Abstract
Ginsenoside Rb1 (Rb1) is the most predominant ginsenoside isolated from the roots of ginseng (Panax ginseng C. A. Meyer). This compound is active in various human biological pathways that are involved in human collagen synthesis and inhibition of cell apoptosis. In this study, the skin-whitening effects of Rb1 were investigated in B16 melanoma cells. Our results showed that Rb1 inhibited melanogenesis in α-melanocyte-stimulating hormone (α-MSH)-stimulated B16 cells in a dose-dependent manner, which collectively indicated that Rb1 may have skin-whitening effects and may be formulated into skin-whitening products for skin care. Accordingly, a ginsenoside collagen transdermal patch was developed as a vehicle to topically deliver Rb1 into pig skin. The percutaneous permeation, retention within skin, and release in vitro of Rb1 from seven transdermal patch formulas were studied. It was determined that the best formula for ginsenoside collagen transdermal patch is made of protein collagen hydrolysate powder (PCHP) 2.0% (w/w), methyl cellulose (MC) 0.5% (w/w), polyethyleneglycol 6000 (PEG6000) 0.5% (w/w), ginsenoside 0.036% (w/w), azone 0.4% (v/w), menthol 0.20% (w/w), and water.
KEY WORDS: ginsenoside rb1, melanin, permeation, skin whitening, transdermal patch
INTRODUCTION
In the field of skin health, much effort has focused on developing reliable skin-rejuvenating and skin-whitening products for patients with unwanted pigments or freckles. Hyperpigmentation and freckles of the skin are a result of abnormal accumulation of melanin, a photoprotective skin pigment that is essential for the human body. Studies have previously reported that the first steps of melanogenesis pathway in humans are the hydroxylation of l-tyrosine to 3-4-dihydroxy-phenylalanine (l-DOPA) and the oxidation of l-DOPA to o-dopaquinone (1). Tyrosinase catalyzes both of the reactions and is known as the key enzyme in melanogenesis. Antioxidants, such as arbutin or kojic acid (2,3), may inhibit these oxidation steps and have been used in the treatment of hyperpigmentation.
Ginsenoside Rb1 (Rb1) (Fig. 1a), an aglycone with a dammarane skeleton steroidal saponin, is isolated from the roots of ginseng (Panax ginseng C. A. Meyer). Recently, ginsenoside Rb1 has been reported to show various biological functions, which include protecting cellular apoptosis from ultraviolet (UV) radiation by inducing DNA repair (4), inhibiting collagen degradation after UV irradiation on mice skin (5), inducing human type I collagen synthesis through activation of SMAD signaling (6), promoting both COL1A2 messenger RNA (mRNA) and protein expression mainly mediated through PPARδ, and reducing microRNA-25 expression to promote COL1A2 mRNA translation (7). The first objective of the study is to show that Rb1 may also inhibit melanin production in mouse B16 melanoma cells by measuring the melanin contents and tyrosinase activities in these cells.
Fig. 1.

The chemical structure of ginsenoside Rb1 (C54H92O23) and appearance of ginsenoside collagen transdermal patches. a The chemical structure of ginsenoside Rb1. b Ginsenoside collagen transdermal patch. c Ginsenoside collagen transdermal patch in cylinder size for percutaneous permeation study
The second objective of the study is focused on developing a suitable topical delivery vehicle for gensinoside Rb1. Protein collagen hydrolysate powder (PCHP) is made from non-polluted and high-quality fish through modern enzymatic biotechnology, which has low molecular weight and is soluble in water and easily absorbed by human body. We found experimentally that the mixture of protein collagen hydrolysate powder, methyl cellulose (MC), and polyethyleneglycol 6000 (PEG6000) by dissolving in water can form a translucent and smooth film and exhibit considerable thermal stability, which can be used as a direct supplement of collagen in skin through pore filtering (8,9) to improve the skin’s appearance and tactility and to remove wrinkles. Therefore, it was of interest for use as a topical drug delivery vehicle for cosmetic products, in particular as a topical patch or a facial mask.
The principle of pharmacology states that the drug molecule must reach its target tissue to exert its effect. In the case of ginsenoside Rb1, it must be able to penetrate the epidermis, a heterogeneous tissue layer, before reaching its target, the melanocytes. Gensinoside Rb1 is a large molecule with poor solubility profiles. In view of ginsenoside Rb1’s poor penetration (10) across skin, the use of penetration enhancers is an enticing consideration to significantly increase its percutaneous permeation. A wide variety of azones as well as menthol have been shown to increase the percutaneous absorption of many drugs (11).
There are some cosmetic products on the market that now include ginseng extract within their ingredients, such as Beauty Junky—Ginseng Face Mask (UK), Aqualabel (Japan), Ginseng Mask Pack (Korea), etc. However, there is no report about any investigation into how the ginseng ingredients from the facial mask reach the skin and the pharmacokinetics of drug release of these mask products. The final objective of this study is to develop a novel ginsenoside transdermal patch formula with optimal Rb1 penetration across skin. The effect of penetration enhancers at different concentrations have been studied on the percutaneous permeation of ginsenoside Rb1 on pig skin in vitro. Based on the principals of experimental design of experiments, the methodology encompasses the use of various types of experimental designs, generation of kinetics equations, and statistical comparisons with a percutaneous permeation coefficient to optimize the ginsenoside collagen transdermal patch formula.
MATERIALS AND METHODS
Materials
B16 melanoma cells were provided by the Shanghai Institute for Biological Science, Chinese Academy of Sciences (Shanghai, China). 3, 4-Dihydroxyphenylalanine (DOPA), Triton X-100, α-melanocyte-stimulating hormone (α-MSH), arbutin (>98% purity), synthetic melanin (>95% purity), Trypan blue solution (0.4%), menthol, methyl cellulose (MC), and polyethyleneglycol 6000 (PEG6000) were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle Media (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), phosphate-buffered saline (PBS) pH 7.4, Trypsin-EDTA (0.05%), penicillin, and streptomycin were purchased from Invitrogen (Life Technologies™, Carlsbad, CA). Protein collagen hydrolysate powder (PCHP) was purchased from Guangzhou Zhuoning Co., Ltd (Guangdong, China). Ginsenoside Rb1 was purchased from Fleton Natural Products (Chengdu, China), and its purity was verified to be over 98% by high-performance liquid chromatography and thin-layer chromatography. Azone (97%) was purchased from Aladin Chemistry Co., Ltd. (Shanghai, China). Acetonitrile was of HPLC grade (Anaqua Chemicals Supply, Houston, TX). Ultrapure water was generated by a Millipore water purification system (Billerica, MA).
