Abstract
Biowaivers are recommended for immediate-release solid oral dosage forms using dissolution testing as a surrogate for in vivo bioequivalence studies. Several guidance are currently available (the World Health Organization (WHO), the US FDA, and the EMEA) where the conditions are described. In this study, definitions, criteria, and methodologies according to the WHO have been applied. The dissolution performances of immediate-release metronidazole, zidovudine, and amoxicillin products purchased in South African and Indian markets were compared to the relevant comparator pharmaceutical product (CPP)/reference product. The dissolution performances were studied using US Pharmacopeia (USP) apparatus 2 (paddle) set at 75 rpm in each of three dissolution media (pH1.2, 4.5, and 6.8). Concentrations of metronidazole, zidovudine, and amoxicillin in each dissolution media were determined by HPLC. Of the 11 metronidazole products tested, only 8 could be considered as very rapidly dissolving products as defined by the WHO, whereas 2 of those products could be considered as rapidly dissolving products but did not comply with the f2 acceptance criteria in pH 6.8. All 11 zidovudine products were very rapidly dissolving, whereas in the case of the 14 amoxicillin products tested, none of those products met any of the WHO criteria. This study indicates that not all generic products containing the same biopharmaceutics classification system (BCS) I drug and in similar strength and dosage form are necessarily in vitro equivalent. Hence, there is a need for ongoing market surveillance to determine whether marketed generic products containing BCS I drugs meet the release requirements to confirm their in vitro bioequivalence to the respective reference product.
KEY WORDS: BCS, dissolution testing, generic drug, immediate-release solid oral dosage forms, WHO criteria
INTRODUCTION
It is reported that various substandard medicines are freely accessible on global markets. Consequently, drug resistance and treatment failure are commonly reported in emerging markets such as in Asian and African countries. The Indian and South African pharmaceutical markets have a plethora of generic products that are generally substituted for innovator products (1,2). Although the quality, safety, and efficacy of marketed drug products in the above-mentioned countries are regulated by their respective regulatory agencies, such as The Central Drugs Standards Control Organization (CDSCO) and the Drugs Controller General (DCG) in India (3) and Medicines Control Council (MCC) in South Africa, substandard products somehow appear in the market. Thereby, clinicians face a challenge due to wide choice of generic products, especially in South Africa where generic substitution is mandated by law (4).
Substitution using generic products can only be done if their bioequivalence is comparable with a reference product, usually the innovator or “Brand” product. Whereas, the reference drug product (reference-listed drug (RLD)) required by the US FDA is listed as the RLD in the Orange book (5); in developing countries, innovator products may not be generally available (6). Hence, the World Health Organization (WHO) (7) has proposed that a well-established drug product may be used as the comparator pharmaceutical product (CPP). In recent years, the possibility of allowing waivers for in vivo bioequivalence studies for certain drug products has created considerable interest (8,9). Initially, dissolution methods and acceptance criteria were only considered for drugs falling within the biopharmaceutics classification system (BCS) I class (10). More recently, consideration has been given to apply biowaivers also for dugs falling into the other three BCS classes. According to the WHO (11) and the EMEA (12), a biowaiver implies that in vivo bioequivalence studies may be substituted by performing in vitro dissolution testing to compare the “test” product against a reference product (RLD or CCP). Such biowaivers ensure good quality generic medicines at lower costs. Currently, several guidance documents such as those issued by the US FDA (13), the WHO (11), and the EMEA (14) are available, wherein the requirements to declare bioequivalence for specific classes of drugs contained in immediate-release drug products are described. Table I depicts the various definitions and criteria, including methodologies, which are recommended to assess in vitro bioequivalence using dissolution studies.
Table I.
