Abstract
In this study, we have evaluated the interactions of zein microspheres with different class of drugs (hydrophobic, hydrophilic, and amphiphilic) using in vitro and in silico analysis. Zein microspheres loaded with aceclofenac, metformin, and promethazine has been developed by solvent evaporation technique and analyzed for its compatibility. The physical characterization depicted the proper encapsulation of hydrophobic drug in the microspheres. The in vitro release study revealed the sustaining ability of the microspheres in the following order: hydrophobic > hydrophilic > amphiphilic. In silico analysis also confirmed the better binding affinity and greater interactions of hydrophobic drug with zein. The above results revealed that zein is more suitable for hydrophobic drugs in the development of sustained drug delivery systems using solvent evaporation technique. The study therefore envisages a scope for identifying the most suitable polymer for a sustained drug delivery system in accordance with the nature of the drug.
KEY WORDS: hydrophilic drugs, hydrophobic drugs, in silico analysis, protein-drug interactions, solvent evaporation, zein microspheres
INTRODUCTION
In the recent years, plant proteins have been emerged as a preferred polymeric carrier system for the controlled drug delivery applications. They are less expensive than animal proteins and have the capacity to entrap various molecules either by adsorption or through covalent linkage. Zein is a water-insoluble plant protein that predominantly present in the endosperm of corn kernels. It is “generally regarded as safe” and food-grade ingredient by FDA. The hydrophobic nature of zein provides good mechanical properties, high thermal resistance, and prevents the contents from atmospheric moisture. Hence, it has been used as an edible coating for foods and pharmaceuticals. Zein has been successfully employed as a promising carrier for various drugs and proteins to achieve sustained or controlled release (1–5). Zein has an equal amount of hydrophilic and lipophilic amino acid composition (6). When the amino acid chain folds, it forms a ribbon-like structure with hydrophobic front and back and hydrophilic edges. The ribbon-like tertiary structure of zein has geometry of 17 nm length, 4.5 nm width, and 1.2 nm thickness with sharply defined hydrophobic and hydrophilic domains (7). This structure allows zein to function as a polymeric amphiphile, and the brick-like shape has a potential to entrap various molecules inside them.
Controlled drug delivery systems, in particular microsphere preparations are based on three different basic techniques—solvent evaporation, phase separation, and spray drying. Phase separation and spray drying has its own disadvantages such as uncontrollable particle size distribution and inability to encapsulate highly thermosensitive compounds (8). In the case of solvent evaporation, it does not require elevated temperature or some phase separation-inducing agents (9). Moreover, proper selection of materials and conditions would result in the development of particles with desired size range and optimum encapsulation efficiency. Selection of suitable polymer for the development of sustained drug delivery system using different class of drug is a great challenge.
Wang et al. (10) investigated the effect of various hydrophilic and lipophilic compounds on zein aggregation and self-assembly. This study revealed that lipophilic compounds increased the curvature of zein and thereby resulting in the development of smaller spherical particles. On the other hand, hydrophilic compounds decreased the curvature and leads to the formation of smooth films. Zein with amphiphilic compounds resulted in the formation of sponges with interconnected channels. This illustrated the behavior and structural development of zein in the presence of various HLB systems. However, there have been no studies with reference to the screening of different class of drug through zein microspheres.
Subhashini et al. (11) applied molecular dynamics simulation to study the drug uptake by different polymers such as alginate, eudragit, etc. Samina et al. (12) studied the encapsulation of prednisolone, paracetamol, and isoniazid in poly (l-lactic acid) microspheres using molecular dynamics technique. Therefore, predictive computational tools can be employed to facilitate the selection of suitable polymer for a particular lead moiety based on its interaction energy. In silico techniques such as molecular modeling, molecular docking, and molecular dynamic (MD) simulation has wide potential to assist in achieving better design and development of controlled release systems.
