Abstract
Desonide is a topical corticoid used in the treatment of skin diseases and is marketed in different pharmaceutical dosage forms. Recently, the poor photostability of a commercially available hair solution after direct exposure to UVA light was verified. In this study, we investigated the ability of the antioxidants ascorbic acid, butylhydroxyanisole (BHA), butylhydroxytoluene (BHT), α-tocopherol, and the UV filter benzophenone-3 (BP-3) to prevent the photodegradation of desonide in hair solution (desonide 0.1%) and the stability of the proposed formulation under environmental conditions. The tested antioxidants were not able to prevent the photolysis of desonide, whereas the addition of 0.3% BP-3 enhanced the photostability of the drug. After 15 h of direct exposure to UVA radiation, the desonide remaining content in the hair solution with BP-3 was approximately 98%. Higher photostability was also verified under UVC radiation. Additionally, the results indicated that the formulation was stable under accelerated and room temperature conditions for 70 days, corresponding to the total period of the study.
KEY WORDS: antioxidants, benzophenone-3, desonide, photostability, stability
INTRODUCTION
Desonide (Fig. 1) is a glucocorticoid with anti-inflammatory and antipruritic activities that is used in the treatment of corticosteroid-responsive dermatoses. These drugs are widely used for the treatment of skin diseases of inflammatory, proliferative, or immunological origin (1).
Fig. 1.
Desonide chemical structure
The topical efficacy of these drugs depends on the potency of the steroid, the permeability coefficient and the drug concentration in the preparation, the vehicle and excipients, and metabolic local processes. There is no consensus about the potency of desonide; it is classified as a corticosteroid of middle (2) or low potency (3,4). In a study published in 2009, desonide was indicated as the topical corticoid most prescribed for the treatment of atopic dermatosis in the USA (5). In Brazil, desonide is marketed as an ointment, cream, gel cream, cream lotion (0.5 mg/g), and hair lotion (1.0 mg/g), and it has been produced by several national laboratories and compounding pharmacies (6). In some countries, desonide is available as foam (5).
The photoreactive potential of glucocorticoids is well known. Photo-oxidation was reported for hydrocortisone, cortisone, and its acetates in the solid state, mediated by the loss of the side chain on C17. For prednisone, triamcinolone, prednisolone, and betamethasone, a photorearrangement from cyclohexadienones to bicyclohexenones has been reported. Considering their photoreactive activity, the protection from light is indicative to this class of drugs (7), as suggested in the compendial monographs. Regarding the photostability of desonide, Iqbal and co-workers reported the photoinstability of desonide solubilized in organic solvents after exposure to UVB and UVC radiation. They verified the lumiketone rearrangement on the cross-conjugated ketone after exposure to UVC radiation and the photocleavage of the C17–C20 bond followed by hydrogen atom abstraction when the drug was exposed to UVB radiation (8).
The three ranges of ultraviolet radiation have different effects and characteristics. UVC radiation (200–280 nm) is called shortwave or far-UV and does not reach the Earth’s surface because of absorption by the stratosphere. However, UVC can be found in artificial radiation sources, such as germicide lamps, and can cause skin damage and rapid photodegradation (9). The UVB component is responsible for causing sunburn and skin cancer, among other biological effects (9,10). UVA radiation is the least energetic band and is known as near-UV. However, 95% of the ultraviolet radiation that reaches the Earth’s surface consists of UVA radiation (320–400 nm), and only 5% is UVB radiation (290–320 nm) (10). Photosensitization by endogenous or exogenous substances and radiation absorption by DNA and proteins may be some of the chemical and biological effects caused by UVA radiation (9).
Recently, Dalla Santa and co-workers verified the photoinstability of a marketed desonide hair solution after direct UVA radiation exposure (352 nm). It was found that after 2 h of irradiation, the residual content of the drug was less than 90% (11). It is important to emphasize that the manufacturer of this pharmaceutical preparation recommends application twice a day, and no information about photostability is provided. Some practical consequences of the photoinstability are the loss of potency, resulting in a therapeutically inactive product and the formation of degradation products, which can be toxic (12).
Photolabile drugs require light protection from handling until the final product. Photoprotection can be acquired through external or internal protection. External protection avoids radiation from reaching the formulation, by appropriate packaging or by the use of a coating, as in the case of capsules and tablets. Internal protection can be acquired through the addition of stabilizers. These substances should be able to absorb the radiation more quickly than the drug, or they can act by suppressing the photoreaction (7,12,13).