Definitions, Criteria, and Methodologies Recommended for Biowaivers Using In Vitro Dissolution Testing for Immediate-Release Solid Oral Dosage Forms Containing BCS Class I Drugs
| WHO (11) | US FDA (13) | EMEA (12) | ||
|---|---|---|---|---|
| Acceptance criteria | ||||
| Definitions | Very rapidly dissolving products | At least 85% of labeled amount released within 15 min or less from the test and the comparator product. Profile comparison not needed | Not applicable | More than 85% released within 15 min in a volume of 900 ml or less in media |
| Rapidly dissolving products | 1. At least 85% of labeled amount released within 30 min or less from the test and the comparator product 2. Profile comparisons using e.g., f 2 testing, are required |
1. No less than 85% of labeled amount of drug substance dissolves within 30 min 2. Two dissolution profiles are considered similar when the f 2 value is ≥5 3. To allow use of mean data, the CV should not be more than 20% at the earlier time points and should not be more than 10% at other time points 4. When both test and reference products dissolve 85% or more of the labeled amount of drug in ≤15 min using all 3 dissolution media, the profile comparison with f 2 is unnecessary |
85% released within 30 min | |
| Testing conditions | ||||
| Dissolution media | Use of 3 different dissolution media, viz: 1. Buffer solution at pH 1.2 2. Buffer solution at pH 4.5 3. Buffer solution at pH 6.8 Temperature: 37 ± 0.5°C Volume: 900 ml or less |
Use of 3 different dissolution media, viz: 1. 0.1 N HCl or simulated gastric fluid USP without enzymes 2. pH 4.5 buffer 3. pH 6.8 buffer or Simulated Intestinal Fluid USP without enzymes Temperature: 37 ± 0.5°C Volume: 900 ml or less |
Use of 3 different dissolution media, viz: 1. pH 1.0–1.2 (usually 0.1 N HCl or SGF without enzymes) 2. pH 4.5 3. pH 6.8 (or SIF without enzymes) Temperature: 37 ± 1°C Volume: 900 ml or less |
|
| Recommended apparatus | Basket apparatus speed at 100 rpm or Paddle apparatus speed at 75 rpm or less |
Basket speed 100 rpm (USP apparatus 1) or Paddle speed 50 rpm (USP apparatus 2) |
Basket apparatus speed at usually 100 rpm or Paddle apparatus speed at usually 50 rpm |
|
| Other conditions | 1. Surfactants should not be used 2. 12 units to be tested 3. Sampling intervals should be short, e.g., 5, 10, 15, 20, 30, and 45 min. Inclusion of the 15 min time point in the protocol is of strategic importance for profile similarity determinations |
1. Minimum of 12 dosage units to be evaluated 2. Samples should be collected at a sufficient number of intervals to characterize the dissolution profile of the drug product (e.g., 10, 15, 20, and 30 min) |
1. No surfactant 2. In the case of gelatin capsules or tablets with gelatin coatings, use of enzymes may be acceptable 3. 12 units to be tested 4. Sampling schedule, e.g., 10, 15, 20, 30, and 45 min |
|
CV coefficient of variation, USP US Pharmacopeia, SIF simulated intestinal fluid, SGF simulated gastric fluid
Löbenberg et al. (6) reported on comparative dissolution study of different generic products containing BCS class 1 drugs—amoxicillin, metronidazole, and zidovudine marketed in selected American countries—Argentina, Peru, Chile, Uruguay, and Mexico to their corresponding products marketed in the USA. The authors report that only 3 of the 12 amoxicillin products (11 generics vs 1 CPP) showed in vitro equivalence to the innovator, and none of the tested metronidazole products exhibited in vitro equivalence to the innovator, whereas all tested zidovudine products were found to be in vitro equivalent to the respective innovator product.
The present work is an extension of the above-mentioned dissolution studies on amoxicillin, metronidazole, and zidovudine generic products but comparing them with innovator/CPP products available in South Africa and India. Since the selected dosage forms have been approved for marketing by the respective regulatory agencies, as a hypothesis, it is expected that these products would be bioequivalent and meet the dissolution criteria. Drug release was tested by validated HPLC analytical methods and assessed for bioequivalence in accordance with the WHO requirements for biowaivers for immediate-release solid oral dosage forms containing BCS class I drugs.
MATERIALS AND METHODS
Reagents and Materials
HPLC-grade acetonitrile and methanol were purchased from Romil Ltd. (Cambridge, Great Britain). Sodium acetate, sodium phosphate, sodium hydroxide, and glacial acetic acid were procured from Merck (Darmstadt, Germany). HPLC-grade water was generated in a MilliQ® System (Millipore, Milford, CT, USA) and Millex HV® hydrophilic PVDF 0.45-μm membrane filters (Millipore, Bedford, MA, USA) were used to filter all solutions. Metronidazole reference standard (RS) (JOC316), zidovudine RS (HOF263), and amoxicillin RS (KOH332) were purchased from the US Pharmacopeia (USP, Rockville, MD, USA). Generic and innovator products of amoxicillin, metronidazole, and zidovudine were purchased from local pharmacies and dealers in their respective countries. Buffer media for dissolution testing were prepared as per USP specifications at pH of 1.2, 4.5, and 6.8 (15). Weight variation of the individual products was carried out as per compendia methods.
Flagyl® 400 (Sanofi-Aventis) was selected as the reference product (comparator pharmaceutical product—CPP) for the South African metronidazole products, whereas Flagyl® 400 obtained in India and manufactured by Abbott Healthcare Pvt. Ltd. was used as the CPP for the Indian products. Retrovir® (GlaxoSmithKline (GSK)) 100- and 300-mg formulations were selected as the CPP for zidovudine for the South African products. Since Retrovir® 100 (GSK) is no longer available in India, Zidovir 100 and 300 mg (Cipla-India (IND)) were used as the CPP for the Indian products. The innovator amoxicillin product is Amoxil® 500-mg capsules (GSK). However, Amoxil® is no longer available in South Africa or in India; hence, Ranmoxy 500-mg capsules (Ranbaxy-South Africa (SA)) and Mox 500-mg capsules (Ranbaxy-IND) were used as the respective CPPs.