In this study, we have attempted to develop hydrophilic, hydrophobic, and amphiphilic drug-loaded zein microspheres using solvent evaporation technique and evaluate its encapsulation and sustaining ability. Aceclofenac, metformin, and promethazine were used as hydrophobic, hydrophilic, and amphiphilic drugs, respectively. Further, we would like to establish the interaction between these compounds using protein-drug interaction with the help of in silico analysis. The three-dimensional structure of zein protein was predicted and grid-based ligand docking with energetics (Glide) module was used for the docking analysis to understand the interactions between zein and the three different drugs.
MATERIALS AND METHODS
Zein was purchased from Sigma Life Sciences (Sigma-Aldrich, St. Louis, USA). Aceclofenac was procured as a gift sample from Ind-swift Ltd. Chandigarh, India. Metformin and promethazine were kindly obtained as a gift sample from the Industrial Chemistry department of CSIR-Central Leather Research Institute, and all other reagents were of reagent grade.
Preparation of Zein Microspheres
The individual drug-loaded zein microspheres were prepared with different drug-polymer ratios (1:5, 1:3, and 1:2) by emulsification and solvent evaporation method as described earlier (13). First, the polymer solution was prepared using 90% ethanol, and, to this, drugs were added separately under stirring. The homogenous drug-polymer solution was then added dropwise to the continuous phase (sesame oil) containing 0.5% span 80 as an emulsifying agent. The emulsification of the above mixture was facilitated using a homogenizer with a rotating speed of 400 rpm. Then, 1 ml of toluene saturated with glutaraldehyde was added as a cross-linking agent and the stirring was continued for 1 h. The dispersed drug and zein were immediately transformed into fine droplets and the solidification of these microparticles was induced by increasing the temperature of the medium. The developed particles were separated by filtration, washed with petroleum ether to remove the oil phase, and desiccated under vacuum for 24 h.
Physical Characterization
The surface morphology of the Zein microspheres was analyzed using a scanning electron microscope (SEM, Hitachi). The drug-loaded microspheres were vacuum dried, mounted onto brass stubs using adhesive tapes, and sputter coated with gold in argon atmosphere to render the particles electrically conductive. Then, the images were taken at the excitation voltage of 5–10 kV. Fourier-transformed infrared spectrum of all drugs, zein, and Acl/Met/Pmt-Zn-Ms were obtained by potassium bromide pellet method using ABB instrument. The pellets were prepared by mixing individual components with KBr and compressed into a pellet by applying pressure (1 t/unit). Spectral scanning was done in the range of 4,000–400 cm−1. Differential scanning calorimetry of drugs, polymer, and Acl/Met/Pmt-Zn-Ms were obtained using TA instruments, Q-200. The samples were placed in aluminum pans and hermetically sealed before the analysis. The thermograms were recorded over a temperature range of 25–250°C by heating the samples at a constant rate of 10°C/min. An inert atmosphere was maintained by purging nitrogen gas at a rate of 20 ml/min.
Pharmaceutical Characterization
Drug Loading and Encapsulation Efficiency
The amount of aceclofenac, metformin, and promethazine entrapped within the microspheres was determined by dissolving the microspheres in 90% ethanol and analyzed using an UV spectrophotometer (Perkin-Elmer, USA) at 273, 232, and 249 nm, respectively. Drug loading and encapsulation efficiency was determined using the following equations:
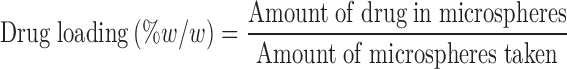 |
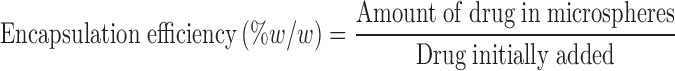 |
In Vitro Release Studies from Zein Microspheres
The in vitro release studies were performed by adopting the procedure followed by Trivedi et al. (14). The dissolution analysis was carried out on the microspheres at 37°C and 100 rpm.