Therefore, in this study, we evaluated the effect of some known stabilizers aiming to prevent the photodegradation of desonide in hair solution. For this purpose, several formulations were prepared, and their photostabilities under UVA and UVC radiation were determined to obtain a new formulation with higher stability. Furthermore, a stability test under room conditions was performed.
MATERIALS AND METHODS
Materials
The desonide (99.67%, lot DS002/0510/EF) that was used to prepare the formulations was obtained from Cosmetrade (Porto Alegre, Brazil). The desonide reference substance (98.55%, lot 0622-3) was purchased from Proactive Molecular Research (Alachua, USA). Methanol and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany) and Tedia (Fairfield, USA), respectively. Ultrapure water (>18 MΩ-cm) that was used to prepare the mobile phase was purified by a Megapurity Water Purification System. Isopropyl alcohol, methylparaben, propylparaben, and benzophenone-3 (BP-3) were obtained from Delaware (Porto Alegre, Brazil). The acetone was purchased from Impex (Diadema, Brazil), and the propylene glycol was purchased from Belga Import and Export of Chemicals Ltd. (Santa Maria, Brazil). Butylhydroxyanisole (BHA), butylhydroxytoluene (BHT), ascorbic acid, and α-tocopherol were obtained from Henrifarma (São Paulo, Brazil), Embacaps (Porto Alegre, Brazil), Delaware (Porto Alegre, Brazil), and Pharma Nostra (Rio de Janeiro, Brazil), respectively. All the reagents were used as received.
Preparation of Formulations F1–F7
Hair solution with a qualitative composition similar to the one commercially available was prepared (F1); alternatively, the antioxidants BHT, BHA, ascorbic acid, and α-tocopherol were added up to the highest concentration that is usually employed in pharmaceuticals (14). These formulations were denominated as F2, F3, F4, and F5, respectively. In addition, hair solutions containing different quantities of benzophenone-3 (BP-3) were prepared (named F6). The compositions of the formulations are indicated in Table I. To prepare the formulations, desonide, methylparaben, and propylparaben were solubilized in propylene glycol, and then isopropyl alcohol and acetone were added. BP-3 was solubilized in acetone, and the antioxidants were solubilized in propylene glycol. After all the components were mixed, acetone was used to obtain the appropriate weight, and the formulations were packed and sealed tightly in amber glass flasks. All the components were at room temperature before mixing.
Table I.
Quali-Quantitative Composition of the Hair Solution Formulation
| Percentage of each component (w/w) | |||||||
|---|---|---|---|---|---|---|---|
| Component | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
| Desonide | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Isopropyl alcohol | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Methylparaben | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Propylparaben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylene glycol | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| BHT | – | 0.1 | – | – | – | – | – |
| BHA | – | – | 0.02 | – | – | – | – |
| Ascorbic acid | – | – | – | 0.1 | – | – | – |
| α-Tocopherol | – | – | – | – | 0.05 | – | – |
| BP-3 | – | – | – | – | – | # | 0.3 |
| Acetone | q.s 100 g | q.s 100 g | q.s 100 g | q.s 100 g | – | q.s 100 g | q.s 100 g |
q.s quantity sufficient, #At concentrations of 2.5, 1.25, 0.6, 0.3, and 0.15%
Assay of Formulations F1–F6
The desonide content in the formulations that were prepared was determined by HPLC (11). The chromatographic system consisted of a Shimadzu LC system (Kyoto, Japan) with a LC-20AT pump, SPD-M20A detector, DGU-20A5 degasser, CBM-20A system controller, and LC-SOLUTION software. An RP-18 column (Luna® 4.6 × 150 mm, 5 μm, Phenomenex, Torrance, CA, USA) was used with a mobile phase composed of an aqueous solution of triethylamine 0.3%, (pH 4.0), methanol, and acetonitrile (50:10:40) at a flow rate of 1.0 mL min−1, injection volume of 20 μL, and detection at 244 nm.
To prepare sample solutions, portions of each hair lotion developed (1.0 g, equivalent to 1.0 mg of desonide) were accurately weighed and then diluted to 50 μg mL−1 with methanol. The content of desonide in the formulations was calculated against a desonide reference substance solution (50 μg mL−1) and was assumed as a reference for the subsequent assays.