Instrumentation and Equipment
HPLC was carried out using a model 2695 separation module equipped with a 2487 dual absorbance detector, a model Pro2 Empower data-acquisition system (Waters, Milford, CT, USA). Analytical column was Luna® C8 (2) column, 150 × 4.6 mm i.d., 5 μ particle size, and a Luna® Security Guard column (Phenomenex, Torrence, CA, USA). The pH testing of the prepared buffers were checked using a GLP 21 Crison pH meter (Crison, LASEC, South Africa). An automated model SR 8 PLUS dissolution apparatus (Hanson Research Corporation, Chartsworth, CA, USA) fitted with an Autoplus™ Multifill™ and a Maximizer Syringe Fraction Collector (Hanson Corporation, Chartsworth, CA, USA) was used for dissolution testing of dosage forms. An utrasonic bath (Model 8845-30, Cole-Parmer Instruments, Chicago, IL,USA) was used for sonication to dissolve the reference standards during preparation of standard solutions.
Generic and Innovator Products
The market samples of generic and CPP products of metronidazole, zidovudine, and amoxicillin are listed in Tables II, III, and IV, respectively.
Table II.
Metronidazole Products Tested
| Country | Company | Product | Dosage form | Batch | Expiry date | Excipients (as indicated on the product label) |
|---|---|---|---|---|---|---|
| South Africa | Sanofi-Aventis | Flagyl® 400 | Tabs | 89A | 03/2012 | NA |
| Pharmacare | Trichazole® 400 | Tabs | 7208139 | 05/2011 | NA | |
| Be-Tabs | Bemetrazole 400 | Tabs | 2131258 | 02/2012 | NA | |
| Adcock-Ingram | Adco-metronidazole 400 | Tabs | AH9001 | 06/2011 | NA | |
| Pharma Script | Acuzole 400 | Tabs | 117 | 07/2012 | NA | |
| India | Sun Life Sciences | Metrosun 400 | Tabs | T110244 | 01/2013 | Sunset yellow FCF and Titanium dioxide |
| Abbott | Flagyl® 400 | Tabs | VC0017 | 05/2015 | Tartrazine and titanium dioxide | |
| Medibest Pharm | Metronidazole 400 | Tabs | MFF1001 | 06/2012 | NA | |
| JB Chemicals | Metrogyl® 400 | Tabs | TM81167 | 03/2015 | Sunset yellow FCF | |
| Quest Labs (FDC) | Metgyl 400 | Tabs | 09 | 02/2013 | Titanium dioxide | |
| Martin & Brown | Metronidazole 400 | Tabs | MTZ24 | 10/2012 | Sunset yellow |
NA not available
Table III.
Zidovudine Products Tested
| Country | Company | Product | Dosage form | Batch | Expiry date | Excipients (as indicated on the product label) |
|---|---|---|---|---|---|---|
| South Africa | GSK | Retrovir® 100 | Caps | X1738 | 05/2012 | Titanium dioxide, gelatin, indigo carmine, Black iron oxide in capsule shell. Starches, microcrystalline cellulose, sodium starch glycollate, magnesium stearate |
| Aspen | Aspen-Zidovudine 100 mg | Caps | E775832 | 08/2012 | NA | |
| Aurobindo | Auro-Zidovudine 100 mg | Caps | ZC1010002-B | 01/2012 | NA | |
| Adcock-Ingram | Adco-Zidovudine 300 mg | Tabs | 5Z | 03/2012 | NA | |
| Aspen | Aspen-Zidovudine 300 mg | Tabs | A739756 | 06/2012 | NA | |
| GSK | Retrovir® 300 | Tabs | 4297 | 04/2011 | NA | |
| Cipla-Medpro | Cipla-Zidovudine 300 | Tabs | G85701 | Shelf life to 31/10/2011 | NA | |
| India | Cipla | Zidovir 300 | Tabs | G94901 | 06/2012 | Titanium dioxide |
| Cipla | Zidovir 100 | Caps | X91256 | 11/2012 | NA | |
| Emcure | Zidine™ 300 | Tabs | 01A11001 | 02/2013 | Titanium dioxide | |
| Aurobindo | Zidovex 300 | Tabs | ZN3011026-A | 05/2013 | Titanium dioxide |
GSK GlaxoSmithKline, NA Not Available
Table IV.