Methods
Cell Culture
B16 melanoma cells were cultured in DMEM with GlutaMAX containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
The cells were seeded at an appropriate cell density in a 24-well or a 96-well plate. After 24 h of incubation, the triplicate cells were treated with the drugs in various concentrations in the absence or presence of 10 nM of α-MSH for another 48 h. Thereafter, the cells were harvested and used for various assays (12).
Cell Viability Studies
MTT (3- (4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay was performed to examine the viability of cells treated with various concentrations of Rb1. B16 cells were seeded at a density of 3 × 103 cells per well in 96-well culture plates. After 24 h of incubation, the cells were treated with Rb1 (15.63, 31.25, 62.5, 125, 250, 500, 1000 μM) for 48 h and then subjected to MTT assay to determine the cell viability according to a method reported previously with slight modifications (13). Briefly, these cells were first treated with 20 μL of MTT (0.5 mg/mL in DPBS) at 37°C for 2.5 h. The MTT supernatant was then sucked away and 100 μL of dimethylsulfoxide (DMSO) was added to dissolve the formazan precipitates, following which the absorbance of the dissolved precipitates were quantified at wavelength 570 nm with the reference wavelength of 630 nm by the Benchmark Plus Microplate Reader (Bio-Rad Laboratories). Cell viability was expressed as a percentage relative to the absorbance value from untreated cells. Each experiment was performed in triplicate and each experiment was repeated three times. The results from the assay were applied to the following experiments.
Measurement of Melanin Content
The amount of melanin in cells studied was measured as described previously, with a slight modification (12,14,15). In brief, B16 cells were seeded at a density of 3 × 104 cells per well of 24-well microplates and incubated at 37°C under 5% CO2 atmosphere for 24 h. These cells were then stimulated with α-MSH (10 nM) and were treated with various concentrations of Rb1 for 48 h. At the end of cell cultivation, the cells were washed with DPBS and lysed in 100 μL of 1% Triton X-100 at 4°C for 30 min. After centrifugation at 16400 rpm for 10 min, 100 μL of supernatant were transferred to a microcentrifuge tube for tyrosinase activity study, and the precipitated melanin pellets were dissolved in 1 M NaOH followed by a 1-h heating at 80°C. Each lysate was then transferred into wells of a 96-well microplate, and its optical density at 405 nm was measured by using a microplate reader. The melanin content was calculated using the standard curve of synthetic melanin. The protein content in the supernatant was determined by using a Bradford assay with bovine serum albumin (BIO-RAD, USA) as the standard. Each experiment was repeated three times. The specific melanin content was adjusted by the amount of protein in the same reaction. Arbutin and kojic acid were used as the positive control for melanogenesis inhibition.
Tyrosinase Activity Assay
Cellular tyrosinase activity was measured as previously described (12,14,16) with slight modification. Briefly, the tyrosinase activity was estimated by measuring the level of l-DOPA. Study cells were harvested, lysed, and centrifugated according to methods previously described. After centrifugation, 75 μL of freshly prepared substrate solution (0.2% l-DOPA) was added to the same volume lysate supernatant. Following 4-h incubation at 37°C, the optical densities (OD) of dopachrome formation was monitored by measuring absorbance at a wavelength of 475 nm. Each treatment was performed in triplicate and each experiment was repeated three times. The specific tyrosinase activity was normalized with regard to protein content in the reaction.
UPLC Determination of Ginsenoside Rb1
A quantitative determination method of ginsenoside Rb1 through ultra-performance liquid chromatography (UPLC) was devised and validated as per Pharmacopoeia of the People’s Republic of China (2010 Edition) by using an ACQUITY UPLC System (Waters, Milford, MA) equipped with DAD detector and ACQUITY UPL® BEH C18 column (1.7 μm, 2.1 mm × 50 mm, Waters). The mobile phase was a mixture of Acetonitrile and Millipore water at a ratio of 28/72 (v/v) (17–19). Different concentration of ginsenoside Rb1 was solved in PBS (pH 7.4) and aliquots of 10 μL from each sample were injected and eluted at a flow rate of 0.3 mL/min. Measurements were taken at a wavelength of 203 nm and the column temperature was maintained at 30°C. The calibration curves, y = 4907.5x − 14034; (y represents area, x represents the concentration of ginsenoside Rb1), were plotted for ginsenoside Rb1 in the range of 2.89–28.9 μg/mL. A good linear relationship was observed between the concentration of Rb1 with a correlation coefficient (r = 0.9994). The limit of quantity (LOQ, S/N = 10) and the limit of detection (LOD, S/N = 3) were lower than 20 and 6 ng, respectively.
Solubility Studies
Solubility study was performed according to the method previously described (20). Briefly, n-octanol and water were mutually saturated for 24 h before the experiment. The solubility of Rb1 was determined in water, PBS (pH 7.4), and n-octanol. Stock solution of Rb1 was prepared in alcohol at 1 M. Thirty microliters of Rb1 was pipetted into three vials and dried under air. One milliliter of either water, PBS (pH 7.4), or n-octanol was then added into the vials, and the mixture was shaken at 37 ± 0.5°C for more than 24 h until equilibration. After the mixture was centrifuged at 16, 400 rpm for 20 min, the supernatant was filtered through 0.2-μM membrane filters and the concentration of drug was measured by UPLC after appropriate dilution. The experiments were performed in triplicate and the results were shown in Table I. Octanol/water partition coefficient (Po/w) of ginsenoside Rb1 was calculated according to the literature (21).
Table I.
Physicochemical Properties of Ginsenoside Rb1
| Properties | Results |
|---|---|
| Molecular weight (g/mol) | 1109.28 |
| Solubilitya in water (mg/mL) | 15.32 ± 0.911 |
| Solubilitya in n-octanol (mg/mL) | 25.88 ± 1.007 |
| Solubilitya in PBS (mg/mL) | 18.68 ± 1.143 |
| Partition coefficient (P o/w) | 1.689 |
| Log P o/w | 0.228 |
PBS phosphate-buffered saline
aThe solubility was tested at room temperature (20 ± 1°C controlled by an air conditioner)
Skin Preparation
The skin samples were obtained from the abdominal regions of pigs from the slaughterhouse under legal requirements. In brief, skin flaps (including epidermis and dermis) were removed from pig abdomens immediately after slaughter. The flaps were washed with cold water before they were stored at −20°C until use (maximum 6 months). Prior to the experiment, the skin flaps were defrosted and equilibrated in PBS for 120 min. The integrity of the skin samples was carefully checked and any defective samples were rejected. All procedures involving animals were carried out according to the guidelines of the Committee on the Use of Human and Animal Subjects in Teaching and Research of Hong Kong Baptist University and the Health Department of Hong Kong Special Administrative Region.