Preliminary Photostability Evaluation
In the preliminary photostability study, we investigated whether antioxidants and the UV filter would be able to protect desonide from photodegradation. For this purpose, 1.0 g aliquots of formulations F1 to F6 (Table I) were dispensed into transparent capped cuvettes (Brand® ultra-micro UV-Cuvettes, 12.5 mm × 45 mm × 12.5 mm) and exposed to UVA radiation in a mirrored chamber for a period of 15 h. At the end of the exposure period, the samples were diluted to an estimated concentration of 50 μg mL−1 with methanol, and the drug content was determined by HPLC using the method mentioned in the section “Assay of formulations F1–F6.” Samples and analyses were performed in triplicate.
Validation of the Analytical Method
To perform the experiments with the most photostable formulation, which was determined during the preliminary photostability study, optimization of the analytical conditions was necessary to decrease the analysis time. From this purpose, a mobile phase with the same qualitative composition but with the proportion changed to 60:10:30 at a flow rate of 1.0 mL min−1 was employed. An RP-18 4.6 × 250 mm, 5-μm column (Phenomenex Luna®, Torrance, CA, USA) and the same chromatographic system described before were used. The modified analytical method was validated by evaluating the specificity, linearity, precision, and accuracy parameters as described below. The chromatographic parameters (theoretical plates, tailing factor, capacity factor, and resolution) were also examined.
Preparation of Desonide Reference Substance and Sample Solutions
The stock solutions with the desonide reference substance (500 μg mL−1) were prepared with methanol because of the solubility of desonide. From these solutions, dilutions with the same solvent were made up to the working concentration (50 μg mL−1). To the sample preparation, 1.0 g of hair solution (equivalent to 1 mg of desonide) was accurately weighed and diluted with methanol to an estimated concentration of 50 μg mL−1.
Validation Parameters
The following parameters were evaluated: specificity, linearity, precision, and accuracy. To verify the method specificity, the interference from the excipients was determined. Thus, solutions of the placebo and desonide hair solutions were prepared in accordance with the sample preparation procedure and analyzed using the developed method. The placebo solution was composed of a mixture of all the excipients of the most photostable formulation. Furthermore, the peak purity index was evaluated using a PDA detector, and values higher than 0.9999 were considered acceptable (15).
Linearity was determined through the analysis of three independent analytical curves, each one with five concentrations (5, 10, 20, 50, and 100 μg mL−1) of the reference substance, diluted as mentioned at the section titled “Preparation of desonide reference substance and sample solutions.” Analytical curves were constructed by plotting the peak areas against the respective concentration of desonide. Linear regression analysis was performed using the least squares method, in which the calibration equation and the correlation coefficient r were determined. Analysis of variance (ANOVA) was used to determine the compliance of the linear model.
The precision of the analytical method was evaluated based on the levels of repeatability and intermediate precision and was expressed as a relative standard deviation. For repeatability, the results from six independent samples of F7 at 100% of the working concentration (50 μg mL−1) in the same experimental conditions (day and analyst) were analyzed. For intermediate precision, the same procedure was repeated on another day and performed by a second analyst.
From the data of specificity, linearity, and precision, the accuracy was inferred (16).
Stability Studies
Photostability Study
To evaluate the photostability of the formulation that was suggested in the previous stages as the most resistant to radiation, 1.0 g aliquots of F7 were accurately weighed into transparent plastic cuvettes (Brand®, ultra-micro UV-Cuvettes, 12.5 mm × 45 mm × 12.5 mm) and exposed to UVA radiation for 15 h. The UVA radiation was determined with a UV measurement equipment (UV-400, Icel, Manaus, Brazil) and the value (approximately 1,350 W h/m2) followed the ICH requirements (energy not less than 200 W h/m2). Over this period, the samples (n = 3/time) were analyzed for their desonide content at 2, 4, 6, 8, and 15 h. The drug content was determined by comparison with samples that were prepared in the same way but not irradiated. The decay of BP-3 over time was also assessed, considering the peak area of zero time as a reference.
F7 was also exposed to UVC radiation (254 nm) for 8 h. For comparison, the simulated commercial formulation (F1) was also exposed to UVC radiation for 4 h, following the procedure that utilized UVA light. The desonide content was assayed along the time of exposure (n = 3/time). The samples that were submitted to the same procedure but protected from light were used as controls.
Accelerated Stability Study
Hair solutions F1 and F7 were submitted to a climatic chamber (Mecalor, São Paulo, Brazil) at 40°C ± 2°C and 75% ± 5% of relative humidity for 70 days. Along this period, at days 7, 14, 30, and 70, aliquots of the products were removed and analyzed for their desonide content and visual appearance. The formulations were packaged in tightly closed amber glass bottles. Samples were prepared and analyzed in triplicate.
