Abstract
Biowaivers for class I drugs according to the biopharmaceutics classification system (BCS) were first introduced in 2000. The in vitro equivalence can be used to document bioequivalence between products. This study compared the in vitro dissolution behavior of two BCS class I drugs, amoxicillin and metronidazole, which are sold in China. Identifying a reference product on the Chinese domestic market was impossible. Three 250-mg and two 500-mg amoxicillin capsules and four metronidazole tablet products were tested. None of the amoxicillin products and three of the four metronidazole tablets were found to be equivalent to each other when the same strengths were compared. The bioequivalence of products that fail the in vitro test can be established via in vivo clinical studies which are expensive and time consuming. Establishing nationally or globally accepted reference products may provide regulatory agencies with an efficient mechanism approving high quality generics.
KEY WORDS: bioequivalence, biopharmaceutics drug classification system (BCS), biowaiver, dissolution, generic drugs
INTRODUCTION
Most developing countries adopt national medical policies that (1) provide their residents access to essential medicines. The World Health Organization’s (WHO) vision for essential drugs is (2) that people worldwide have the right to healthcare under the “principles of accessibility, availability, appropriateness, and assured quality to medical goods and services.”
To achieve this goal, it is important to accelerate drug approval and make generics available. Usually, a generic manufacturer must conduct clinical bioequivalence testing of the generic product. This is done by comparing it with an innovator product and establishing therapeutic equivalence. These studies can be extremely expensive and time consuming. The United States (US) Food and Drug Administration (FDA) introduced biowaivers based on the biopharmaceutical drug classification system (BCS) in 2000 (3). Bioequivalence between products can be established using dissolution testing as surrogate (4). In 2006, the WHO published a proposal (5), which allows generic drug approval based on biowaivers. This proposal is based on the availability of innovator products or well-documented generics as reference products. However, many innovator products or their therapeutical equivalents are unavailable in many countries, particularly in developing countries. Generally, an interchangeable multisource (generic) product is therapeutically equivalent when exhibiting both pharmaceutical equivalence and bioequivalence to an innovator product (6). Moreover, a pharmaceutically equivalent product contains the same active ingredients and salt form, requires the same dosage form and route of administration, and has the same strength as its comparator product (7).
Currently, governments worldwide struggle to offer high efficacy health-care services at affordable costs. For many countries, producing generic products instead of buying innovator products provides an efficient mechanism for reducing drug costs by approximately 50 to 89% (8,9).
China is the world’s largest developing country and accounts for 20% of the global population. The Chinese domestic drug market is growing rapidly. In 2011, China overtook Germany to become the third largest pharmaceutical market in the world, and it is predicted to overtake Japan by 2015 to become the second largest pharmaceutical market after the USA (10,11). From 2013 to 2020, the Chinese market will continue to rapidly expand (10,12,13). However, the size of the Chinese pharmaceutical market primarily results from its large population and increasing aging population rather than its maturity, as it is still an emerging market (10,12).
A previous study suggested that if Chinese public hospitals exchange four different innovator products with their generic equivalents, they could reduce medical costs by US$370 million (9). Therefore, the use of generic products can reduce the cost of drug therapy. In the next 5 years, patents worth US$77 billion are estimated to expire for certain innovator products. This wave of patent expirations will significantly boost generic manufacturers and generic sales (12).
The centralized drug regulatory authority in China, the China Food and Drug Administration (CFDA), is responsible for supervising the regulations related to food, drugs, medical devices, and cosmetics in the provinces, autonomous regions, and municipalities. The CFDA correlates to the FDA in the USA. Specific to China, the CFDA classifies the generic products into the following categories: (1) generic products based on traditional Chinese medicines or natural drug injections, (2) generic chemical products, and (3) biosimilars (14). The presented research only focuses on generic chemical products. In fact, generic drugs are the mainstay of China’s pharmaceutical market and are likely to remain so for a long time. There are 7,019 pharmaceutical companies in China (15), most of which produce generic drugs. China will most likely continue to rely upon the widespread prescription of generics through its public insurance plan to maintain overall healthcare expenditures (12). The CFDA approved 644 domestic drug registrations in 2011, 67.7% of which were for generic drugs (16). Thus, ensuring that large amounts of pharmaceutical products in the market are therapeutically equivalent is a challenge for the different level regulatory agencies in China. The practitioners have no knowledge which reference product was used to make a generic since no reference products are officially listed.