Preparation of Ginsenoside Collagen Transdermal Patch
Ginsenoside collagen transdermal patches were prepared by a three-step procedure. (I) MC was firstly dissolved in 5 g water with slow stirring at room temperature (the lab was kept at 20 ± 1°C by the air condition). The MC solution was heated to 70°C by a 75°C water bath, then PCHP, and PEG6000 were dispersed in the 70°C solution. (II) Rb1 (100 mg) was dissolved in water to form 10.0-mL Rb1 solution. Various penetration enhancers, 360-μL Ginsenoside Rb1 solution, and adequate water were added to the former 10 g polymeric aqueous dispersion under gentle agitation to ensure homogeneity of the formula. (III) The homogeneous mixture (10 g) was pulled and spread onto a 10.0 × 7.5 (cm2) glass plate and fully covered the whole flat area of the glass plate, which would form a slightly white ginsenoside collagen transdermal patch at room temperature in 5 min. The patches were translucent and contained a high concentration of ginsenoside Rb1 (Fig. 1b). The recipes of the ginsenoside collagen transdermal patches used in the following experiments are shown in Table II. Formula 7 was the control group which contained no enhancer in the transdermal patch.
Table II.
Composition of Ginsenoside Collagen Transdermal Patch (10 g)
| Formula | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| PCHP (g) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| MC (g) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| PEG6000 (g) | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Rb1 (mg) | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Azone (mL) | 0.02 | 0.04 | 0.04 | 0.04 | |||
| Menthol (g) | 0.02 | 0.04 | 0.02 | 0.04 | |||
| Ultrapure water | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Total weight (g) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Rate of In Vitro Percutaneous Permeation: Measurement and Characterization
Skin permeation experiments were conducted in two types of diffusion cells, modified Franz diffusion cell (vertical) and Side-Bi-Side diffusion cell (horizontal) (PermeGear, Inc, Hellertown, PA). During the experiment, the receptor chamber contained 6 mL of magnetically stirred PBS (pH 7.4) solution. The temperature of the compartments was maintained at 37 ± 0.5°C through the use of a circulating water bath. The skin flap was mounted onto the diffusion cell, with the epidermal surface facing the donor chamber. Excess skin at the sides was trimmed off to minimize lateral diffusion. The exposed surface area of the skin sample for the permeation of the transdermal patch was 0.67 cm2. The ginsenoside collagen transdermal patch was cut into a cylindrical frame with a diameter of 0.9 cm and height of 0.3 cm (Fig. 1c). The patch was mounted directly onto the skin surface if in vitro percutaneous permeation was carried in vertical modified Franz diffusion cells. In case where the horizontal Side-Bi-Side diffusion cells were used, the donor compartment entrance was covered with parafilm® membrane (Menasha, USA), and the transdermal patch was clamped between the donor compartment and skin surface. This ensures that the exposed surface area of the skin samples were similar between the two devices. An aliquot of receiver medium (300 μL) was collected every 30 min from the receptor chamber for 4 h, followed by the replenishment of the same volume of fresh, preheated receiver medium after each sampling interval (17,22). Three replicates were performed for each diffusion experiment. The collected samples were directly injected to an UPLC system for drug analysis.
Cumulative amounts (Q, Eq. 1) of drug permeated in microgram per square centimeter were plotted against time. The cumulative penetration rate (F) of ginsenoside Rb1 was calculated following Eq. 2. The permeation characteristics of Rb1 may be modeled by zero-order kinetics equation, first-order kinetics equation, Higuchi equation, or Weibull equation (23). The ginsenoside collagen transdermal patch percutaneous permeation in vitro would fit to the model that has the highest fitting correlation coefficients (r) among four kinetic equations.
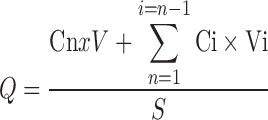 |
1 |
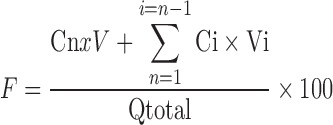 |
2 |
Q is the cumulative amounts of ginsenoside Rb1 permeated across the skin in time t = n; Cn is the ginsenoside Rb1 concentration permeated in the receptor chamber in time t = n; V is the volume of receptor chamber (6 mL); Ci is the ginsenoside Rb1 concentration permeated in the receptor chamber in time t = n − 1; Vi is the sample volume every time (300 μL); S is the exposed surface area of the skin for the permeation of the transdermal patch (0.67 cm2); F is the cumulative rate of ginsenoside Rb1 permeated in the receptor chamber; and Qtotal is the total content of ginsenoside Rb1 in the ginsenoside collagen transdermal patch sample.
Ginsenoside Rb1 Retained in Skin Studies
The amount of ginsenoside Rb1 retained in the skin samples was determined by UPLC analysis. The study was performed according to the method of Daniela Monti et al. (24). Briefly, at the end of the in vitro permeation experiments, the skin samples were removed from the diffusion cells, rinsed with PBS solution to remove excess formulation residue from the skin surface, and gently wiped dry with cotton wool tampons. Each skin sample was then treated with 2-mL PBS solution and was homogenized for 5 min. The mixture was shaken with 20 μL H2SO4 (2 M) and 4 mL n-butanol for 30 m. After centrifugation (16,400 rpm, 4°C, 10 min), the supernatant was transferred into another vial and was evaporated on a rotary evaporator until dry. PBS was used to dissolve the analyte for UPLC analysis after appropriate dilution. For validation of the extraction procedure (25), a slice of blank pig skin was submitted to the assay and the retention time of endogenous compounds in was compared with that of Rb1 in order to verify that there were no interferences in analyzing the molecule. Moreover, a known aliquot of Rb1 was added to blank skin, and the extraction recovery was determined by computing the ratio of the amount of Rb1 extracted from the skin to the amount added. The percentage of recovery was 95.92 ± 2.91% (mean ± standard deviation (SD)).
In Vitro Release Studies
Drug release studies from ginsenoside Rb1 collagen transdermal patch were performed by a simple and reproducible method which has been developed to measure in vitro release of the drug from a delivery vehicle using a diffusion cell and synthetic membrane (26). This methodology employs a Franz vertical diffusion cells, a commercially available cellulose acetate synthetic membrane, and an aqueous receptor phase (27). To determine in vitro drug release, a known amount of ginsenoside collagen transdermal patch was applied on the donor chamber. Aliquots of the receptor media were removed after specific time intervals and analyzed, and the cumulative rate of drug released (expressed by %; F) was plotted against time. The procedure was similar to the procedure used to measure in vitro permeation of Rb1 through pig skin flaps. The only difference is that instead of skin samples, 0.2-μm Whatman® cellulose membranes were used. Before the experiment, the membranes were soaked in PBS for 1 h. Each experiment was repeated three times. The percentage of ginsenoside Rb1 released in the receptor medium was calculated with Eq. 2.
