) have been reported in a study involving 15 staphyloccocal strains (63).
Blank niosomes failed to inhibit biofilm formation; however, the optical density of biofilms formed in presence of blank niosomes dispersed in nutritive broth was less than that of biofilms formed in presence of nutritive broth alone (Table IV). A 50% reduction in OD630 values was observed using broth as positive control, pointing to a passive physical barrier effect of niosomes.
Table IV.
Optical Density (OD) Values of Stained Biofilms Formed by Different Staphylococcal Strains Placed in Broth and in Broth Containing Blank Niosomes
| Organism | OD of biofilm formed in broth | OD of biofilm formed in broth containing blank niosomes |
|---|---|---|
| S21 | 0.304 | 0.161 |
| S23 | 0.234 | 0.120 |
| S15 | 0.211 | 0.095 |
| S5 | 0.265 | 0.141 |
| S. aureus | 0.132 | 0.098 |
Accordingly, biofilm inhibition by niosomal VCM could be explained by a dual drug-based and vesicle-based functionality. A direct antibacterial VCM effect, obviously enhanced by niosomal encapsulation, was associated with a physical barrier effect conferred by cosurfactant niosomes partially competing with bacterial adhesion. A passive bacterial adhesion inhibitory effect on abiotic surfaces has been demonstrated for hydrophilic polyethylene glycol and polyethylene oxide polymer coatings (9–11, 64). Preferential adsorption of liposomal vesicles on immobilized S. aureus biofilms at the biofilm-bulk phase interface has been demonstrated by confocal microscopy (65).
Eradication of Surface-Borne Biofilm
The ability of VCM-eluting niosomes to eradicate surface-borne model biofilms was assessed in terms of Minimal Biofilm Eradication Concentrations (MBECs, Table III). Niosomal VCM could eradicate preformed biofilms at 1/4 the required concentration of free drug for S21, S23, and S. aureus and at 1/2 the required concentration of free VCM for S5. However, for S15, MBECs were similar for both niosomal and free VCM. MBECs were significantly higher compared to MICs and MBICs for the same bacterial strains verifying the need for higher VCM concentrations to eradicate surface-borne biofilms. The generally enhanced activity of niosomal VCM relative to the free drug corroborated reported ability of vesicular systems to adhere to bacterial biofilms, improving access of the released drug to the biofilm (14, 20, 21).
Biofilm Growth Inhibition
The ability of VCM-eluting niosomes to inhibit the growth of surface-borne model biofilms of three staphylococcal isolates by adhesion to biofilm during a relatively short exposure period was assessed in comparison to a VCM/blank niosomes physical blend and free VCM. Compared to the biofilm eradication study, conditions of biofilm growth inhibition were more challenging as the biofilm was treated initially with niosomes or controls for only 2 h after which the biofilms were washed and incubated in an antibiotic-free growth medium for 24 h. The study was performed at VCM concentrations ranging from half to double the MIC value for the respective strains (MICs are shown in Table III). For the physical blend, VCM concentrations were calculated in proportion to the MIC of free VCM. Results were expressed as percent Biofilm Growth Inhibition (% BGI) (41).
Results indicated that despite limited contact time (2 h), biofilm growth was stunted by the three treatments in a VCM concentration-dependent manner in the order: VCM-eluting niosomes > VCM/blank niosomes blend > free VCM (Fig. 6, a–c). The percent growth inhibition ranged from 55 to 67% at the highest VCM concentration. The difference between treatments was evident and consistent at all concentrations for the three organisms. The data generated suggest that VCM in niosomes, after a short term (2 h) contact with the biofilm, can reach and kill some of the bacterial cells embedded in the immobilized biofilm more efficiently than VCM solution.
Fig. 6.