The objective of this study was to evaluate the performance of two widely used BCS I drug products, amoxicillin and metronidazole, which are marketed in China. The dissolution behaviors of these products were compared to establish bioequivalence between products as used for biowaivers.
METHODS
Chemicals
The amoxicillin (K0H332) and metronidazole (H0F263) reference standards (RS) were obtained from US Pharmacopeia (USP, Rockville, MD, USA). Acetonitrile HPLC Grade, potassium phosphate, potassium hydroxide, and sodium acetate were purchased from Caledon (Georgetown, ON). Hydrochloric acid, glacial acetic acid, sodium hydroxide, sodium chloride, and HPLC-grade phosphoric acid were purchased from Fisher Scientific (Fairlawn, NJ). All chemicals were USP grade except where specified.
Test Products and Media
All products were purchased from a pharmaceutical market in China and were tested prior to their expiration dates. The various strengths of the amoxicillin capsules and metronidazole tablets are listed in Tables I and II, respectively. The authors used three 250-mg amoxicillin products, A, B, and C and two 500-mg amoxicillin products, D and E, for the evaluation. Four metronidazole tablets were tested, A, B, C, and D. Simulated gastric fluid (SGF), acetate buffer (pH 4.5 USP), and simulated intestinal fluid (SIF) were prepared without enzymes according to the procedure for USP test solutions.
Table I.
Amoxicillin Capsules
| Product | Manufacturer | Lot number | Expire date | Approval number |
|---|---|---|---|---|
| Amoxicillin 250 mg | Liaoyuan Yulongyadong Pharmaceutical Co., Ltd. | 120302 | 02/15 | H22025159 |
| Amoxicillin 250 mg | Shijiazhuang Kanghewei Pharmaceutical Co., Ltd. | 120310 | 02/15 | H13020051 |
| Amoxicillin 250 mg | Hong Kong United Laboratories Co., Ltd. | 18858 | 02/16 | HC20090039 |
| Amoxicillin 500 mg | Zhuhai United Laboratories (Zhongshan) Co., Ltd. | 20505011 | 04/16 | H20003263 |
| Amoxicillin 500 mg | North China Pharmaceutical Group Corporation | 1111203 | 10/14 | H20043535 |
Table II.
Metronidazole Tablets
| Product | Manufacturer | Lot number | Expire date | Approval number |
|---|---|---|---|---|
| Metronidazole 200 mg | Feiyunling Pharmaceutical Co., Ltd. | 120401 | 03/14 | H52020150 |
| Metronidazole 200 mg | Shanxi Tongda Pharmaceutical Inc. | 111201 | 11/14 | H14022863 |
| Metronidazole 200 mg | Grand Pharm (China) Co., Ltd | 120543 | 04/15 | H42021947 |
| Metronidazole 200 mg | Huazhong Pharmaceutical Co., Ltd. | 20120524 | 04/14 | H42040388 |
Dissolution Test
A VK 7020 dissolution tester with six vessels, a VK 8000 auto-sampler station (Agilent Technologies, Carey, NC), and a USP apparatus 2 were used for the studies. For the capsule products, the Japanese Pharmacopeia Basket Sinkers (Quality Lab Accessories, Brigewater, NJ) which are compliant with USP were utilized. Preheated and degassed media (900 mL) were used to fill each vessel without the inclusion of air. The test was initiated at 75 rpm once the temperature was confirmed in all vessels. The medium (1.25 mL) was collected and filtered, and 1 mL was transferred into a vial from each vessel at 10, 15, 20, 30, 45, and 60 min. The remaining fluid was discarded, and the drug concentration was computationally corrected because the medium was not replaced after sampling.