Statistical Analysis
Data were expressed as the mean ± SD of at least three experiments. Analysis of variance was carried out (SPSS 13.0) followed by Student’s t test. A value of P < 0.05 was considered statistically significant.
RESULT AND DISCUSSION
Ginsenoside Rb1 Decreases Melanin Content Through Inhibition of Tyrosinase Activity
Figure 2a shows the effect of ginsenoside Rb1 on the viability of B16 melanoma cell. Rb1 does not inhibit the growth of B16 cells at the dosage between 15.63 and 500 μM, while Rb1 at higher concentrations (1,000 μM) showed significant (P < 0.001) cytotoxicity toward B16 cells, reducing the cell viability to 4.67%.
Fig. 2.
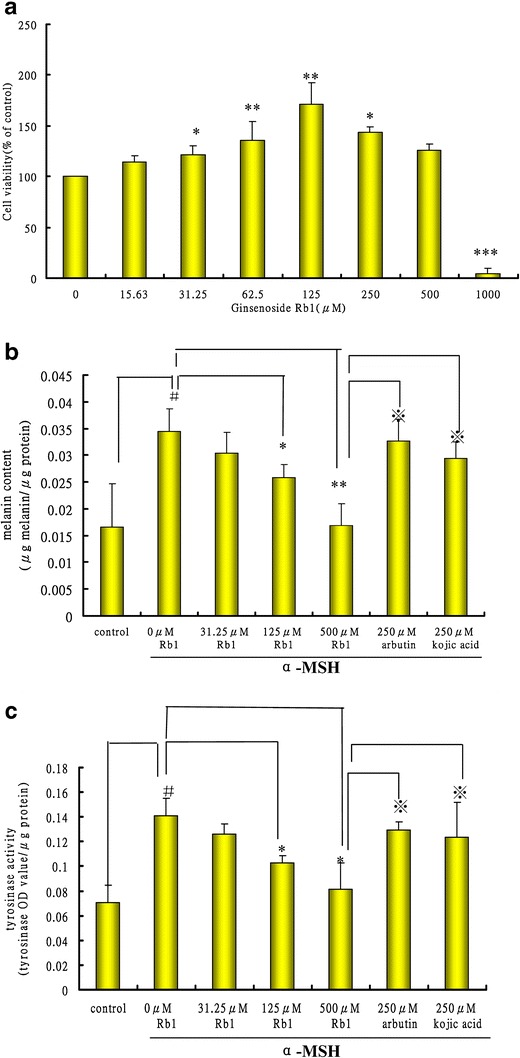
Effects of ginsenoside Rb1 on cell viability, melanin production, and tyrosinase activity in B16 cells. a Viability of B16 cells after treatment with ginsenoside Rb1. The cells were seeded in 96-well plates for 24 h and then treated with various dosages of ginsenoside Rb1 (15.63–1000 uM) for 48 h. The cell viability was then examined by the MTT assay. b Effect of ginsenoside Rb1 on cellular melanin synthesis. c Effect of ginsenoside Rb1 on tyrosinase activity. The cells were exposed to α-MSH (10 nM) in the presence or absence of ginsenoside Rb1. B16 cells with and without α-MSH treatment (absence of ginsenoside Rb1) were used as two negative controls. The melanin content, protein content, and tyrosinase activity of each reaction were determined by a spectrometric method as described in the “Materials and Methods” section. Data were expressed as the mean ± SD of three independent experiments carried out in triplicate. Statistically significant difference *P < 0.05, **P < 0.01, ***P < 0.001 compared with 0-uM Rb1 group stimulated byα-MSH; # P < 0.05 compared with control group withoutα-MSH treatment; ※ P < 0.05 compared with arbutin group or kojic acid group
During the initial search of prospective cosmetic compounds, it is important to consider the agent’s possible cytotoxicity to melanocytes. According to the cell viability studies, Rb1 is nontoxic to melanocytes within the range of concentrations used in subsequent assays. Figure 2b shows the inhibitory effect of Rb1 on melanogenesis in B16 melanoma cells. α-MSH is a hormone that stimulates melanin production in cells. In our study, the B16 cells treated with α-MSH but no Rb1 produced significantly more melanin than the B16 control cells sample without Rb1. The results also show that Rb1 significantly inhibited the melanogenesis-stimulating effect of α-MSH in a dose-dependent manner. The addition of 31.25 μM ginsenoside Rb1 showed a comparable degree of inhibition of melanogenesis compared to 250 μM arbutin or 250 μM kojic acid. We also examined α-MSH-induced tyrosinase activity, a key enzyme catalyzing the initial steps in the biosynthetic pathway of melanin pigments (28). B16 cells showed a markedly increased level of tyrosinase activity upon exposure to α-MSH alone (Fig. 2c). Rb1 also suppressed tyrosinase activity in B16 cells in a dose-dependent manner and was more effective than arbutin or kojic acid in higher doses (>125 uM). The result suggests that Rb1 at a noncytotoxic concentration has a strong inhibitory effect on melanogenesis in melanoma cells through downregulation of tyrosinase activity. Currently available positive skin-whitening agents such as arbutin or kojic acid only exhibited comparably weaker inhibitory activity. The result implies that ginsenoside Rb1 could be a promising candidate as an effective skin-whitening agent due to its potent inhibition of melanogenesis at a low, nontoxic concentration.
The Relationship between Skin Permeability and Solubility of Ginsenoside Rb1
Table I shows some molecular characteristics of Rb1. The molecular weight of Rb1 is 1,109.29 g/mol. Bos JD et al. (29) and Riviere J. E. et al. (30) found that the molar mass and the molecular size of a molecule was related to its diffusion coefficient and melting point and therefore was related to its solubility. Optimal permeability was observed in compounds with low molar mass, ideally less than 500 g/mol. Therefore, the large molecular size of ginsenoside Rb1 would adversely affect the rate of percutaneous delivery into skin.