Amoxicillin Products Tested
| Country | Company | Product | Dosage form | Batch | Expiry date | Excipients (as indicated on the product label) |
|---|---|---|---|---|---|---|
| South Africa | Cipla | Promoxil 500 | Caps | BM0014 | 12/2011 | NA |
| Adco | Adco-Amoxycillin 500 | Caps | 100623 | 04/2013 | NA | |
| Mylan-Xixia | Zoxil 500 | Caps | ZCBH0005 | 12/2013 | NA | |
| Austell | Austell-Amoxicillin 500 | Caps | X79026Z | 09/2012 | NA | |
| Be-Tabs | Betamox 500 | Caps | 2178740 | 06/2012 | NA | |
| Ranbaxy | Ranmoxy 500 | Caps | 2106753 | 11/2012 | NA | |
| India | MHS Pharma | AmoxyRite 500 | Caps | PP1122 | 06/2012 | NA |
| Ranbaxy (Rexcel) | Mox 500 | Caps | 2288602 | 05/2013 | NA | |
| Golden Cross Pharma (Cipla) | Novamox 500 | Caps | DT1180 | 02/2013 | NA | |
| Alkem | Almox 500 | Caps | AMCM01271B | 10/2012 | NA | |
| Mankind | Moxikind® 500 | Caps | MKC1011 | 04/2012 | NA | |
| Makers Labs | Exylin 500 | Caps | ACV1001AU | 05/2013 | NA | |
| Interphar Health care | Moxvid 500 | Caps | S751426 | 05/2013 | NA | |
| Unichem | Mymox* 500 | Caps | MMC11001 | 12/2012 | NA |
NA Not Available
Dissolution Testing
Dissolution testing was performed using a USP apparatus 2 (paddle). The paddle was set at 75 rpm, and 900 ml of dissolution media was used to test all samples. Prior to testing, the dissolution media was preheated and degassed to prevent air bubble formation during transfer of buffers into the vessels. Dissolution testing was started after the temperature of 37°C (±0.5°C) was confirmed in all vessels. At pre-set time points of 10, 15, 20, 30, 45, and 60 min, samples were withdrawn by the automated system through a 45-μm UHMW polyethylene external probe end filters which were immersed in the dissolution media. Tablet samples were immersed into the media directly, and USP sinkers (316 stainless steel with inside length of 25–26 mm, inside diameter of 12.0 ± 0.2 mm, and wire diameter of 1.0 mm) were used for capsules. Samples of 1.5 ml were withdrawn by the autosampler in combination with the Multifill fraction collector from each vessel at each time point. The volume of media sampled was not replenished into the vessels after each withdrawal. Hence, drug concentration was corrected by calculation for the withdrawn volume. The collected samples were transferred into a 1.8-ml vial which was eventually placed in the autosampler for injection into the HPLC. Sample concentrations were determined by validated HPLC methods.
Analytical Quantitation
Metronidazole
The quantitation of metronidazole dissolution samples was carried by following HPLC chromatographic conditions: Mobile phase consisted of water:methanol mixture (85:15), which was pumped at a flow rate of 1 ml/min through a Luna® C8 (2) (5 μ, 150 mm × 4.6 mm i.d.) column. The autosampler system injected 10 μl of standard and sample solutions into the chromatograph. The UV detector was maintained at an absorption wavelength of 319 nm. Prior to sample estimation, the HPLC method was validated for its suitability in all three dissolution media for linearity, accuracy, precision, and recovery studies according to the USP general chapter Validation of Compendial Procedures <1225> (16). The linear range was selected based on an expected lowest release concentration of about 3.7% and highest of 120% of drug content dissolved in 900 ml of each dissolution medium at pH 1.2, 4.5, and 6.8. The observed correlation coefficient was r2 = 0.999, and coefficients of variation were 1.68, 2.12, and 1.84% in pH 1.2, 4.5, and 6.8 buffers, respectively.
Zidovudine
Zidovudine was tested using HPLC chromatographic conditions that consisted of water:acetonitrile mixture (85:15) as the mobile system, which was pumped at a flow rate of 1 ml/min through a Luna® C8 (2) (5 μ, 150 mm × 4.6 mm i.d.) column. The autosampler system was set to inject 10 μl each of the standard followed by samples into the chromatograph. The UV detection was set at an absorption wavelength of 265 nm. Prior to routine analysis, the HPLC method was validated for its suitability by testing for linearity, accuracy precision, and recovery studies according to the USP general chapter Validation of Compendial Procedures <1225> (12). The linear range was set based on lowest expected release of about 3.7% and highest of 120% of drug content in 900 ml of each dissolution medium of pH 1.2, 4.5, and 6.8. The observed correlation coefficient was r2 = 0.999, and coefficients of variation were 1.72, 1.26, and 2.24% in pH 1.2, 4.5, and 6.8 buffers, respectively.