HPLC Assay
The quantity of drug released from the products was determined using a high-performance liquid chromatography (HPLC) system. The flow rate was 1 mL/min, and the injected sample size was 10 μL. The run time for each drug was approximately 3–3.5 min. The retention time ranged from 2 to 2.5 min. A LiChrosphere RP 60 Select B column (12.5 × 5 mm) (Merck, Darmstadt) with a guard column was used.
Amoxicillin
For the HPLC assay of amoxicillin, UV detection occurred at 219 nm. The mobile phase was 5% acetonitrile and a 95% pH 5.0 buffer. The pH 5.0 buffer was 6.8 g KH2PO4 in 900 mL water with the pH was adjusted to 5.0 ± 0.1 to yield 45% KOH with an adjusted volume of 1,000 mL. The correlation coefficient of the calibration curve was at least 0.999 for each medium over the expected concentration range from 3.75 to 120% with a coefficient of variation (CV%) of 1.95 in SGF, 2.00 in pH 4.5 buffer, and 2.56 in SIF.
Metronidazole
The HPLC assay of the metronidazole utilized UV detection at 228 nm, and the mobile phase consisted of water/acetonitrile (66:34). The calibration curve was linear for the concentration range between 3.75 and 120% with a correlation coefficient of at least 0.999 for each medium and CV%s of 1.73 in SGF, 2.02 in pH 4.5 buffer, and 1.59 in SIF.
Uniformity of Dosage Units
Weight variation was used to assess uniformity between test units; homogeneous drug distribution was assumed, and weight variation was used to link potential drug release differences to weight differences of the test units. The weights of 18 to 36 capsules or tablets were recorded for each tested product. The weight variations were calculated as relative standard deviations (RSD) using the following equations:
in which s represents the standard deviation, Xi is the individual weight, is the mean of all weights, and n is the number of samples measured (17). Generally, the RSDs are ≤6.0%.
Data Analysis
All dissolution data were evaluated using an Excel spreadsheet, and the results were plotted for each product. If two products both exhibited a drug release above 85% within 15 min, indicating a very rapidly dissolving product as defined by the WHO (4), then they were considered similar for that medium. Therefore, if the average drug release for six units of a drug product at 15 min was below 85%, an additional six samples were evaluated for that product, and the similarity factor (f2) was calculated for two products which were compared with each other using DDSolver, an Excel-plug-in module (18). The in vitro equivalence among the various test products was established when the dissolution profiles were similar in all three test media according to the f2 evaluation.
RESULTS
Amoxicillin
No innovator product could be identified for the Chinese market. Therefore, the available products were compared only to each other.
The 250-mg amoxicillin capsules varied in weight between 369.3 and 388.5 mg, with an observed RSD between 1.0 and 2.6%. The mean weights of the two 500-mg amoxicillin products were 693.6 and 684.4 mg, with RSDs of 0.7 and 1.7%, respectively.
The drug release profiles of the three 250-mg amoxicillin capsules are shown in Fig. 1. Figure 2 presents the dissolution behavior of the 500-mg amoxicillin products. Both figures indicate that the amoxicillin is chemically unstable in SGF, which is consistent with the findings of Loebenberg et al. (19) who studied amoxicillin products in the Americas.
Fig. 1.