The solubility of the drug, which depends on the chemical structure itself and the vehicle, is important in determining the rate of delivery into the skin. As shown in Table I, Rb1 had low solubility in water (15.32 ± 0.911 mg/mL) and moderately solubility in n-octanol (25.88 ± 1.007 mg/mL). Partition is the term applied to the distribution of a substance between two adjacent but different phases at equilibrium. The partition coefficient Po/w of a drug between n-octanol and water is used in pharmaceutics (31). For practical purposes, the logarithm of the partition coefficient (log Po/w), a measurement of how well a substance partitions between in lipid and water, determines the route of drug penetration through the skin. It was reported that molecules which exhibit intermediate partition coefficients (log Po/w values between 1 and 3) are sufficiently soluble in the lipid domains of the stratum corneum to permit diffusion across those domains while still being sufficiently hydrophilic to be soluble in the formulation and to allow partitioning into the viable tissue of the dermis. Since ginsenoside Rb1’s log Po/w (0.228) is not in the specified scope (1~3), it is expected that the Rb1’s cutaneous permeation would be slow and difficult. Therefore, a viable topical Rb1 delivery system should include certain penetration enhancers to increase Rb1’s absorption through skin.
Characteristics of Ginsenoside Collagen Transdermal Patch
The prepared ginsenoside collagen transdermal patch was slightly white in color with mint-aromatic odor. The pH of all the seven transdermal patch formulations was found in the range of 7.0–7.4. Each transdermal patch (10 g) contained 3.6 mg of ginsenoside Rb1.
In Vitro Percutaneous Permeation
Skin Permeation Studies
To optimize the ginsenoside collagen transdermal patch, seven formulas with different penetration enhancers were prepared (Table II). The percutaneous permeation of ginsenoside Rb1 from the ginsenoside collagen transdermal patch formulas 1–7 across pig skin was studied in the modified Franz vertical diffusion cell, which has become a popular method to assess in vitro release of drug from semisolid dosage forms (32,33). Figure 3a showed the overall cumulative amounts of ginsenoside Rb1 from formulas 1–7 permeated across pig skin within 4 h. All results were expressed as means ± standard deviations. The results revealed that formula 5 (0.4% azone + 0.2% menthol), formula 6 (0.4% azone + 0.4% menthol), and formula 3 (0.4% azone) exhibited significantly enhanced percutaneous drug delivery of ginsenoside Rb1. Among all the formulas, formula 5 had the highest enhancing effect on the permeation of ginsenoside Rb1 through the pig skin. When 0.2% menthol or 0.4% menthol were used alone as an enhancer in the formula, there were little improvement in the permeation of ginsenoside Rb1. However, when a combination of 0.4% azone plus 0.2% menthol were used as enhancers (formula 5), the percutaneous permeation of ginsenoside Rb1 was significantly higher. The finding suggested that the optimal penetration enhancement of Rb1 may be achieved through a certain combination of azone and menthol. Formula 6 showed that the addition of menthol beyond 0.2% (with 0.4% azone) did not further increase Rb1 penetration. In all seven formulas, less than 10% of the Rb1 penetrated across the skin from the patch in 4 h, which indicated that the rate of Rb1 penetration was very slow despite the use of enhancers.
Fig. 3.
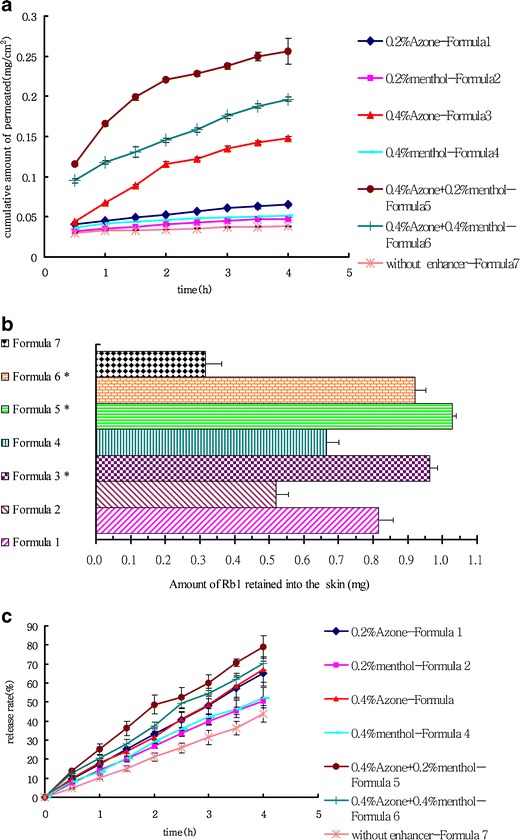
Permeation profile of ginsenoside Rb1 in ginsenoside collagen transdermal patchs with or without different penetration enhancers through pig skin or Whatman® cellulose membranes and the storage ginsenoside Rb1 within pig skin at the end of permeation experiment. a Permeation profiles through pig skin of ginsenoside Rb1 from formulas 1–7. b The amount of ginsenoside Rb1 retained into pig skin after application of formulas 1–7 for 4 h. c Permeation profile of ginsenoside Rb1 in formulas 1–7 across Whatman® cellulose membranes. Data were expressed as the mean ± SD of three independent experiments. Statistically significant difference at *P < 0.05 compared to the control group (formula 7)
In order to develop a transdermal drug delivery system of ginsenoside collagen transdermal patch, it is necessary to establish its permeation profile. The characterization of permeation profiles by a model function was attempted using different kinetic models (zero order, first order, Higuchi square root, and Weibull model) which were derived by Korsmeyer et al. (34), wherein Mt/Mα is the fractional permeation of drug, Mt is the amount permeated at time t, Mα is the total amount of drug contained in the ginsenoside crystal collagen transdermal patch, t is time, K is the kinetic constant, and n is the diffusional release exponent indicative of the operating release mechanism.
Zero-order equation, first-order equation, Higuchi equation, and Weibull equation of formulas 1–7 were shown in Table III. In each formula, the linear relative coefficient (r2) of Higuchi equation was higher than that of other three equations. Therefore, the rate of ginsenoside Rb1 permeated from formulas 1–7 transdermal patches across skin in vitro can best be described by Higuchi equation.
Table III.