Room Temperature Stability
F1 and F7 were maintained under laboratory conditions (20°C ± 2°C) for 70 days to evaluate their stabilities under room conditions. At days 7, 14, 30, and 70, aliquots of the products were removed, and the content of desonide was determined by HPLC. The visual appearance was also evaluated each time. Immediately after preparation, the samples were tightly closed and packaged in amber glass bottles, remaining protected from the light. Samples were prepared and analyzed in triplicate.
Statistical Analysis
The data were statistically evaluated using Student’s t test, ANOVA and Tukey’s post-hoc test at a 5% level of significance.
RESULTS AND DISCUSSION
Assay of the Formulations and Preliminary Photostability Study
It is well known that corticosteroids are susceptible to oxidation and that oxidative reactions can be catalyzed by light (7). Moreover, the stabilization of betamethasone-17 valerate, another corticosteroid, using the antioxidant BHT in topical preparations was recently reported (17). Thus, considering the previous results from a commercial desonide formulation (11), we initially investigated the effects of some antioxidants on the stability of desonide. For this purpose, we prepared desonide hair solutions with added antioxidants with different mechanisms of action (F2–F5) or desonide hair solutions added by a UV filter (F6) and exposed to UVA radiation (15 h). For comparison, a hair solution with the same composition but without these additives was submitted to the same conditions (F1). Immediately after preparation (zero time), the developed formulations were analyzed using the HPLC method (11). All of the prepared formulations presented desonide contents in the range of 90%–110% of the expected value. These values were considered as a reference for the subsequent assay. The results comparing the desonide content at zero time and after 15 h of irradiation are presented in Fig. 2. As shown, the antioxidants were unable to protect desonide from photodegradation because a significant decay in desonide content was observed. After 15 h of exposure, the desonide percentage in all the developed formulations decreased to 43% and 62% of the theoretical value.
Fig. 2.
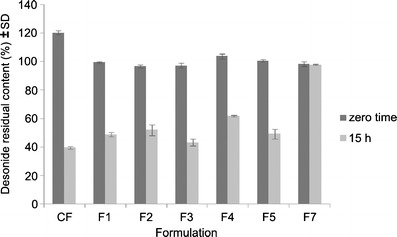
Residual content of desonide in hair solutions after UVA exposure (15 h): commercial formulation (CF), F1 (simulated commercial formulation), F2 (BHT formulation), F3 (BHA formulation), F4 (ascorbic acid formulation), F5 (α-tocopherol formulation), and F7 (BP-3, 0.3% formulation)
On the other hand, in the formulations with BP-3, no decrease in desonide content was observed (Table II). The BP-3 was used in a wide range of concentrations (2.5% to 0.15%), and all of them proved to be effective, keeping the desonide content higher than 90% of the theoretical value (1.0 mg/g). However, a significant difference in desonide content between the formulation containing 0.15% BP-3 and all the others was observed (p < 0.05). Thus, the formulation containing 0.30% BP-3 was chosen as the one with the best photostability profile, and it was used in the stability studies. Therefore, stability studies were performed with this preparation, preceded by the validation of the analytical procedure.
Table II.
Desonide Content (%) in Hair Solution F6 (n = 3/Formulation) after Exposure to UVA Radiation (15 h)
| BP-3 concentration (%) | Drug content (%) (zero time) | Desonide content (%)* (after 15 h UVA) |
|---|---|---|
| 2.5 | 100.35 ± 1.57 | 99.87 ± 0.69 a |
| 1.25 | 101.22 ± 1.03 | 98.90 ± 1.22 a |
| 0.6 | 100.59 ± 0.79 | 98.99 ± 1.87 a |
| 0.3 | 98.49 ± 1.00 | 97.66 ± 0.66 a |
| 0.15 | 99.68 ± 2.29 | 95.63 ± 0.06 b |
*Means followed by the same letter do not differ (p > 0.05)
Validation of the Analytical Procedure
Modifications in the method conditions were necessary to decrease the retention time of BP-3. Thus, the mobile phase was changed and the method was validated by evaluating the specificity, linearity, precision, and accuracy. The analysis of the placebo solution indicated the specificity of the method because no interference of the excipient was observed and the peak purity index was greater than 0.9999.