A specified quantity of Acl/Met/Pmt-Zn-Ms (100 mg) was suspended in 100 ml of dissolution medium consisting of 0.1 N hydrochloric acid (pH 1.2, simulated gastric fluid) and the dissolution was done for 2 h. At the end of the second hour, disodium hydrogen phosphate and potassium dihydrogen phosphate were added to make the pH 6.8 (simulated intestinal fluid); the dissolution was continued for 4 h. At the end of the above said period, the pH of the medium was changed to 7.4 by adding disodium hydrogen phosphate and sodium chloride and the release study was continued. One milliliter of medium was withdrawn at specified time intervals and the same volume of fresh PBS was replaced to maintain the sink conditions. The withdrawn samples were diluted suitably and estimated using UV spectrophotometer for aceclofenac, metformin, and promethazine at 273, 232, and 249 nm, respectively.
In Silico Analysis
Zein-3D Structure Prediction
The three-dimensional structure of the zein protein was predicted using an online server named “Bhageerath”, an energy-based protein structure prediction server (15). The amino acid sequence of zein (CAA32512.1) was obtained from NCBI database and submitted to the online server for modeling. The web-based tool comprises eight modules configured to function independently or in a conduit.
Preparation of Target Protein
The Glide (16,17) technique was used for protein preparation, grid generation, and ligand docking in this study. The modeled three-dimensional structure of the zein protein was refined using “protein preparation wizard” in the Glide, Maestro 9.2 (Schrödinger, LLC, New York, USA, 2011). Water molecules were removed, bond orders and formal charges were added for hetero groups, and hydrogen atoms were also added to all atoms in the receptor structure. The side chain of amino acids that is not close to the binding site cavity and those does not participate in the salt bridges was neutralized. After completing protein preparation, the receptor structure was properly refined to optimize the hydrogen bonding network using OPLS_2005 force field. Finally, minimization of protein was performed until the average root mean square deviation of the nonhydrogen atoms reached 0.30 Å.
Ligand Preparation
The structure of drug molecules aceclofenac (ChemSpider ID: 64809), promethazine (ChemSpider ID: 4758), and metformin (ChemSpider ID: 3949) were obtained from the ChemSpider database (http://www.chemspider.com/). These retrieved ligand molecules were properly cleaned and prepared by using ligPrep module in Maestro 9.2 (Schrödinger, LLC). The cleanup and optimization process include conversion of structure from 2D to3D, addition of hydrogen atoms, removal of counter ions, ionization state at the pH7.0, generation of stereoisomer, removal of noncompliant structure, and energy minimization. The OPLS_2005 force filed were used for minimization, which produces the low energy conformers of the ligands. Ionizer was used to generate ionized state of all compounds at target pH 7.0 ± 2.0. This prepared low-energy conformers of the ligand were taken as the input for docking analysis.
Active Site Prediction
Binding pocket information of the target protein was predicted by CASTp (http://sts.bioengr.uic.edu/castp/calculation.php) in order to locate the binding site amino acids; it also gives an interactive visualization of the computed pocket (Fig. 1a). The cavity identification number is given in arbitrary manner and they sort according from the largest to smallest in a row. CASTp, mainly analyze Connolly’s surface area and volume of the cavities. One of best active site was selected based on the maximum surface area (894.8) and volume of the cavity (1855.9). Amino acids that are participated in the selected cavity were also identified (Fig. 1b). The grid for docking analysis was generated using these amino acids information in the active site cavity. Seventy-five percent of active site residues were found to be hydrophobic. This confirmed the hydrophobic nature of the predicted active site cavity. It was also experimentally proved that the zein proteins comprised of more number of hydrophobic moieties in its structure (18). Therefore, the identified active site cavity was authenticated and found to be reliable for further docking analysis.
Fig. 1.