Pharmacokinetics Equations of Each Formula
| Formulations | Zero-order equation | First-order equation | Higuchi equation | Weibull equation | |
|---|---|---|---|---|---|
| Formula 1 | 0.2% azone | Q = 0.0071t + 0.0384; r 2 = 0.9872 | Ln (1 − Q) = −0.0075t − 0.0391; r 2 = 0.9876 | Q = 0.0195t 1/2 − 0.0262; r 2 = 0.9906 | Ln Ln [1 / (1 − Q)] = 0.2384 Ln t − 3.0472; r 2 = 09724 |
| Formula 2 | 0.2% menthol |
Q = 0.0044 t + 0.0311 r 2 = 0.9785 |
Ln (1 − Q) = −0.0046t − 0.0316; r 2 = 0.9791 | Q = 0.0123t 1/2 − 0.0233; r 2 = 0.9890 | Ln Ln [1 / (1 − Q)] = 0.1972 Ln t − 3.3032; r 2 = 0.9708 |
| Formula 3 | 0.4% Azone | Q = 0.0298t + 0.041; r 2 = 0.9397 | Ln (1 − Q) = −0.00331t − 0.0405; r 2 = 0.9458 | Q = 0.0838t 1/2 − 0.0129; r 2 = 0.9872 | Ln Ln [1 / (1 − Q)] = 0.63 Ln t − 2.637; r 2 = 0.9823 |
| Formula 4 | 0.4% menthol | Q = 0.0039t + 0.037; r 2 = 0.9391 | Ln (1 − Q) = −0.0041t − 0.0377; r 2 = 0.9403 | Q = 0.0111t 1/2 − 0.0299; r 2 = 0.9962 | Ln Ln [1 / (1 − Q)] = 0.1646 Ln t − 3.1668; r 2 = 0.9856 |
| Formula 5 | 0.4% azone + 0.2% menthol | Q = 0.0362t + 0.1278; r 2 = 0.8714 | Ln (1 − Q) = −0.045t − 0.1349; r 2 = 0.8868 | Q = 0.1039t 1/2 − 0.0594; r 2 = 0.9671 | Ln Ln [1 / (1 − Q)] = 0.4118 Ln t − 1.7373; r 2 = 0.9596 |
| Formula 6 | 0.4% azone + 0.4% menthol | Q = 0.0288t + 0.0862; r 2 = 0.9900 | Ln (1 − Q) = −0.0338t − 0.0882; r 2 = 0.9934 | Q = 0.079t 1/2 − 0.0365; r 2 = 0.9936 | Ln Ln [1 / (1 − Q)] = 0.3802 Ln t − 2.0766; r 2 = 0.9854 |
| Formula 7 | Without enhancer | Q = 0.0022t + 0.0301; r 2 = 0.9483 | Ln (1 − Q) = −0.0023t − 0.0305; r 2 = 0.9487 | Q = 0.0061t 1/2 + 0.0262; r 2 = 0.9675 | Ln Ln [1 / (1 − Q)] = 0.1139 Ln t − 3.4083; r 2 = 0.9573 |
Fick’s law of diffusion (35,36), which is widely used to describe the properties of drug percutaneous permeation, was applied to evaluate the drug diffusion mechanism. The steady-state skin flux (J) (μg/cm2/h) was determined as the slope obtained by the linear regression of cumulative amounts of drugs (Q) against the time (h) based on J = (dQ/dt)/S; where S is a diffusion area of skin tissue (cm2) for Rb1 permeation, and dQ is the amount of permeated Rb1 through the skin in the time of dt.
The permeability coefficient (Pm) (cm/h) was calculated as Eq. 3.
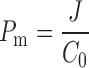 |
3 |
where C0 is the initial concentration of ginsenoside Rb1 in the transdermal patch.
On the other hand, the enhancement ratio (ER) was calculated by Eq. 4.
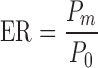 |
4 |
where P0 is the permeability coefficient of ginsenoside Rb1 from ginsenoside collagen transdermal patch without penetration enhancers (formula 7).
Using different permeation parameters of ginsenoside Rb1 from formulas 1–7 across pig abdominal skin, the flux, permeability coefficient, and enhancement ratio were calculated from the permeation data and the values were shown in Table IV.
Table IV.
Permeation Parameters of Ginsenoside Rb1 from 1–7 Formula Across Pig Skin
| Formulations | J × 10−3 μg/(cm2/h) | P m × 10−3 cm/h | ER | |
|---|---|---|---|---|
| Formula 1 | 0.2% azone | 19.5 ± 1.3 | 3.37 ± 0.21 | 3.210 ± 0.234 |
| Formula 2 | 0.2% menthol | 12.3 ± 1.1 | 2.12 ± 0.15 | 2.019 ± 0.157 |
| Formula 3 | 0.4% azone | 83.8 ± 5.4 | 1.92 ± 0.11 | 1.828 ± 0.174 |
| Formula 4 | 0.4% menthol | 11.1 ± 0.9 | 1.92 ± 0.32 | 1.828 ± 0.143 |
| Formula 5 | 0.4% azone + 0.2% menthol | 103.9 ± 87.4* | 17.95 ± 1.51 | 17.095 ± 1.347* |
| Formula 6 | 0.4% azone + 0.4% menthol | 79.0 ± 6.3 | 13.65 ± 1.24 | 13.000 ± 1.132* |
| Formula 7 | Without enhancer | 6.1 ± 0.7 | 1.05 ± 0.09 | 1 |
J flux, P m permeability coefficient, ER enhancement ratio
*Statistically significant difference at P < 0.05, compared to the control group (formula 7)
**Statistically significant difference at P < 0.01, compared to the control group (formula 7)
Formula 5 (0.4% azone + 0.2% menthol) demonstrated the highest steady state flux (J) with (0.1039 ± 0.0874) (μg/cm2/h), while formula 7 (without enhancers) had the lowest steady state flux (J) with (0.0061 ± 0.0007) (μg/cm2/h). Formula 3 (0.4% azone) had the second highest steady state flux (J) after formula 5. Moreover, it can be clearly seen from Table IV that formula 5 (0.4% azone + 0.2% menthol) has highest enhancement activity (ER = 17.095).
Drug retention Within the Skin
The amount of ginsenoside Rb1 retained in skin after application of the patch was shown in Fig. 3b and Table V. As evidenced from the histogram in Fig. 3b, the amount of Rb1 accumulated was highest (0.92 ± 0.032 mg) in the skin mounted with formula 5 (0.4% azone + 0.2% menthol). Furthermore, 69.12% of ginsenoside Rb1 from formula 5 remained within pig skin, which was the highest of all seven formulas.
Table V.