A typical chromatogram obtained by the proposed method is shown in Fig. 3. Chromatographic parameters were evaluated and fulfilled the required values (18), calculated using the USP method: theoretical plates = 4,591 ± 27.02; tailing factor = 1.08 ± 0.03; capacity factor = 2.93 ± 0.05; and resolution = 3.0 ± 0.04. The linearity study demonstrated the linear range of the method from 5 to 100 μg mL−1 with an adequate correlation coefficient (r = 0.9999). The statistical analysis (ANOVA) demonstrated significant linear regression (p < 0.05) and non-significant linear deviation (p > 0.05) with the linear regression equation y = 43,415x−6,772, in which x is the concentration and y the peak area (mAU).
Fig. 3.
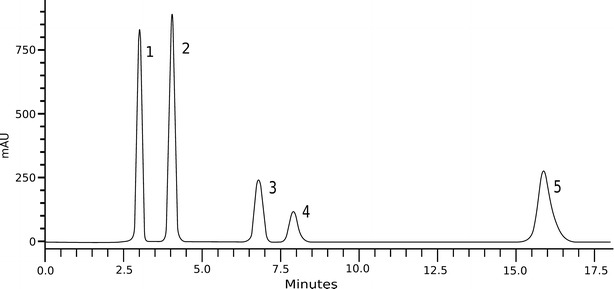
Chromatogram of F7 obtained by the method proposed. 1 acetone, 2 methylparaben, 3 propylparaben, 4 desonide, 5 benzophenone-3
The precision was evaluated in levels of repeatability and intermediate precision and was expressed as the relative standard deviation (RSD). The repeatability RSD values were 0.38 (n = 6, analyst 1, day 1) and 0.27 (n = 6, analyst 2, day 2). The intermediate precision RSD value was 1.85 (n = 12). These results indicated the precision of the analytical method with the average content of 98.21 ± 1.37 (n = 12). Once the specificity, linearity, and precision were well established, the method was inferred as accurate (16).
Stability Studies
Photostability Study
For the photostability study, F7 was exposed to UVA radiation (352 nm) in a light chamber for 15 h in transparent cuvettes. During this time, samples (n = 3/time) were analyzed using the validated analytical procedure. Additionally, the peak area of BP-3 was also monitored to verify its decay during exposure to UVA radiation.
The results indicated that BP-3 stabilized desonide in the hair solution because after 15 h of exposure, the residual content of desonide was 98.61% (Table III). Under the same conditions, the residual content of desonide in the commercial pharmaceutical preparation was approximately 39%, with a t90 value of 1.7 h (11). An assay of F7 submitted to the same procedure but protected from light indicated that degradation occurs without the influence of temperature because the desonide content remaining in these samples was approximately 99%. We verified just a small decay on BP-3 peak area along the 15 h of exposure (Fig. 4). This result was expected from the photostability studies of several UV filters including BP-3, which indicated the photostability of this substance (7).
Table III.
Results of Desonide Assay in Hair Solution F7 in the Photostability Study (n = 3/Time, UVA, 352 nm)
| Time of exposure (h) | Theoretical concentration (mg/g) | Drug content (mg/g) | Drug content (%) |
|---|---|---|---|
| 0 | 1.0 | 1.002 ± 0.72 | 100.20 ± 0.72 |
| 2 | 0.995 ± 1.28 | 99.53 ± 1.28 | |
| 6 | 0.980 ± 0.23 | 98.03 ± 0.23 | |
| 8 | 0.979 ± 0.18 | 97.92 ± 0.18 | |
| 15 | 0.986 ± 0.64 | 98.61 ± 0.64 |
Fig. 4.
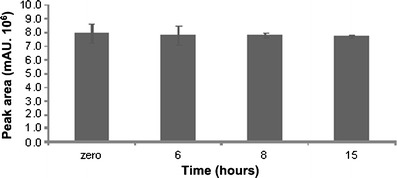
BP-3 peak area on photostability study of F7 (UVA, 352 nm)
Considering that the radiation absorbed by the drug can cause the photochemical process that may subsequently result in photodegradation, blocking of the wavelengths absorbed by the drug may prevent photodecomposition (12). Substances with absorption spectra similar to that of the drug can be incorporated into the formulation to protect it from photodegradation. This is known as the overlay spectral principle (19). The use of UV filters as photostabilizers has already been reported for some substances in different preparations (20,21). BP-3 is an organic filter capable of absorbing both UVA and UVB radiation (22). As shown in Fig. 5, the desonide maximum absorption band (244 nm) is overlapped by one BP-3 maximum absorption band (241 nm).
Fig. 5.