Amoxicillin
The HPLC method was validated for its suitability in all three media for their linearity, accuracy, precision, and recovery studies as per USP general chapter Validation of Compendial Procedures <1225> (16). The linearity range was set based on lowest expected drug release concentration of about 3.7% and highest of 120% in 900 ml of each dissolution medium at pH 1.2, 4.5, and 6.8. The observed correlation coefficient was r2 = 0.999, and coefficients of variation which were about 2.46, 2.87, and 1.47% in respective buffers of pH 1.2, 4.5, and 6.8. HPLC assay used the following chromatographic conditions: buffer composed of 6.8 g KH2PO4 in 900 ml water after which the pH was adjusted with 0.1 NaOH to 4.5 ± 0.1, and the volume was made up to 1,000 ml. The buffer was further mixed with 5% acetonitrile which was pumped at a flow rate of 1 ml/min through a Luna® C8 (2) (5 μ, 150 mm × 4.6 mm i.d.) column. Equal quantities (10 μl) of standards and samples were injected into the chromatograph, and the responses were detected at a UV wavelength of 229 nm.
Study Design
The mechanical systems of the dissolution apparatus was calibrated by performing the USP performance verification test (PVT) in accordance with the general chapter Dissolution <711> (17). Thus ensuring the apparatus was performing at optimum conditions and complies with the compendial standards established for dissolution test procedures.
Selection Criteria for the Comparator Pharmaceutical Product (CPP)
According to the World Health Organization (WHO), a suitable reference product is an innovator product for which quality, safety, and efficacy have been established in a well-regulated country. When the requisite reference product cannot be identified, an alternative CPP or suitable reference product may be considered. As per the WHO recommendations: “The alternative CPP should have been approved in ICH or associated countries and has been “pre-qualified” by the WHO. Furthermore, it has extensive documented use in clinical trials and reported in the peer-reviewed scientific journals. It should have a long and unproblematic period of post-market surveillance and must conform to compendial quality standards” (5).
The authors used the FDA’s Orange book (18) to select suitable reference products when this study was initiated, the Orange book listed Amoxil® 500-mg capsules manufactured by GlaxoSmithKline as the reference-listed drug (RLD). However, during the course of this study, the Orange book lists Amoxil® 500-mg capsules under discontinued products. Hence, to continue with the study with suitable reference products, the authors chose amoxicillin, manufactured by Ranbaxy as the CPP since the product is listed in the Orange book as bioequivalent to Amoxil® 500 mg (19). In the case of metronidazole, although the Orange book only lists the 250 and 500 mg strengths with Pfizer as the manufacturer, Flagyl® 400-mg tablets manufactured by Sanofi-Aventis was used as the CPP. Retrovir® 100-mg capsules and 300-mg tablets containing zidovudine, both manufactured by GlaxoSmithKline, were used as the respective CPPs (20).
Data Analysis
The generated analytical data was processed using a Microsoft Excel® spread sheet, and the results were calculated for all samples of each product tested. The criterion for evaluating the dissolution profiles was determined as per FDA guidance.
In vitro equivalence between the CPP and relevant test product(s) obtained from their respective domestic markets was established based on the dissolution profiles of the test and CPP. Acceptance criteria based on the similarity factor (f2) when tested and the reference product in all three dissolution media were established as similar due to rapid dissolution of the active drug.
RESULTS AND DISCUSSION
Metronidazole
The dissolution profiles of products marketed in South Africa (SA) vs the reference product were shown in Fig. 1.
Fig. 1.
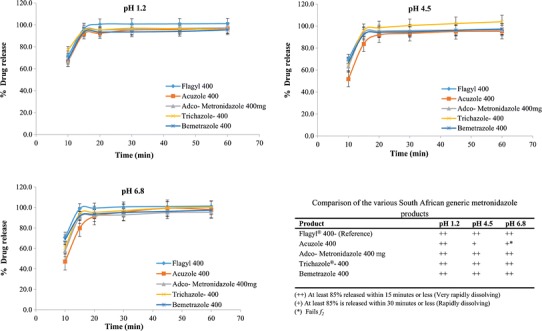
Dissolution profiles of the CPP and metronidazole products marketed in South Africa
Although Flagyl® 400 manufactured by Sanofi-Aventis (SA) was used as the reference product for this study, Abbott Pharma markets their metronidazole product under the same trade name in India. When the dissolution of both Flagyl® (South Africa) and Flagyl® (India) were tested, both products showed a release of >85% in 15 min in all three media. Hence, the two reference products were shown to be equivalent.
The first set of data in Fig. 1 (South African products) shows that all products dissolved very rapidly in pH 1.2 and met the requirement by exceeding a release of 85% metronidazole in 15 min. In pH 4.5, Trichazole® 400, Bemetrazole 400, and Adco-metronidazole 400 mg products dissolved very rapidly (i.e., >85% in 15 min), but Acuzole 400 dissolved rapidly (85% or more in 30 min) requiring more than 15 min to release. In pH 6.8, Bemetrazole 400 and Trichazole® 400 products met the very rapidly dissolving requirement. Although Acuzole 400 dissolved rapidly, it failed the f2 (43.92) with the CPP.