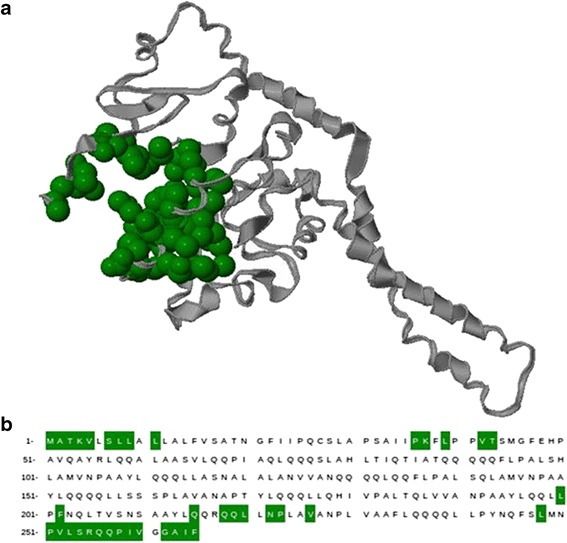
a The 3D structure of zein protein with the identified active site cavity was shown. The protein and active site cavity were shown in ribbon (gray) and cartoon (green) representation, respectively. b Amino acid residues encompass in the active site cavity were shown in green
Docking Analysis
Docking consists of five steps—protein preparation, ligand preparation, receptor grid generation, docking procedure, and, finally, viewing the docking results using the pose-viewer. Glide (Schrödinger, LLC) module from Maestro 9.2 was used for the docking analysis after ensuring the suitability of the target and ligand using protein preparation wizard and Ligprep. Ligand molecules were docked into the active site of the receptor, by generating grid around the cavity. The receptor grid file was generated using grid-receptor generation program. The ligands were docked with the grid using the Glide standard precision (SP) mode. The final evaluation of ligand-protein binding was done with Glide score.
RESULTS
In the present study, Acl/Met/Pmt-Zn-Ms were prepared with various drug-polymer ratios. The scanning electron micrographs (SEM) of blank, aceclofenac-, metformin-, and promethazine-loaded microspheres were shown in Fig. 2a–d, respectively. In Fig. 2c, the metformin crystallized on the surface of the microspheres were seen whereas in aceclofenac-loaded zein microspheres, there were no such phenomena. The surface of the promethazine-loaded microspheres appeared to be porous in nature. The average particle size of Acl-Zn-Ms, Met-Zn-Ms, and Pmt-Zn-Ms was found to be 136 ± 25.92, 94 ± 18.36, and 68 ± 30.28 μm, respectively. Fourier transform infrared (FTIR) spectrum of zein shows characteristic peaks around 1,645, 1,541, and 1,236 cm−1 corresponding to amide I, II, and III, respectively. FTIR spectrum of aceclofenac (Fig. 3b) showed characteristic C=O stretching at 1,772 and 3,318 cm−1, carboxylic group absorption band at 1,717 cm−1, N–H stretching at 1,589 cm−1, O–H bending around 1,055 cm−1, and C=C stretching at 2,362 cm−1. The FTIR spectrum of Acl-Zn-Ms (Fig. 3e) was predominantly occupied with the characteristic polymer bands, and this confirmed the presence of required secondary interactions between the polymer and drug. FTIR spectrum of metformin (Fig. 3c) showed characteristic primary N–H stretching at 3,369 cm−1, secondary N–H stretching at 3,170 cm−1, and C–N stretching at 1,622 and 1,570 cm−1. FTIR spectrum of Met-Zn-Ms (Fig. 3f) showed characteristic peaks of both the drug and polymer. The FTIR spectrum of promethazine (Fig. 3d) showed characteristic C–H and N–H stretching at 2,920 and 2,380 cm−1, respectively, CH3 and CH2 bending at 1,450 cm−1, and C–N stretching of tertiary amine at 1,334 cm−1. The out plane C–H bending of aromatic rings was presented at 757 cm−1. An endothermic peak around 