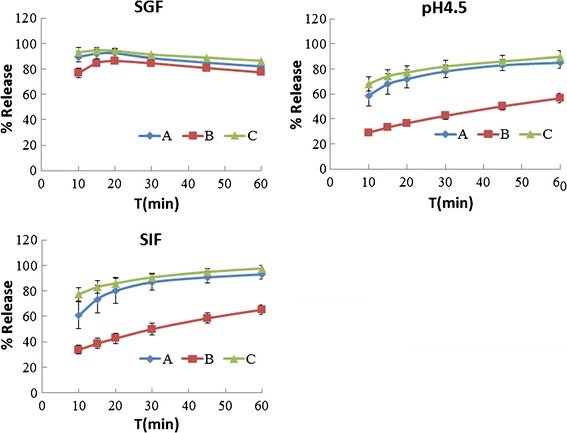
Dissolution behavior of 250-mg amoxicillin capsules
Fig. 2.
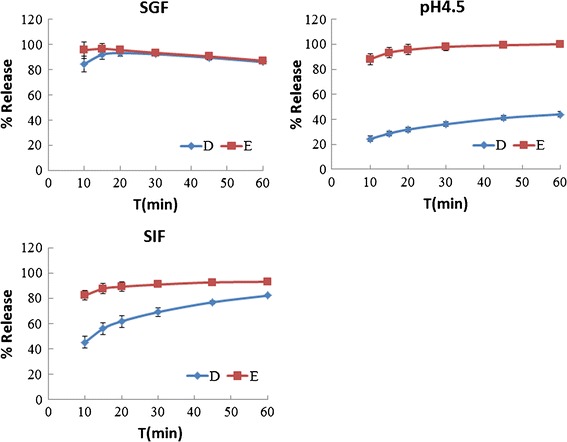
Dissolution behavior of 500-mg amoxicillin capsules
As shown in Fig. 1, the 250-mg amoxicillin products A, B, and C dissolved rapidly in SGF only. All three products were less than 85% dissolved in 15 min in the pH 4.5 buffer and in SIF. All three products failed their f2 comparisons in SIF (f2 = 23.21, 46.66, and 17.91). In the pH 4.5 buffer, product B was unlike products A and C (f2 = 24.12 and 20.27), whereas product A was similar to C (f2 = 60.84).
Figure 2 provides the drug release profile of the 500-mg amoxicillin product E, which rapidly dissolved in all three media. The 500-mg product D dissolved rapidly only in SGF with a drug release of less than 85% after 15 min in the pH 4.5 buffer and SIF. The two 500-mg products were also different.
Therefore, the 250- and 500-mg amoxicillin capsules were not equivalent to the other products of the same strength in vitro.
Metronidazole
The variations in the weights of all tested metronidazole tablets ranged from 249.2 to 289.9 mg, with an RSD ranging from 1.1 to 3.5%. The in vitro drug release behavior of the 200-mg metronidazole tablets is presented in Fig. 3. Products B, C, and D dissolved very rapidly in all three media, and they were considered equivalent to each other in vitro. Product A dissolved rapidly only in the pH 4.5 buffer and SIF, with less than 85% dissolved after 15 min in SGF. Therefore, the drug release profile of product A was unlike the other three products.
Fig. 3.
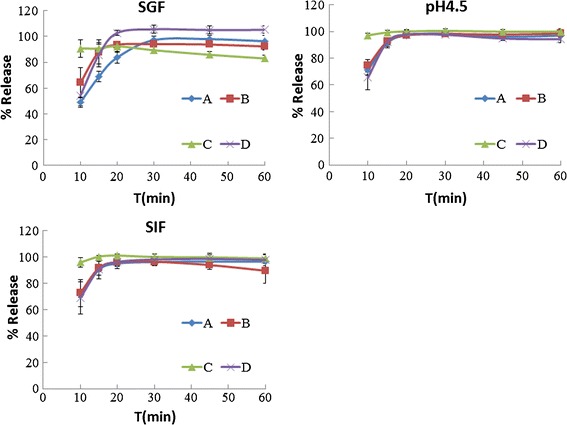
Dissolution behavior of metronidazole tablets
DISCUSSION
For amoxicillin, neither an amoxicillin comparator pharmaceutical product (CPP) listed in the WHO Guidance on the selection of comparator pharmaceutical products for equivalence assessment of interchangeable multisource (generics) products (20), Amoxil from SmithKline Beecham, nor the listed reference drug in the FDA’s Orange Book (7), Amoxicillin from TEVA, was available on the pharmaceutical market in China (21) or Canada where the study was undertaken (22). For metronidazole, the strength of the metronidazole purchased from China is 200 mg, different to the strength of 250 mg that sold in North America. According to the WHO guidance (20), the innovator product for metronidazole cannot be identified and no specific CPP exists. In 2007, the CFDA defined that the reference products used for generics development in China should be innovator products if they are available on the domestic market; otherwise, market-leading products can be used instead (23). Identifying market-leading products as reference products for generics remains challenging when the innovator products are discontinued or unavailable in a country’s pharmaceutical market (24). Therefore, this study can only determine whether the tested products are in vitro equivalents to each other.