The dissolution profiles of the metronidazole products in India are shown in Fig. 2. Metgyl required 60 min to release 85% in all three pH media and thus did not meet the biowaiver requirements. Metrosun 400, metronidazole 400 (Medibest), Metrogyl® 400, and metronidazole I.P. 400 (Mortin & Brown) dissolved very rapidly in pH 1.2 and in pH 4.5 buffers and thus met the requirement by exceeding a release of 85% in 15 min. In pH 6.8, Metrosun 400, metronidazole 400 (Medibest), and Metrogyl® 400 dissolved very rapidly and passed the f2 requirement of ≥50. However, metronidazole I.P. 400 (Mortin & Brown) dissolved rapidly but did not meet the f2 requirements (49.15). Hence, only 8 of the total 11 tested metronidazole products released more than 85% in 15 min (very rapid dissolving) in all three media and thus met biowaiver requirements. Metronidazole I.P. 400 (India) and Acuzole (South Africa) were very rapidly dissolving in pH 1.2 but were only rapidly dissolving in pHs 4.5 and 6.8 and failed the f2 requirements.
Fig. 2.
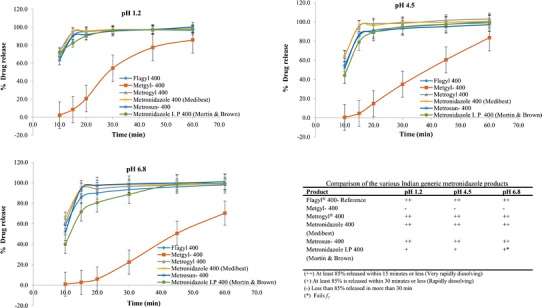
Dissolution profiles of the CPP and metronidazole products marketed in India
Zidovudine
Retrovir® 10-mg and 300-mg formulations were manufactured by GSK and were used as CPP for South African products. The dissolution profiles of all zidovudine products vs relevant the CPP for 100-mg dose strength are shown in Fig. 3. All the zidovudine 100-mg formulations, Auro-zidovudine (SA), Aspen-zidovudine (SA), and Zidovir 100 (India) dissolved very rapidly in all three media and were considered to be in vitro equivalent using Retrovir® as CPP.
Fig. 3.

Dissolution profiles of the CPP and zidovudine 100 mg products marketed in South Africa and India
The dissolution profiles for Retrovir® 300 mg (CPP) and generic zidovudine formulations purchased in South Africa are shown in Fig. 4. The drug release from generic formulations and the CPP from the Indian market are shown in Fig. 5. The tested products dissolved very rapidly and met the requirement by exceeding a release of 85% in 15 min in all three media. Thus, these formulations may be considered as being in vitro equivalent as per the biowaiver recommendations.
Fig. 4.

Dissolution profiles of the CPP and zidovudine 300 mg products marketed in South Africa
Fig. 5.

Dissolution profiles of the CPP and zidovudine 300 mg products marketed in India
Amoxicillin
The dissolution profiles of products marketed in South Africa vs CPP are shown in Fig. 6. Promoxil 500, Austell-amoxicillin 500, Ranmoxy 500, and Betamox 500 were all rapidly dissolving only in pH 1.2 but failed the rapidly dissolving requirements in pHs 4.5 and 6.8. Similarly, all the other amoxicillin products did not meet the biowaiver criteria.
Fig. 6.

Dissolution profiles of the CPP and amoxicillin products marketed in South Africa
Comparison of the Indian generic amoxicillin products and their dissolution profiles of the products are shown in Fig. 7. AmoxyRite 500, Novamox 500, Almox 500, Moxikind® 500, and Mox 500 were all rapidly dissolving products in pH 1.2 only. The remaining Indian products failed the biowaiver requirements. Interestingly, although Ranbaxy sells amoxicillin products as different brands in different markets (Ranmoxy 500-SA) and (Mox 500-India) and was used as the respective CPPs, they showed variation in their dissolution behaviors under all test conditions. In fact, when the dissolution of both these products were tested, (n = 12) neither met the 85% release in the 15-min criterion (very rapidly dissolving) in any of the three media. However, the Indian CPP product, Mox 500, released 95.0% within 30 min, thereby falling under the rapidly dissolving category at pH 1.2. But only dissolved 63.8 and 76.0% in pHs 4.5 and 6.8, respectively. However, Ranmoxy® 500 (SA) dissolved 88.1% in pH 1.2 within 30 min which complies with the rapidly dissolving criterion in pH 1.2 but only 51.6 and 60.9% in pHs 4.5 and 6.8, respectively. In fact, in terms of the requirements for a biowaiver, Ranmoxy®500 and Mox 500 would not be considered to be in vitro equivalent.
Fig. 7.