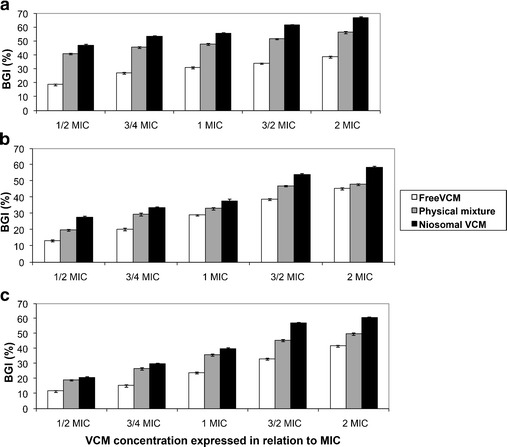
Percent Biofilm Growth Inhibition (% BGI) of free and niosomal VCM determined in staphylococcal isolates: a S23, b S21, and in c St. S. aureus. The physical mixture contains free drug and blank niosomes
While biofilm growth inhibition by free VCM represents a direct antibacterial effect, the greater effect of VCM/blank niosomes blend provided more evidence for the passive barrier effect of cosurfactant niosomes. The further greater activity of VCM-eluting niosomes was due to a passive barrier effect coupled with an active controlled VCM delivery effect into the biofilm.
Effect Of Vancomycin Subminimum Inhibitory Concentrations (sub-MIC) On Biofilm Formation by Non-Biofilm-Forming Bacteria
Biofilm induction by sub-MICs has been documented for some antibiotics including VCM as a defensive reaction of bacteria (66). This is of clinical relevance as bacteria may be exposed to sub-MIC antibiotic concentrations during systemic and local antibiotic therapy and antibiotic treatment of abiotic surfaces. In this study, it was observed that sub-MIC concentrations of free VCM induced biofilm formation by non-biofilm-forming staphylococcal isolates. This effect was compared to that of niosomal VCM using three non-biofilm-forming clinical Staphylococcal isolates (S30, S35, and MRSA) and standard S. epidermidis ATCC 12228. Sub-MICs of both free and niosomal VCM induced biofilm formation, at biofilm-forming concentrations (BFCs) ranging from 0.25 to 4 μg/mL and 0.015 to 0.25 μg/mL for free and niosomal VCM, respectively (Table III). For all strains tested, the formed biofilms, in case of VCM niosomes, were of lower OD630 (Fig. 7), implying interference of niosomes with induction of biofilm formation at low antibiotic concentrations. This adds to the benefits of VCM-eluting niosomes in inhibiting biofilm formation on abiotic surfaces including long-term indwelling medical devices.
Fig. 7.

Biofilm formation, expressed as optical density values at 630 nm, induced by sub-MICs of free and niosomal VCM determined in non-biofilm-forming staphylococcal strains and clinical isolates: a St. S. epidermidis, b S30, c MRSA, and d S35. The control is broth
CONCLUSION
The VCM-eluting cosurfactant (Span 60/Tween 40) niosome formulation presented offers promise as an antimicrobial delivery system for the control of colonization and biofilm formation on abiotic surfaces, an innovative niosome application. The mixed bilayer composition provided sufficient structural integrity promoting retention of the hydrophilic amphiphile, Tween 40, in an invariable vesicle/supernatant ratio and VCM for at least 12 months at 4°C. Microbiological findings obtained with model staphylococcal biofilms suggest a combined passive niosome barrier effect reducing bacterial adhesion and active antibiotic-mediated effect enhanced by possible interaction of the vesicle bilayers with microbial cells in biofilms. This was manifested as a noticeable antibiofilm activity encompassing inhibition of biofilm formation, eradication of surface-borne biofilms, biofilm growth inhibition, and interference with biofilm induction by sub-MIC VCM concentrations. Findings support VCM-eluting cosurfactant niosomes as a stable and inexpensive delivery system offering promise as an alternative approach to the control of abiotic surfaces infection.