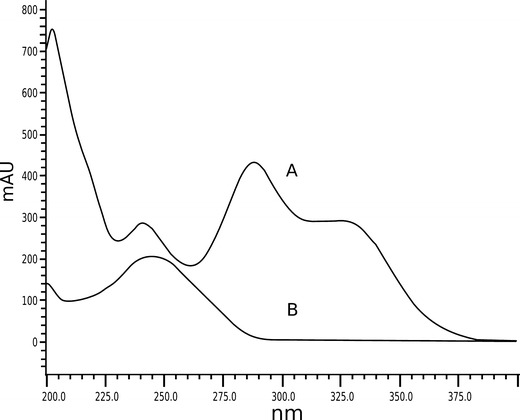
UV spectrum of BP-3 (A) and desonide (B) in hair solution F7
Studies on the photostability of desonide have shown that although the UV absorption spectrum of the drug is limited to the UVC range, the drug is unstable to UVB radiation (8) and UVA (11,23), similar to what we observed in this study. Pregna-1,4-diene-3,20-diones, such as desonide, present two separated chromophore groups: the cross-conjugated dienone in ring A and the ketone at C20. Ricci et al. (24) described the general patterns in the photochemistry of these compounds under UV radiations. These substances were irradiated at 254, 310, and 366 nm (UVC, UVB, and UVA radiation, respectively). The cross-conjugated ketone absorbs predominantly at both 254 and 366 nm to form lumiderivatives. This reaction was reported for prednisolone, whose λ max (242 nm) and spectrum are similar to desonide. Another possible reaction involves the cleavage of C17−C20 or the hydrogen abstraction from the 18-methyl group, in some cases.
The photochemistry of desonide was studied under aerobic and anaerobic conditions under UVC (254 nm) and UVB (310 nm) radiation, in acetonitrile and 2-propanol (8). The results obtained corroborate the ones reported before (24). Moreover, desonide degradation was evidenced in formulations directly exposed to UVA radiation (11,23).
According to Moore (9), the absorption spectrum is described by the maximum absorption wavelength and the molar absorptivity at that wavelength. However, the spectrum of a drug molecule is usually broad, and any overlap of the absorption spectrum with the output of the photon source colliding with it has the potential to lead to photochemical change.
In order to investigate the influence of the components of the proposed formulation on desonide photostability, solutions of desonide in the excipients were prepared. We prepared four solutions: desonide in isopropanol (S1); desonide in propylene glycol (S2); desonide in a mixture of isopropanol, acetone, and propylene glycol (S3); and simulated commercial formulation (S4), which differs from S3 because it contains the preservatives methylparaben and propylparaben. The solutions were exposed to UVA for 15 h and the following levels of residual desonide were obtained: 64.1, 64.0, 61.2 and 52.5% for S1, S2, S3, and S4, respectively. The content of S4 differed significantly from the other ones (p < 0.05), suggesting that the presence of parabens could accelerate the photodegradation of desonide.
An additional photostability study was developed that employed UVC radiation (254 nm). Although most UVC radiation is filtered by ozone in the upper atmosphere, the determination of the chemical and biological effects of this radiation has been receiving more attention in recent years, not only for better knowledge of the photochemistry of this radiation but also because of the specific damage caused by it (9). Considering that UVC is the most energetic ultraviolet radiation, causing rapid photodegradation, the use of this radiation is interesting because it is possible to expose the formulation to an extreme condition, allowing the evaluation of its effect. We compared the F1 and F7 formulations under this irradiation condition. BP-3 was able to protect against the desonide UV photolysis, even under such adverse conditions, but it was unable to completely prevent photolysis because the remaining drug content after 4 and 8 h of irradiation were 71.3% and 53.0%, respectively. On the other hand, in the F1 formulation, the residual content of desonide after 4 h of irradiation was 44.4%.
The degradation kinetics of the two analyzed formulations was also determined to define the reaction order and the reaction rate. For most substances, degradation reactions occur as zero order, first order, or pseudo first order (23). Analysis of the correlation coefficients that were obtained by plotting the drug concentration versus time, log of drug concentration versus time and the inverse of concentration versus time indicated that the degradation reaction of F7 follows first-order kinetics (y = −0.075x + 3.905, r = 0.9954). The slope of the straight line indicated the reaction constant (k) and expresses the fraction of drug that reacts per time; from the k value, the t90 was estimated as 1.41 h. The degradation kinetics study of F1 showed that it follows zero-order kinetics (y = −6.746x + 49.38, r = 0.9954) with a t90 value of 0.64 h. These results suggest that the BP-3 modified the kinetics of the degradation reaction of the formulations and duplicated the t90 value in the used conditions.