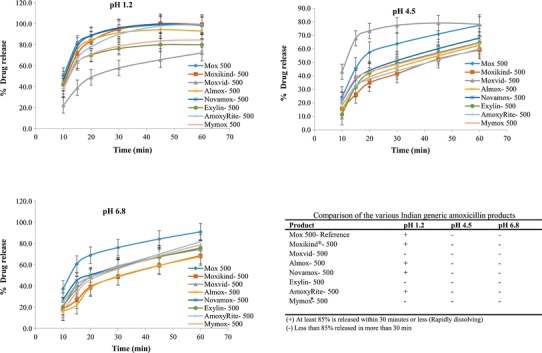
Dissolution profiles of the CPP and amoxicillin products marketed in South Africa
This study clearly challenges the presumptions relating to dissolution properties of generic products containing BCS I drugs. Even more challenging, as emphasized by Löbenberg et al. (6), is the choice/identification of a suitable/acceptable CPP when the RLD (USA) or relevant innovator product is no longer manufactured in the respective country. In our instance, the originally listed amoxicillin CPP from the Orange book was withdrawn from the market during the planning of this study, and the Orange book did not indicate the replacement CPP. Hence, the authors selected a reference product for use as the CPP using the WHO criteria (vide supra). A further intrigue is that Amoxil® 500 mg, manufactured by GlaxoSmithKline in India and listed in the Orange book, was not commercially available in either of the countries. Hence, it was not possible to obtain a reference product (i.e., the innovator product) for use as an appropriate CPP. Consequently, the resulting CPPs for India and South Africa were chosen according to the WHO requirements for use as acceptable respective “alternative” CPPs.
Interestingly, in the case of metronidazole products, it was found that two different products with the same trade name, Flagyl®400, are being marketed in India and South Africa. The Indian innovator product is manufactured by Abbott Pharma and the South African by Sanofi-Aventis (SA). Notwithstanding, both products were found to meet the very rapidly dissolving criteria in all three media. Eight of eleven metronidazole tablets (400 mg) including two CPPs were tested, five from South Africa and six from India. Of those five South Africa products, four met the dissolution requirements in all three pH media, whereas one product, Acuzole, did not comply at pH 4.5 or 6.8 (n = 12). When Acuzole was compared to the relevant CPP, Flagyl®400 mg (Sanofi-Aventis), the f2 value in pH 6.8 is 43.92.
Of the six Indian metronidazole products, four met the very rapidly dissolution requirements, whereas one product, Metgyl 400, failed to even meet the 85% in 30 min requirement in all three media. Another product, metronidazole I.P. 400 (Mortin & Brown), was found to be rapidly dissolving in all three pHs. However, whereas the f2 values passed in pH 1.2 (66.44) and pH 4.5 (63.98), the f2 value in pH 6.8 was just below the acceptance value of 50 (49.15) and, as such, deemed to have failed the biowaiver requirements. Hence, whereas previous studies (6) on these products available in the Americas found that none of the five generic metronidazole products (three generics from Argentina and two generics from Mexico vs one CPP) were in vitro bioequivalent. This is in contrast to our findings that of nine products tested (four generics vs one CPP from South Africa and five generics vs one CPP from India), three products from each of South Africa and India were found to be in vitro equivalent. In the case of the zidovudine products from South Africa and India containing 100 mg of the API, all products were very rapidly dissolving in all three media and thus complied with the biowaiver requirements. These results concur with those of Löbenberg et al. (6) where all the generic zidovudine products in the Americas showed >85% dissolution within 15 min and thus were also found to be in vitro equivalent. Although there is an innovator product, Retrovir® 100-mg capsules, marketed in South Africa by GSK, an innovator zidovudine product could not be identified in India. Hence, the Cipla product, Zidovir 100-mg capsules, marketed in India was chosen as the CPP. Similarly, Retrovir® 300-mg (GSK) tablets were available in South Africa; an innovator tablet product containing 300 mg zidovudine was not marketed in India; hence, the Cipla product, Zidovir 300-mg tablets, was chosen as the CPP.
All the tested amoxicillin products from the South African and Indian markets were not in vitro equivalent and therefore failed to meet the biowaiver requirements, whereas Löbenberg et al. (6) found that 3 of the 11 generic amoxicillin products tested against 1 CPP were found to be in vitro equivalent. The South African and Indian amoxicillin products used as CPPs only met the dissolution requirements in pH 1.2 (rapidly dissolving) but did not meet the dissolution requirements in pH 4.5 or 6.8. As a result, these two CPPs were not deemed to be in vitro equivalent. On the other hand, the two different metronidazole products with the same trade name but manufactured by different innovator companies, Sanofi-Aventis in South Africa and Abbott in India, were found to be “in vitro” equivalent.