Contributor Information
Heba S. Barakat, Email: h_barakat76@yahoo.com
Mervat A. Kassem, Email: mrvtkssmalex@yahoo.com
Labiba K. El-Khordagui, Phone: +2034871317, Email: lakhalil@gmail.com, Email: labiba.elkhordagy@alexu.edu.eg
Nawal M. Khalafallah, Email: nawalkhalaf@dataxprs.com.eg
References
- 1.McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol. 2008;60(12):1551–71. doi: 10.1211/jpp.60.12.0001. [DOI] [PubMed] [Google Scholar]
- 2.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64(1):175–88. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 3.Chilukuri DM, Shah JC. Local delivery of vancomycin for the prophylaxis of prosthetic device-related infections. Pharm Res. 2005;22(4):563–72. doi: 10.1007/s11095-005-2497-7. [DOI] [PubMed] [Google Scholar]
- 4.Gordon RJ, Weinberg AD, Pagani FD, Slaughter MS, Pappas PS, Naka Y, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127(6):691–702. doi: 10.1161/CIRCULATIONAHA.112.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Int Med. 2011;171:1821–8. doi: 10.1001/archinternmed.2011.441. [DOI] [PubMed] [Google Scholar]
- 6.Habib A, Le KY, Baddour LM, Friedman PA, Hayes DL, Lohse CM, et al. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol. 2013;111(6):874–9. doi: 10.1016/j.amjcard.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 7.Sousa C, Henriques M, Oliveira R. Mini-review: antimicrobial central venous catheters-recent advances and strategies. Biofouling. 2011;27(6):609–20. doi: 10.1080/08927014.2011.593261. [DOI] [PubMed] [Google Scholar]
- 8.Van der Mei HC, Leonard AJ, Weerkamp AH, Rouxhet PG, Busscher HJ. Surface properties of Streptococcus salivarius HB and nonfibrillar mutants: measurement of zeta potential and elemental composition with X-ray photoelectron spectroscopy. J Bacteriol. 1988;170(6):2462–6. doi: 10.1128/jb.170.6.2462-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mat Res. 2000;51(3):343–51. doi: 10.1002/1097-4636(20000905)51:3<343::AID-JBM7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol. 2010;59(3):227–38. doi: 10.1111/j.1574-695X.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee I, Pangule RC, Kane RS. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23(6):690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27(11):2450–67. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Keeling WB, Myers AR, Stone PA, Heller L, Widen R, Back MR, et al. Regional antibiotic delivery for the treatment of experimental prosthetic graft infections. J Surg Res. 2009;157(2):223–6. doi: 10.1016/j.jss.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 14.Tamilvanan S, Venkateshan N, Ludwig A. The potential of lipid- and polymer-based drug delivery carriers for eradicating biofilm consortia on device-related nosocomial infections. J Controlled Release. 2008;128(1):2–22. doi: 10.1016/j.jconrel.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Sousa C, Botelho CM, Oliveira R. Nanotechnology applied to medical biofilms control. In: Méndez-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances: Formatex Research Center, 2011. p. 878-88
- 16.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet J-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother. 2008;61(4):869–76. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 17.Lellouche J, Friedman A, Lahmi R, Gedanken A, Banin E. Antibiofilm surface functionalization of catheters by magnesium fluoride nanoparticles. Int J Nanomedicine. 2012;7(1):1175–88. doi: 10.2147/IJN.S26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27(11):2440–9. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29(31):4157–63. doi: 10.1016/j.biomaterials.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Robinson AM, Bannister M, Creeth JE, Jones MN. The interaction of phospholipid liposomes with mixed bacterial biofilms and their use in the delivery of bactericide. Colloids Surf, A: Physicochem Eng Aspects. 2001;186(1–2):43–53. doi: 10.1016/S0927-7757(01)00481-2. [DOI] [Google Scholar]