Based on the results, none of the amoxicillin products were in vitro equivalents at the same dose and dosage form tested. To ensure the therapeutic equivalence of these products, an in vivo study must be performed despite the possibility to request a biowaiver.
All of the tested products were approved by the regulatory agency, and they are presumably bioequivalent in vivo. However, our results illustrate that the in vitro behaviors of various amoxicillin brands were not similar. If an innovator product is discontinued or is unavailable on the Chinese market, then it is impossible to know for the practitioner which reference product was used. Furthermore, it is not known whether different reference products exist and whether these products are bioequivalent to each other.
Figure 3 also indicates that product A of metronidazole dissolves slower in SGF than in the pH 4.5 buffer and SIF, which is surprising because metronidazole is a weak base with a maximum dissolution at approximately pH ≤ 2.0 (25). This result may depend on the excipients used in the formulation.
Generally, establishing a reference product and applying a biowaiver for equivalence assessment simplify the approval process for several generic products, aid generic manufacturers in reducing their drug development costs, and eliminate the ethical question raised by hundreds of bioequivalence studies necessary if biowaivers are not used.
According to the Regulation on the Drug Registration promulgated by the CFDA in 2007 (14), once the drug quality is controlled by defined processes and standards, 18–24 subjects are typically required for each chemical generic product in a bioequivalence study. According to the Pharmacopoeia of the People’s Republic of China, there are three different strengths (125, 250, and 500 mg) of amoxicillin capsules (26) and three different strengths (100, 200, and 250 mg) of metronidazole tablets (27) sold in China. Therefore, as expected, the CFDA lists 372 types of amoxicillin capsules and 692 types of metronidazole tablets marketed in China from different manufacturers with different strengths (28). For each product, the overall approval process may take 1 to 2 years. Even if the cost of clinical testing performed in China is only half or one fifth of that in the USA (29), for the massive amount of generic products marketed in China, the overall cost of clinical trials remains large. These costs could be saved by establishing the reference products and in vitro equivalences to waive the in vivo bioequivalence studies for qualifying generic drug products.
During this study, the CFDA released (December 2012) a recommendation that suggested reforming the drug approval review process by enhancing its efficiency (30), providing more effective and safe medical services, and ensuring citizen rights to high-quality health care. Based on this initial statement, the CFDA (February 2013) issued a scheme to evaluate generic quality consistency and establish a standard to reevaluate and control the quality of the essential generic products that were approved prior to 2007 (31). In this scheme, the CFDA will define the standard in vitro evaluation methods of generics by 2015, identify the universal standard reference products, and allow biowaiver studies. All essential generic products that were approved prior to 2007 will be reevaluated according to the new regulations. After being reevaluated, the generic approval time in China will decrease 8 to 12 months.
Therefore, establishing a reference product is valuable for the development of pharmaceutical manufacturers in China. This benefits both the government, which may reduce the approval processing time, and the manufacturers, who may reduce their development costs. These reference products may also aid the regulatory agencies in overseeing the quality of various product batches.