87°C was observed in the differential scanning calorimetry (DSC) thermogram of zein (Fig. 4a). The DSC thermogram (Fig. 4b) of aceclofenac showed an endothermic peak at 154°C and the Acl-Zn-Ms (Fig. 4e) showed a single endothermic peak corresponding to cross-linked zein at 107°C. The DSC thermogram (Fig. 4c) of metformin showed an endothermic peak at 230°C and the Met-Zn-Ms (Fig. 4f) showed two endothermic peaks at 83°C and 214°C corresponding to melting temperatures of zein and metformin, respectively. The DSC thermogram of promethazine (Fig. 4d) showed an endotherm at 226°C corresponding to its melting peak, a crystallization peak at 276°C, and a sharp endothermic peak at 288°C. The thermogram of Pmt-Zn-Ms (Fig. 4g) showed an endothermic peak at 174°C and 203°C, which is lesser than the melting peaks of the raw drug. Moreover, the endothermic peak of zein appeared as a small shoulder peak at 89°C. The drug loading and encapsulation efficiency of zein microspheres were given in Table I. The drug loading of aceclofenac, promethazine, and metformin was found to be 6.85 ± 0.56, 6.08 ± 0.72, and 5.68 ± 0.08, respectively. The in vitro release pattern of aceclofenac, metformin, and promethazine from the developed zein microspheres were depicted in Fig. 5a–c, respectively. It appeared biphasic and might be attributed to the permeability and hydrophobic nature of zein. The release profile of aceclofenac from zein microspheres showed that there is no burst release observed in the initial hours and in 0.1 N HCl, only 12% of drug was released from the microspheres. The zein microspheres prolonged the release of aceclofenac to almost 72 h. The release profile of metformin from zein microspheres revealed that in the initial 2 h at 0.1 N HCl, a burst release of drug (32%) was observed. At pH 6.8 and 7.4, the drug continued to get released and at the end of 8 h almost 90% of drug was released from the zein microspheres. At simulated gastric pH (1.2), we found a burst release of 67% of promethazine and at the end of 2 h almost 70% of drug got released in to the medium. At the end of 6 h in pH 6.8, nearly 83% of drug was released and after 8 h of study in simulated intestinal fluid almost 89% of drug was released from the delivery systems.
Fig. 2.
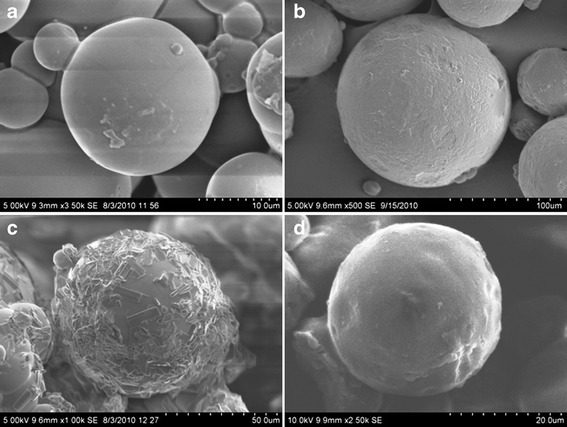
Scanning electron micrographs of zein microspheres loaded with a blank, b aceclofenac, c metformin, and d promethazine
Fig. 3.
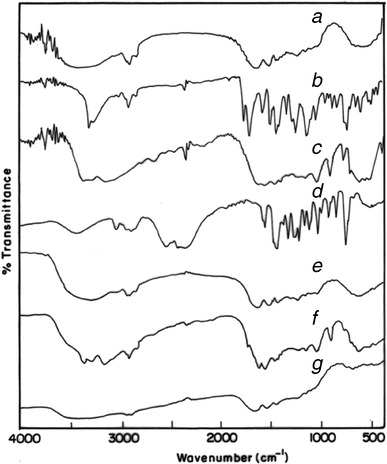
FT-IR spectrum of a zein, b aceclofenac, c metformin, d promethazine; e, f, and g were aceclofenac, metformin, and promethazine-loaded zein microspheres, respectively
Fig. 4.
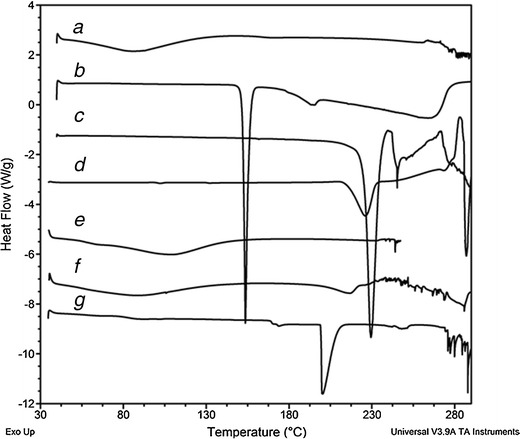
DSC thermograms of a zein, b aceclofenac, c metformin, d promethazine; e, f, and g were aceclofenac, metformin, and promethazine-loaded zein microspheres, respectively
Table I.