- 21.Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):2016–22. doi: 10.1128/AAC.01547-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X-M, Zhang Y, Chen F, Khutsishvili I, Fehringer E, Marky L, et al. Prevention of orthopedic device-associated osteomyelitis using oxacillin-containing biomineral-binding liposomes. Pharm Res. 2012;29(11):3169–79. doi: 10.1007/s11095-012-0812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interface Sci. 2012;183–184:46–54. doi: 10.1016/j.cis.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Satish J, Amusa AS, Gopalakrishna P. In vitro activities of fluoroquinolones entrapped in non-ionic surfactant vesicles against ciprofloxacin-resistant bacteria strains. J Pharm Technol Drug Res. 2012;1(1):1–11. doi: 10.7243/2050-120X-1-5. [DOI] [Google Scholar]
- 25.Akbari HS, Abedi D, Pardakhty A, Mohsen S, Shafizadegan V. Antimicrobial properties of non-ionic surafactant vesicles containing ciprofloxacin. Res Pharm Sci. 2012;7(5):S15. [Google Scholar]
- 26.Kopermsub P, Mayen V, Warin C. Potential use of niosomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res Int. 2011;44(2):605–12. doi: 10.1016/j.foodres.2010.12.011. [DOI] [Google Scholar]
- 27.Barakat HS, Darwish IA, El-Khordagui LK, Khalafallah NM. Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev Ind Pharm. 2009;35(5):631–7. doi: 10.1080/03639040802498864. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh KS, Chellampillai B, Pawar AP. Studies on nonionic surfactant bilayer vesicles of ciclopirox olamine. Drug Dev Ind Pharm. 2010;36(8):946–53. doi: 10.3109/03639040903585150. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, Tatsui T, Shimanouchi T, Umakoshi H. Membrane interaction between Span 80 vesicle and phospholipid vesicle (liposome): span 80 vesicle can perturb and hemifuse with liposomal membrane. Colloids Surf B: Biointerfaces. 2013;106:258–64. doi: 10.1016/j.colsurfb.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf, B. 2003;30(1–2):129–38. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 31.Abdelbary G, El-gendy N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech. 2008;9(3):740–7. doi: 10.1208/s12249-008-9105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdallah M, Sammour O, Ghamry H, Abu-Selem M. Preparation and in-vitro evaluation of diclofenac sodium niosomal formulations. IJPSR. 2013;4(5):1757–65. [Google Scholar]
- 33.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato H, Nagai Y, Yamamoto K, Sakabe Y. Determination of polysorbates in foods by colorimetry with confirmation by infrared spectrophotometry, thin-layer chromatography, and gas chromatography. J AOAC. 1989;72(1):27–9. [PubMed] [Google Scholar]
- 35.Manconi M, Sinico C, Valenti D, Lai F, Fadda AM. Niosomes as carriers for tretinoin: III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm. 2006;311(1–2):11–9. doi: 10.1016/j.ijpharm.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 36.Collee JE, Miles RS. Mackie and McCartney practical medical microbiology. New York: Edinburgh: Churchill Livingstone, 1989.
- 37.Brooks GF, Butel JS, Morse SA. Jawetz, Melnick, & Adelberg’s medical microbiology. New York: McGraw-Hill Medical; 2007. [Google Scholar]
- 38.Stepanović S, Vuković D, Dakić I, Savić B, Svabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–9. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 39.Carsenti-Etesse H, Durant J, Entenza J, Mondain V, Pradier C, Bernard E, et al. Effects of subinhibitory concentrations of vancomycin and teicoplanin on adherence of staphylococci to tissue culture plates. Antimicrob Agents Chemother. 1993;37(4):921–3. doi: 10.1128/AAC.37.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In: CLSI, editor.: Wayne PA USA 2006. p. Approved standard M7–A.
- 41.Vyas SP, Sihorkar V, Jain S. Mannosylated liposomes for bio-film targeting. Int J Pharm. 2007;330(1–2):6–13. doi: 10.1016/j.ijpharm.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Kim H-J, Michael Gias EL, Jones MN. The adsorption of cationic liposomes to Staphylococcus aureus biofilms. Colloids Surf, A: Physicochem Eng Aspects. 1999;149(1-3):561–70. doi: 10.1016/S0927-7757(98)00765-1. [DOI] [Google Scholar]
- 43.Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm. 2012;423(2):303–11. doi: 10.1016/j.ijpharm.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Yoshioka T, Sternberg M, Moody M, Florence AT. Niosomes from Span surfactants: relations between structure and form. J Pharm Pharmacol. 1992;44:1044–50. [Google Scholar]
- 45.Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377(1–2):1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Center for Drug Evaluation and Research, FDA. Vancomycin solubility study, report to Office of Generic Drugs. February 5, 2008.