Accelerated Stability Study
For the accelerated stability study, three batches of F1 and F7 were maintained in a climatic chamber for 70 days and analyzed at predetermined times (7, 15, 30, and 70 days). Immediately after preparation, F1 was transparent and colorless, whereas F7 was transparent and slightly yellowish, most likely due to benzophenone-3, which is yellow in color. After the formulations were exposed to the humidity and temperature conditions mentioned above, alterations in the color or appearance were not observed.
As shown in Table IV, the assay of desonide demonstrated the decay of drug content over time for both formulations. At the end of day 70, the residual content of desonide in F7 was 94.13%, whereas for F1, the observed content was 88.33%. At the end of the stability study, the desonide content in F7 remained within the most common shelf life specification, which indicates the loss of 10% of the content as the maximum acceptable (23–25).
Table IV.
Results of Desonide Content Under Accelerated and Room Temperature Conditions (n = 3/Formulation)
| Drug content (%) ± SD Accelerated condition | Drug content (%) ± SD Room temperature | |||
|---|---|---|---|---|
| F1 | F7 | F1 | F7 | |
| 0 | 100.00 ± 1.07 | 100.00 ± 1.15 | 100.00 ± 3.41 | 100.00 ± 1.75 |
| 7 | 100.20 ± 0.70 | 105.03 ± 0.87 | 100.29 ± 0.35 | 101.38 ± 1.02 |
| 15 | 91.61 ± 0.55 | 95.50 ± 2.60 | 100.82 ± 1.64 | 99.70 ± 1.50 |
| 30 | 95.86 ± 5.90 | 94.87 ± 1.67 | 103.85 ± 2.53 | 98.89 ± 1.88 |
| 70 | 88.33 ± 0.84 | 94.13 ± 1.14 | 102.37 ± 2.90 | 99.84 ± 1.74 |
Stability at Room Temperature
To perform this evaluation, three batches of formulations F1 and F7 were maintained under laboratory conditions for 70 days. The temperature was monitored and maintained at 20°C ± 2°C. As occurred in the accelerated stability study, alterations to the color or appearance of the formulations over the period were not observed. Regarding the drug content, the same decay found in the accelerated conditions was not observed. This result was expected, considering that exposure to higher temperatures increases the chemical degradation rate or physical change in pharmaceuticals (13,23,24,26).
CONCLUSION
In this study, the effects of different stabilizers on the photostability of desonide in hair solution were evaluated. We verified that the antioxidants used were not able to protect desonide from degradation after exposure to UVA radiation. However, BP-3 at low concentrations was able to prevent desonide from photodegradation. In addition, the developed formulation showed suitable stability because the desonide content in formulation F7 fell within the most usual compendial limits during the accelerated and room temperature stability test. Thus, we can conclude that this formulation can be considered as an alternative to the available ones.
Acknowledgments
The authors thank FIT/UFSM for the financial support.
References
- 1.Grau PS. Corticoides tópicos: actualización. Med Cutan Iber Lat Am. 2006;34(1):33–8. [Google Scholar]
- 2.Ross TC, Geuer S, Ross S, Brost H. Recent advances in treatment strategies for atopic dermatitis. Drugs. 2004;64(23):2639–66. doi: 10.2165/00003495-200464230-00003. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt EL, Sutter SH, Drake LA. Farmacologia dermatológica. In: Goodman LS, editor. Goodman & Gilman: as bases farmacológicas da terapêutica. Seção XIV: dermatologia. 10. Rio de Janeiro: McGraw-Hill; 2004. pp. 1351–2. [Google Scholar]
- 4.Nichols WK. Hormônios e antagonistas hormonais. In: Gennaro AR, editor. A ciência e a prática da farmácia. 20. Rio de Janeiro: Guanabara Koogan; 2004. p. 421. [Google Scholar]
- 5.Gelbard CM, Hebert AA. Desonide hydrogel: advances in vehicle technology. Expert Rev Dermatol. 2009;4(1):23–7. doi: 10.1586/17469872.4.1.23. [DOI] [Google Scholar]
- 6.Dicionário de especialidades farmacêuticas. 41. Rio de Janeiro: EPUC; 2013. [Google Scholar]