Drug Loading and Encapsulation Efficiency in Zein Microspheres
| Drug | Drug/polymer | Drug loading (% w/w)a | Encapsulation efficiency (% w/w)b |
|---|---|---|---|
| Aceclofenac sodium | 1:5 (100:500) | 4.79 ± 0.45 | 21.54 ± 2.06 |
| 1:3 (100:300) | 4.38 ± 0.66 | 11.82 ± 1.77 | |
| 1:2 (250:500) | 6.85 ± 0.56 | 20.53 ± 1.68 | |
| Metformin hydrochloride | 1:5 | 5.56 ± 0.16 | 27.8 ± 0.64 |
| 1:3 | 5.69 ± 042 | 17.07 ± 1.36 | |
| 1:2 | 5.68 ± 0.08 | 14.26 ± 2.94 | |
| Promethazine hydrochloride | 1:5 | 5.86 ± 0.34 | 23.44 ± 0.92 |
| 1:3 | 5.94 ± 0.26 | 16.63 ± 1.36 | |
| 1:2 | 6.08 ± 0.72 | 19.54 ± 3.60 |
aDrug loading (% w/w) = amount of drug in microspheres/amount of microspheres taken
bEncapsulation efficiency (% w/w) = amount of drug in microspheres/drug initially added
Fig. 5.
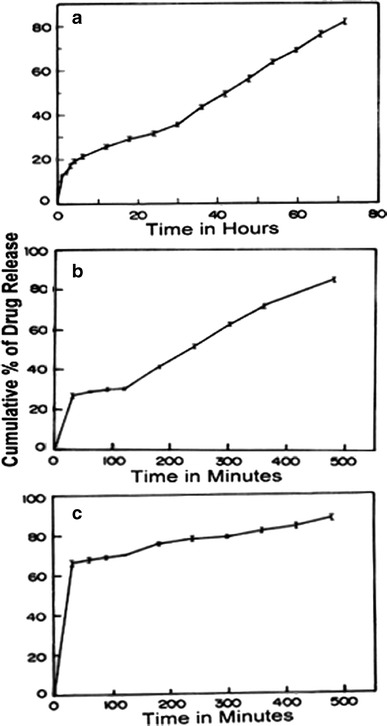
In vitro release profiles from a aceclofenac, b metformin, and c promethazine-loaded zein microspheres (data were the mean ± SD of three measurements)
In Silico Analysis
The Glide score of the docking study was found to be −5.358 kcal/mol for aceclofenac, −4.596 kcal/mol for promethazine, and −3.113 kcal/mol for metformin. The docking results showed that among the three drug molecules, aceclofenac has greater binding affinity compared to other two drugs. The three-dimensional structure of these docking complexes was shown in Fig. 6 along with corresponding 2D image. The different type of interaction between the drugs and the receptor protein was given in the Table II. Aceclofenac drug molecule showed a strong hydrogen bond interaction with Asn222 and Lys37 amino acids of target protein (Fig. 6a). Promethazine showed good binding affinity with receptor protein using hydrophobic and polar interactions only (Fig. 6b). In the case of metformin (Fig. 6c), the drug molecule showed multiple interactions with the target protein using hydrogen, hydrophobic, and electrostatic interaction.
Fig. 6.