The cumulative rate of ginsenoside Rb1 retained into skin after 4 hours in vitro permeation experiments
| Formula number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Cumulative rate in skin (%) | 49.54 ± 2.57 | 31.83 ± 2.06 | 61.20* ±126 | 40.28 ± 2.17 | 69.12* ±0.43 | 60.59* ±1.99 | 19.70 ± 2.73 |
* Statistical significant difference at p < 0.05 compared to the control Formula 7
When azone and menthol were mixed in the appropriate ratio (2/1 v/w) as the penetration enhancers in a ginsenoside collagen transdermal patch, the amount of ginsenoside Rb1 retained into the skin from transdermal patch increased markedly. Some literatures (37–39) reported that azone formed microcavities within the lipid bilayers and increased the free-volume fraction; it could even penetrate into and mix with the lipids. The effect was demonstrated in the 1990s using different analytical methods. Menthol acted by modifying the solvent nature of the stratum corneum, in turn modifying the driving force for diffusion, and improved drug partitioning (40). In addition, menthol as a volatile substance may extract some of the lipid fraction from the stratum corneum and improve drug flux through skin. Therefore, the incorporation of azone and menthol in the ginsenoside collagen transdermal patch could enhance the ginsenoside Rb1 permeation that could store more in epidermis and dermis within 4 h.
In Vitro Release Studies
When the ginsenoside Rb1 collagen transdermal patch is put on the face, the active agent must be released from mask before it can contact the epidermal surface, permeate through the stratum corneum, and remain in the inner layers of skin. The release profiles for ginsenoside Rb1 from all seven formula s were shown in Fig. 3c.
In 4 h, ginsenoside collagen transdermal patch formulas 1 to 7 released 65.06, 50.37, 67.02, 52.27, 79.01, 70.26, and 43.69% of ginsenoside Rb1, respectively. The release profiles of ginsenoside Rb1 from transdermal patch were fitted into zero-order kinetic model. The data of the drug released to the skin and permeated across skin can be described by Higuchi equation. On the other hand, the data of the drug released to the synthetic membrane and permeated across the membrane were fitted into zero-order kinetic model. The two different models may indicate the different permeation mechanism of real skin and the synthetic membrane. The linear correlation coefficients (r) of formulas 1–7 were 0.9996, 0.9989, 0.9987, 0.9974, 0.9932, 0.9973, and 0.9991, respectively. The release experiments showed that Rb1 was released from transdermal patch at a constant rate across all seven formulas. In 4 h, the released quantity of Rb1 was different from each other and from the formulas, but all were much higher than that of the permeation through the skin from the parallel formula. Therefore, the released Rb1 from the patch may stay in the skin for interaction to the melanocyte cells.
CONCLUSIONS
Based on the criteria of attaining the maximum value of Q, skin retention, flux, enhancer ratio, and the composition of mask with ginsenoside Rb1 concentration of 0.036% (w/w), PCHP 2.0% (w/w), MC 0.50% (w/w), PEG6000 0.50% (w/w), azone 0.40% (v/w), menthol 0.20% (w/w), and water 96.364% was found to fulfill the requisite of an optimum formulation.
The present study clearly demonstrated for the release profile of ginsenoside Rb1 from the ginsenoside collagen transdermal patch and its pharmacokinetic profile of permeation through skin. We have not found such research in other transdermal patch products on the market.
In order to promote ginsenoside collagen transdermal patch in market, further preclinical studies are recommended to study the safety of the mask on human skin, such as skin sensitization study by Freund’s complete adjuvant (FCA) and skin irritation study by using white rabbits.
Acknowledgments
This work was supported by Jiangsu Overseas Research and Training Program for University Prominent Young and Middle-aged Teachers and Presidents sponsored by Jiangsu Provincial Department of Education, and also by Hong Kong Baptist University FRG2/08-09/089. The authors also would like to greatly thank Mulin Yang for his proof reading and English correction and Dr. Yu Hua and Yuen Tsz-kin from the Center for Cancer and Inflammation Research of Hong Kong Baptist University for their patient technical guidance in B16 cell culture test.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Martinez-Esparza M, Jimenez-Cervantes C, Solano F, Lozano JA, Garcia-Borron JC. Mechanisms of melanogenesis inhibition by tumor necrosis factor-alpha in B16/F10 mouse melanoma cells. Eur J Biochem. 1998;255(1):139–46. doi: 10.1046/j.1432-1327.1998.2550139.x. [DOI] [PubMed] [Google Scholar]
- 2.Leyden JJ, Shergill B, Micali G, Downie J, Wallo W. Natural options for the management of hyperpigmentation. J Eur Acad Dermatol Venereol. 2011;25(10):1140–5. doi: 10.1111/j.1468-3083.2011.04130.x. [DOI] [PubMed] [Google Scholar]
- 3.Callender VD, St Surin-Lord S, Davis EC, Maclin M. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12(2):87–99. doi: 10.2165/11536930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Cai BX, Jin SL, Luo D, Lin XF, Gao J. Ginsenoside Rb1 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Biol Pharm Bull. 2009;32(5):837–41. doi: 10.1248/bpb.32.837. [DOI] [PubMed] [Google Scholar]
- 5.Kang TH, Park HM, Kim YB, Kim H, Kim N, Do JH, et al. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123(3):446–51. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Jung E, Lee J, Huh S, Kim J, Park M, et al. Panax ginseng induces human type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. 2007;109(1):29–34. doi: 10.1016/j.jep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Hoi-Hin K, Ying-Kit YP, Nai-Ki M, Ngok-Shun WR. Ginsenoside Rb1 induces type I collagen expression through peroxisome proliferator-activated receptor-delta. Biochem Pharmacol. 2012;84:532–9. doi: 10.1016/j.bcp.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Longo C, Galimberti M, De Pace B, Pellacani G, Bencini PL. Laser skin rejuvenation: epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Laser Med Sci. 2013;28(3):769–76. doi: 10.1007/s10103-012-1145-9. [DOI] [PubMed] [Google Scholar]
- 9.Mizuta S, Nishizawa M, Sekiguchi F, Matsuo K, Yokoyama Y, Yoshinaka R. Enzymatic solubilization of collagen in the skin of diamond squid Thysanoteuthis rhombus: application of a fungal acid protease. Fish Sci. 2013;79(5):841–8. doi: 10.1007/s12562-013-0658-x. [DOI] [Google Scholar]
- 10.Han M, Sha X, Wu Y, Fang X. Oral absorption of ginsenoside Rb1 using in vitro and in vivo models. Planta Med. 2006;72(5):398–404. doi: 10.1055/s-2005-916211. [DOI] [PubMed] [Google Scholar]