CONCLUSIONS
Although differences in dissolution behavior exist between some metronidazole, and in particular, most of the amoxicillin generic products tested, both currently and previously (6), zidovudine products appear to be the most consistent in terms of their dissolution behavior. It is clear from all these studies that the appropriate choice of a CPP is extremely important to ensure that products which are approved on the basis of a biowaiver have the necessary quality, safety, and efficacy properties. In particular, since metronidazole, zidovudine, and amoxicillin products are included in the essential drug list (EDL) (21), it is essential that these products are rigorously tested to avoid possible serious consequences for patients if such products are found to be substandard. Based on the results of the present studies, it is somewhat of a concern that even some approved products containing BCS I drugs were not found to be in vitro bioequivalent when tested in accordance with the recommendations for a biowaiver.
Acknowledgments
This study was supported by the Biopharmaceutics Research Institute (BRI), Rhodes University, South Africa, and Dr. Prabhakar Kore Basic Sciences Research Center (BSRC), KLE University, India.
References
- 1.Newton NP. Impact of poor-quality medicines in the ‘developing’ world. Trends Pharmacol Sci. 2010;31(3):99–101. doi: 10.1016/j.tips.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nsimba SED. Problems associated with substandard and counterfeit drugs in developing countries: a review article on global implications of counterfeit drugs in the era of anti-retroviral (ARVs) drugs in a free market economy. East Afr J Public Health. 2008;5(3):205–10. doi: 10.4314/eajph.v5i3.39004. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health and family Welfare-India. Reward Scheme for whistle blowers in the fight against the menace of spurious or fake drugs, cosmetics and medical devices. 2010; p.1–9. http://www.cdsco.nic.in/writereaddata/whistle%20Blowe%20(3).pdf.
- 4.National Drug Policy for South Africa. http://www.doh.gov.za/docs/policy/drugsjan1996.pdf. 1996. Accessed 10 Feb 2011.
- 5.FDA, CDER. Guidance for industry: Dissolution testing of immediate release solid oral dosage forms. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidancesucm070237.pdf. 1997. Accessed 12 Mar 2012.
- 6.Löbenberg R, Chacra NB, Stippler ES, Shah VP, Stefano AJD, Hauck WW, et al. Towards global standards for comparator pharmaceutical product: case studies of amoxicillin, metronidazole, and zidovudine in the Americas. AAPS J. 2012;14(3):462–71. doi: 10.1208/s12248-012-9350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Fortieth report: annex 7: multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. Geneva: WHO; 2006. pp. 347–90. [Google Scholar]
- 8.Löbenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12. doi: 10.1016/S0939-6411(00)00091-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupta E, Barends DM, Yamashita E, Lentz KA, Harmsze AM, Shah VP, et al. Review of global regulations concerning biowaivers for immediate release solid oral dosage forms. Eur J Pharm Sci. 2006;29(3–4):315–24. doi: 10.1016/j.ejps.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.FDA, CDER. Guidance for industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms containing certain active moieties/active ingredients based on a biopharmaceutics classification system. http://www.fda.gov/ohrms/dockets/98fr/990121gd.pdf. 1999. Accessed 28 Dec 2013.
- 11.WHO, General notes on biopharmaceutics classification system (BCS)—based biowaiver applications. http://apps.who.int/prequal/info_applicants/BE/BW_general_2012October.pdf. October 2012. Accessed 28 Dec 2013.
- 12.Verbeeck RK, Musuamba FT. The revised 2010 EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. J Pharm Pharm Sci. 2012;15(3):376–88. doi: 10.18433/j3vc8j. [DOI] [PubMed] [Google Scholar]
- 13.FDA, CDER. Guidance for industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. August 2000. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070246.pdf Accessed 18 May 2011.
- 14.EMEA, CHMP, CPMP/EWP/QWP/1401/98 Rev. 1/Corr, Guideline on the investigation of bioequivalence. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 28 Dec 2013.
- 15.United States Pharmacopoeia, Buffer solutions. http://www.uspbpep.com/usp29/v29240/usp29nf24s0_ris1s119.html. Accessed 18 May 2011.
- 16.USP-NF. Validation of compendial procedures <1225>, https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c1225%20USP36.pdf. 2007. p.1-10.17. Accessed 18 May 2011.
- 17.USP. Dissolution <711>. US Pharmacopeial Convention, Rockville, MD. http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/2011-02-25711DISSOLUTION.pdf. 2011, p. 1-8. Accessed 18 May 2011.
- 18.FDA. Orange book: approved drug products with therapeutic equivalence evaluations. http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. 2012. Accessed Jan 2012.
- 19.FDA. Orange book: approved drug products with therapeutic equivalence evaluations. http://www.accessdata.fda.gov/scripts/cder/ob/docs/temptn.cfm. 2011. Accessed 18 May 2011.
- 20.FDA. Orange book: approved drug products with therapeutic equivalence evaluations. http://www.accessdata.fda.gov/scripts/cder/ob/docs/tempai.cfm. 2011. Accessed 18 May 2011.
- 21.WHO. Model list of essential medicines. 18th list http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf. 2013. Accessed 29 Dec 2013.