- 47.Ruckmani K, Sankar V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech. 2010;11(3):1119–27. doi: 10.1208/s12249-010-9480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabin J, Prieto G, Ruso JM, Hidalgo-Alvarez R, Sarmiento F. Size and stability of liposomes: a possible role of hydration and osmotic forces. Eur Phys J E. 2006;20(4):401–8. doi: 10.1140/epje/i2006-10029-9. [DOI] [PubMed] [Google Scholar]
- 49.Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Gotz F, Manosroi W, et al. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392(1–2):304–10. doi: 10.1016/j.ijpharm.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 50.Dukhin AS, Goetz PJ. How non-ionic "electrically neutral" surfactants enhance electrical conductivity and ion stability in non-polar liquids. J Electroanal Chem. 2006;588(1):44–50. doi: 10.1016/j.jelechem.2005.12.001. [DOI] [Google Scholar]
- 51.Takács-Novák K, Noszál B, Tókés-Kövesdi M, Szász G. Acid-base properties and proton-speciation of vancomycin. Int J Pharm. 1993;89(3):261–3. doi: 10.1016/0378-5173(93)90252-B. [DOI] [Google Scholar]
- 52.Di Marzio L, Marianecci C, Petrone M, Rinaldi F, Carafa M. Novel pH-sensitive non-ionic surfactant vesicles: comparison between Tween 21 and Tween 20. Colloids Surf, B: Biointerfaces. 2011;82(1):18–24. doi: 10.1016/j.colsurfb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech. 2012;13(2):723–31. doi: 10.1208/s12249-012-9783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull. 2011;34(7):945–53. doi: 10.1248/bpb.34.945. [DOI] [PubMed] [Google Scholar]
- 55.Lu D, Rhodes DG. Mixed composition films of Spans and Tween 80 at the air-water interface. Langmuir. 2000;16(21):8107–12. doi: 10.1021/la000396s. [DOI] [Google Scholar]
- 56.Hao Y-M, Ka L. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403(1–2):245–53. doi: 10.1016/j.ijpharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 57.Lawson MC, Shoemaker R, Hoth KB, Bowman CN, Anseth KS. Polymerizable vancomycin derivatives for bactericidal biomaterial surface modification: structure-function evaluation. Biomacromolecules. 2009;10(8):2221–34. doi: 10.1021/bm900410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson M, Hoth K, DeForest C, Bowman C, Anseth K. Inhibition of staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin Orthop Relat Res. 2010;468(8):2081–91. doi: 10.1007/s11999-010-1266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C-M, Chen C-H, Pornpattananangkul D, Zhang L, Chan M, Hsieh M-F, et al. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32(1):214–21. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Ciprofloxacin nano-niosomes for targeting intracellular infections: an in vitro evaluation. J Nanoparticle Res. 2013;15(4):1–14. doi: 10.1007/s11051-013-1556-y. [DOI] [Google Scholar]
- 62.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant material. Biomaterials. 2012;33(26):5967–82. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 63.Kotulova D, Slobodnikova L, Longauerova A. Susceptibility of Staphylococcus aureus biofilms to vancomycin and gentamicin. Int J Antimicrob Agents. 2007;29:S170. doi: 10.1016/S0924-8579(07)70545-9. [DOI] [Google Scholar]
- 64.Nejadnik MR, van der Mei HC, Norde W, Busscher HJ. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials. 2008;29(30):4117–21. doi: 10.1016/j.biomaterials.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Ahmed K, Gribbon P, Jones MN. The application of confocal microscopy to the study of liposome adsorption onto bacterial biofilms. J liposome Res. 2002;12(4):285–300. doi: 10.1081/LPR-120016195. [DOI] [PubMed] [Google Scholar]
- 66.Laureti L, Matic I, Gutierrez A. Bacterial responses and genome instability induced by subinhibitory concentrations of antibiotics. Antibiotics. 2013;2(1):100–14. doi: 10.3390/antibiotics2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]