- 11.Moghimi HR, Williams AC, Barry BW. A lamellar matrix model for stratum corneum intercellular lipids. 5. Effects of terpene penetration enhancers on the structure and thermal behaviour of the matrix. Int J Pharm. 1997;146(1):41–54. doi: 10.1016/S0378-5173(96)04766-7. [DOI] [Google Scholar]
- 12.Chang TS, Chen CT. Inhibitory effect of homochlorcyclizine on melanogenesis in alpha-melanocyte stimulating hormone-stimulated mouse B16 melanoma cells. Arch Pharm Res. 2012;35(1):119–27. doi: 10.1007/s12272-012-0113-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Jang JY, Park C, Kim BW, Choi YH, Choi BT. Curcumin suppresses alpha-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int J Mol Med. 2010;26(1):101–6. doi: 10.3892/ijmm_00000427. [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Chou GX, Wang H, Chu JH, Yu ZL. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18(1):32–5. doi: 10.1016/j.phymed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Yun CY, Kim D, Lee WH, Park YM, Lee SH, Na M, et al. Torilin from torilis japonica inhibits melanin production in alpha-melanocyte stimulating hormone-activated B16 melanoma cells. Planta Med. 2009;75(14):1505–8. doi: 10.1055/s-0029-1185803. [DOI] [PubMed] [Google Scholar]
- 16.Hunt G, Todd C, Cresswell JE, Thody AJ. Alpha-melanocyte-stimulating hormone and its analog Nle(4)Dphe(7)alpha-Msh affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–11. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Chen T, Liu XS, Chen Y, Wang LH. Penetration effect of ostrich oil as a promising vehicle on transdermal delivery of sinomenine. J Oleo Sci. 2013;62(9):657–64. doi: 10.5650/jos.62.657. [DOI] [PubMed] [Google Scholar]
- 18.TangJun W-b W. A rapid method for the simultaneous determination of five saponins in Xueshuaotong injection with ultra performance liquid chromatography. Chin J Pharm Anal. 2008;28(1):97–9. [Google Scholar]
- 19.Yao-xuan X, Ya-zhu L. UPLC determination of ginsenoside Rg1, ginsenoside Rb1and notoginsenoside R1 in Panax Notoginseng. J Guangdong Pharmaceutical Univ. 2011;27(5):489–92. [Google Scholar]
- 20.Yang Z, Gao S, Wang JR, Yin TJ, Teng Y, Wu BJ, et al. Enhancement of oral bioavailability of 20(S)-ginsenoside Rh2 through improved understanding of its absorption and efflux mechanisms. Drug Metab Dispos. 2011;39(10):1866–72. doi: 10.1124/dmd.111.040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JH, Liu M, Jin HJ, Deng LD, Xing JF, Dong AJ. In vitro enhancement of lactate esters on the percutaneous penetration of drugs with different lipophilicity. AAPS PharmSciTech. 2010;11(2):894–903. doi: 10.1208/s12249-010-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Gu XC. In vitro percutaneous permeation of the repellent DEET and the sunscreen oxybenzone across human skin. J Pharm Pharm Sci. 2007;10(1):17–25. [PubMed] [Google Scholar]
- 23.Yao-Dong Y. Design and development of sustained-released preparation and controlled-released preparation (Huan shi kong shi zhi ji de she ji yu kai fa) Beijing: China Medical Science and Technology Press; 2006. [Google Scholar]
- 24.Monti D, Tampucci S, Chetoni P, Burgalassi S, Saino V, Centini M, et al. Permeation and distribution of ferulic acid and its alpha-cyclodextrin complex from different formulations in hairless rat skin. Aaps Pharmscitech. 2011;12(2):514–20. doi: 10.1208/s12249-011-9609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahl J, Braun M, Siebert J, Kietzmann M. The percutaneous permeation of a combination of 0.1% octenidine dihydrochloride and 2% 2-phenoxyethanol (octenisept (R)) through skin of different species in vitro. BMC Vet Res. 2011;7. [DOI] [PMC free article] [PubMed]
- 26.Fares H, Zatz J. Measurement of drug release from topical gels using two types of apparatus. J Pharm Technol. 1995;1:52–6. [Google Scholar]
- 27.Bunge AL. Release rates from topical formulations containing drugs in suspension. J Control Release. 1998;52(1–2):141–8. doi: 10.1016/S0168-3659(97)00211-3. [DOI] [PubMed] [Google Scholar]
- 28.Schweikardt T, Olivares C, Solano F, Jaenicke E, Garcia-Borron JC, Decker H. A three-dimensional model of mammalian tyrosinase active site accounting for loss of function mutations. Pigment Cell Res. 2007;20(5):394–401. doi: 10.1111/j.1600-0749.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- 29.Bos JD, Meinardi MMHM. The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 30.Riviere JE, Brooks JD. Predicting skin permeability from complex chemical mixtures: dependency of quantitative structure permeation relationships on biology of skin model used. Toxicol Sci. 2011;119(1):224–32. doi: 10.1093/toxsci/kfq317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster M, Bolzinger MA, Fessi H, Briancon S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur J Dermatol. 2009;19(4):309–23. doi: 10.1684/ejd.2009.0676. [DOI] [PubMed] [Google Scholar]
- 32.Sanghvi P, Collins C. Comparison of diffusion studies of hydrocortisone between the Franz cell and the enhancer cell. Drug Dev Ind Pharm. 1993;19(13):1573–85. doi: 10.3109/03639049309069327. [DOI] [Google Scholar]
- 33.Corbo M, Schultz T, Wong G, Buskirk GV. Development and validation of in vitro release testing methods for semisolid formulations. Pharm Technol. 1993;17(9):112–28. [Google Scholar]
- 34.Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N. Mechanism of solute release from porous hydro-matrices and other factors may be responsible. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 35.Crank J. The mathematics of diffusion. London: Oxford University Press; 1979. [Google Scholar]
- 36.Ling Z, Zi-ping Y, Li Z, Li C, Yu-jing F. Effects of different penetration enhancers on transdermal behavior of L-carnitine in vitro. Chin J New Drugs. 2012;21(5):559–62. [Google Scholar]
- 37.Cornwell PA, Barry BW, Bouwstra JA, Gooris GS. Modes of action of terpene penetration enhancers in human skin differential scanning calorimetry, small-angle X-ray diffraction and enhancer uptake studies. Int J Pharm. 1996;127(1):9–26. doi: 10.1016/0378-5173(95)04108-7. [DOI] [Google Scholar]
- 38.Ongpipattanakul B, Burnette R, Potts R, Francoeur M. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm Res. 1991;8:350–4. doi: 10.1023/A:1015845632280. [DOI] [PubMed] [Google Scholar]
- 39.Bouwstra J, Peschier L, Brussee J, Bodde H. Effect of nalkyl-azocycloheptan-2-ones including azone on the thermal behaviour of human stratum corneum. Int J Pharm. 1989;52:47–54. doi: 10.1016/0378-5173(89)90087-2. [DOI] [Google Scholar]
- 40.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–18. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]