- 7.Albini A, Fasani E. Drugs: photochemistry and photostability. Cambridge: The Royal Society of Chemistry; 1998. pp. 11–4. [Google Scholar]
- 8.Iqbal J, Husain A, Gupta A. Photochemistry of desonide, a non fluorinated steroidal anti-inflammatory drug. Chem Pharm Bull. 2006;54(6):836–8. doi: 10.1248/cpb.54.836. [DOI] [PubMed] [Google Scholar]
- 9.Moore DE. Photophysical and photochemical aspects of drug stability. In: TØnessen HH, editor. Photostability of drugs and drug formulations. 2. Boca Raton: CRC Press LLC; 2004. pp. 10–40. [Google Scholar]
- 10.Polefka TG, Meyer TA, Agin PP, Bianchini RJ. Effects of solar radiation on the skin. J Cosm Dermatol. 2011;11:134–43. doi: 10.1111/j.1473-2165.2012.00614.x. [DOI] [PubMed] [Google Scholar]
- 11.Dalla Santa F, Sperotto LE, Braga MP, Dalcin TCS, Codevilla CS, Meneghini LZ, et al. Development and validation of a simple stability-indicating LC-method and UVA phostability study of desonide hair lotion. Curr Anal Chem. 2013;9:659–67. doi: 10.2174/15734110113099990011. [DOI] [Google Scholar]
- 12.TØnessen HH. Photostability testing of drugs and drug formulations—why and how? In: TØnessen HH, editor. Photostability of drugs and drug formulations. 2. Boca Raton: CRC Press LLC; 2004. pp. 1–7. [Google Scholar]
- 13.Yoshioka S, Stella VJ. Stability of drugs and dosage forms. New York: Kluwer Academic Publishers; 2002. pp. 61–78. [Google Scholar]
- 14.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 5. London: Pharmaceutical Press; 2006. p. 8. [Google Scholar]
- 15.Watson DG. Pharmaceutical analysis. A textbook for pharmacy students and pharmaceutical chemists. Edinburgh: Elsevier Churchill Livingstone; 2005. pp. 250–3. [Google Scholar]
- 16.ICH. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline: guideline on validation of analytical procedure Q2R1 methodology; 2005. p. 9–10.
- 17.Khattak RS, Shaikh D, Ahmad I, Usmanghani K, Sheraz MA, Ahmed S. Photodegradation and stabilization of bethametasone-17 valerate in aqueous/organic solvents and topical formulations. AAPS PharmSciTech. 2012;14(1):177–82. doi: 10.1208/s12249-012-9902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDER. Center for drug evaluation and research. Reviewer guidance: Validation of chromatographic methods; 1994 p. 8–26.
- 19.Thoma K, Klimeck R. Photostabilization of drugs in dosage forms without protection from packaging materials. Int J Pharm. 1991;67:169–75. doi: 10.1016/0378-5173(91)90429-R. [DOI] [Google Scholar]
- 20.Brisaert M, Vercarmmen PJ. Investigation on the photostability of a tretinoin lotion and stabilization with additives. Int J Pharm. 2000;199:49–57. doi: 10.1016/S0378-5173(00)00366-5. [DOI] [PubMed] [Google Scholar]
- 21.Gaspar LR, Maia Campos PMBG. Photostability and efficacy studies of topical formulations containing UV filters combination and vitamin A, C and E. Int J Pharm. 2007;343:189–91. doi: 10.1016/j.ijpharm.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Index. 14. Rahway: Merck & Co; 2006. p. 182. [Google Scholar]
- 23.Braga MP, Dalcin TCS, Rosa P, Codevilla CS, Silva CB, Rolim CMB, et al. Assay and photodegradation kinetics of desonide lotion by an LC-UV stability-indicating method. J Liq Chromatogr R T. 2014;37:1968–84. doi: 10.1080/10826076.2013.825855. [DOI] [Google Scholar]
- 24.Ricci A, Fasani E, Mella M, Albini A. General patterns in the photochemistry of pregna-1,4-dien-3,20-diones. J Org Chem. 2003;68:4361–6. doi: 10.1021/jo034070a. [DOI] [PubMed] [Google Scholar]
- 25.Carstensen JT, Rhodes CT. Drug stability. Principles and practices. New York: Informa Healthcare; 2007. p. 3–17.
- 26.Lachman L, Deluca P, Akers AJ. Testes de estabilidade e fundamentos de cinética química. In: Lachman L, Lieberman HA, Kanig JL, editors. Teoria e prática na indústria farmacêutica, v.2. Lisboa: Fundação Lacouste Gulbenkian; 2001. pp. 1277–355. [Google Scholar]