CONCLUSION
Three of the four tested metronidazole tablets exhibited in vitro equivalence, whereas none of the amoxicillin products tested exhibited in vitro equivalence. To determine the bioequivalence of these amoxicillin products, clinical trials must be performed. Compared with clinical trials, the in vitro equivalence studies are a less time-consuming and a lower-cost method of ensuring bioequivalence of between drug products. The difficulty in identifying a reference product on the Chinese domestic market may result in the use of different reference products by different generic manufacturers. It is not known whether such reference products are bioequivalent to each other. Establishing a universal reference product for interchangeable multisource products is valuable and may aid governments in providing high-quality generics. Establishing nationally or even globally accepted reference products may also provide the regulatory agencies with an efficient mechanism for approving high-quality generics.
REFERENCES
- 1.World Health Organization. Country pharmaceutical situations: fact book on WHO level 1 indicators 2007. Geneva: WHO. 2009. http://apps.who.int/medicinedocs/documents/s16874e/s16874e.pdf Accessed 20 Nov 2012.
- 2.World Health Organization. Essential medicines and health product. 2012. http://www.who.int/medicines/en/ Accessed 20 Nov 2012.
- 3.Food and Drug Administration. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. 2000. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf. Accessed 20 Nov 2012.
- 4.World Health Organization. Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. Annex 7 of WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva: WHO Technical Report Series No.937, 2006; 40th report: p.347-90. http://apps.who.int/prequal/info_general/documents/TRS937/WHO_TRS_937__annex7_eng.pdf Accessed 20 Nov 2012.
- 5.World Health Organization. Proposal to waive in vivo bioequivalence requirements for WHO model list of essential medicines—immediate-release, solid oral dosage forms. Annex 8 of WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva: WHO Technical Report Series No.937, 2006; 40th report: p.391-461. http://apps.who.int/prequal/info_general/documents/TRS937/WHO_TRS_937__annex8_eng.pdf Accessed 20 Nov 2012.
- 6.Williams RL, Chen ML, Hauck WW. Equivalence approaches. Clin Pharmacol Ther. 2002;72:229–37. doi: 10.1067/mcp.2002.126705. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations, 33rd ed. Rockville: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Pharmaceutical Science Office of Generic Drugs. 2013. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf Accessed 20 Apr 2013.
- 8.Spino M, Tsang YC, Pop R. Dissolution and in vivo evidence of differences in reference products: impact on development of generic drugs. Eur J Drug Metab Pharmacokinet. 2000;25:18–24. doi: 10.1007/BF03190051. [DOI] [PubMed] [Google Scholar]
- 9.Cameron A, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Switching from originator brand medicines to generic equivalents in selected developing countries: how much could be saved? Value Health. 2012;15:664–73. doi: 10.1016/j.jval.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.GBI research. China pharmaceutical market outlook—government incentives, healthcare reform and a rapidly ageing population provide strong stimulus for growth. 2013. http://www.researchandmarkets.com/research/pgk2g8/china Accessed 20 Apr 2013.
- 11.Hirschler B. China seen as no. 2 drugs market by 2015. Reuters. 2010 Nov 8 http://uk.reuters.com/article/2010/11/08/us-summit-china-drugs-idUKTRE6A73SL20101108 Accessed 8 May 2013.
- 12.Deloitte China. The next phase: opportunities in China's pharmaceuticals market. 2012. http://www.deloitte.com/view/en_CN/cn/ind/lshc/723313bbb0943310VgnVCM3000001c56f00aRCRD.htm Accessed 20 Apr 2013.
- 13.Vesiongain. Chinese pharmaceutical market 2013–2023. 2013. http://www.visiongain.com/Report/1018/Chinese-Pharmaceutical-Market-2013-2023 Accessed 8 May 2013.
- 14.China Food and Drug Administration. Regulation on drug registration (《药品注册管理办法》) Order 28, 2007.Chinese. http://www.sfda.gov.cn/WS01/CL0053/24529.html Accessed 21 Apr 2013.
- 15.Database of pharmaceutical manufacturer (药品生产企业). China Food and Drug Administration, Beijing. 2013. Chinese. http://app1.sfda.gov.cn/datasearch/face3/dir.html Accessed 21 Apr 2013.
- 16.China Food and Drug Administration. Annual report of registration and approval of 2011(2011药品注册审批年度报告). China Food and Drug Administration. 2012. Chinese. http://www.sda.gov.cn/WS01/CL0050/75250.html Accessed 10 Feb 2013.
- 17.Uniformity of dosage units <905>. In United States Pharmacopeia and National Formulary USP 36–NF 31. Rockville: The United States Pharmacopeial Convention; 2013. p. 431–3.
- 18.Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–71. doi: 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löbenberg R, Chacra NB, Stippler ES, Shah VP, DeStefano AJ, Hauck WW, et al. Toward global standards for comparator pharmaceutical products: case studies of amoxicillin, metronidazole, and zidovudine in the Americas. AAPS J. 2012;14:462–72. doi: 10.1208/s12248-012-9350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Guidance on the selection of comparator pharmaceutical products for equivalence assessment of interchangeable multisource (generic) products. Annex 11 of WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva: WHO Technical Report Series No.902, 2002; 36th report: p.161-80. http://apps.who.int/medicinedocs/documents/s19641en/s19641en.pdf Accessed 20 Nov 2012.
- 21.Database of imported drug product (进口药品). China Food and Drug Administration, Beijing. 2013. Chinese. http://app1.sfda.gov.cn/datasearch/face3/dir.html Accessed 20 Nov 2012.
- 22.Drug Product Database Online Query. Health Canada, Ottawa. 2012. http://webprod5.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp Accessed 20 Nov 2012.
- 23.China Food and Drug Administration. Guideline of development of chemical generics with existing state standard (已有国家标准化学药品研究技术指导原则). China Food and Drug Administration. 2007. Chinese. http://www.cde.org.cn/zdyz.do?method=largePage&id=2076 Accessed 1 May 2013.
- 24.Williams RL, Shah VP. Continuing equivalence: is there an end to the story? J Generic Med. 2008;5:297–304. doi: 10.1057/jgm.2008.19. [DOI] [Google Scholar]
- 25.Wu Y, Fassihi R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int J Pharm. 2005;290:1–13. doi: 10.1016/j.ijpharm.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Amoxicillin capsules (阿莫西林胶囊). In Pharmacopoeia of the People's Republic of China 2010, vol. 2 (中华人民共和国药典2010版二部). Beijing: Chinese Pharmacopeia Commission. p. 403–4. Chinese.
- 27.Metronidazole tablets (甲硝唑片). In Pharmacopoeia of the People's Republic of China 2010, vol.2 (中华人民共和国药典2010版二部). Beijing: Chinese Pharmacopeia Commission. p. 153–4. Chinese.
- 28.Database of domestic drug product (国产药品). China Food and Drug Administration, Beijing. 2013. Chinese. http://app1.sfda.gov.cn/datasearch/face3/dir.html Accessed 21 Apr 2013.
- 29.Cyranoski D. Pharmaceutical futures: made in China? Nature. 2008;455:1168–70. doi: 10.1038/4551168a. [DOI] [PubMed] [Google Scholar]
- 30.China Food and Drug Administration. View of reforming the drug approval process to encourage drug innovation (Draft) (关于深化药品审评审批改革进一步鼓励药物创新的意见) (征求意见稿). China Food and Drug Administration.2012. Chinese. http://www.sda.gov.cn/WS01/CL0778/77409.html Accessed 1 May 2013.
- 31.China Food and Drug Administration. Evaluation scheme of generic quality consistency(仿制药质量一致性评价工作方案). China Food and Drug Administration.2013. Chinese. http://www.sfda.gov.cn/WS01/CL0844/78516.html Accessed 1 May 2013.