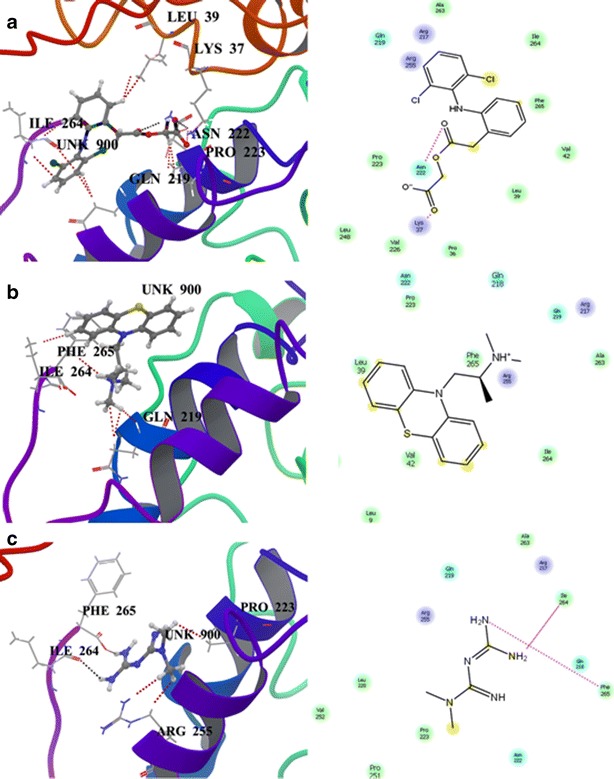
3D structure of docking complexes between zein and a aceclofenac, b promethazine, and c metformin along with corresponding 2D image
Table II.
Interaction Analysis of Drug with the Receptor Protein-Zein
| Type of interaction | Aceclofenac sodium | Promethazine HCl | Metformin HCl |
|---|---|---|---|
| Glide score | −5.358 | −4.596 | −3.113 |
| Hydrophobic | 3 | 2 | 1 |
| Hydrogen bonding | 2 | Nil | 2 |
| Polar | 1 | 1 | 1 |
| Electrostatic | 1 | Nil | Nil |
DISCUSSION
The suitability of the polymer, zein, in developing sustained release formulation using hydrophobic, hydrophilic, and amphiphilic drugs using solvent evaporation method was studied using in vitro and in silico analysis. The hydrophilic drug metformin has a poor miscibility with zein in ethanol, and, hence, during rapid solvent evaporation, the drug crystallized on the surface of the microspheres (19). The hydrophobic drug aceclofenac, having good compatibility with zein in ethanol, resulted in the development of microspheres with smooth surface. This indicated the proper encapsulation of drug in the polymer matrix. In the promethazine-loaded zein microspheres, the outer layer of the spheres was not looking smooth and there were some uneven surfaces which may be due to the deposition of drug on its surface. Further, the presence of drug on the surface may be confirmed using FTIR and DSC analysis. The FTIR studies confirmed the strong secondary interactions between aceclofenac and zein and poor secondary interactions between the metformin and zein. The strong secondary interactions ensured the sustainable release of the drug and increase the compatibility among the components in the developed microspheres (20). In case of Pmt-Zn-Ms, the IR spectrum (Fig. 3g) was predominantly occupied by the characteristic peaks of zein with no or minor contribution from promethazine. The findings confirmed the presence of secondary interactions between zein and promethazine and further revealed the presence of required compatibility among the two components. The absence of drug peak depicted the presence of aceclofenac in amorphous form and the uniform molecular dispersion of drug in the polymer matrix. The presence of drug endothermic peak in the thermogram of Met-Zn-Ms (Fig. 4f) indicated the crystalline nature of the substance in the formulation. The result is in line with the findings of the morphological analysis. This also revealed the improper encapsulation of the hydrophilic drug in the polymer matrix. The Pmt-Zn-Ms thermogram revealed that the drug is present in its crystalline form and, further, appearance of drug’s endothermic peak in the thermogram revealed the improper encapsulation of drug in the polymer matrix. The drug-loading studies depicted that the drug loading remains constant in the case of metformin and promethazine-loaded microspheres in spite of changing the concentration of drug. With respect to aceclofenac-loaded microspheres, the drug loading increases with increasing the concentration of the drug. This may be attributed to the uniform uptake of the drug by the polymer zein. In silico analysis also revealed that aceclofenac has more number of hydrophobic interactions with the zein compared to promethazine and metformin. Duncan et al. (21) stressed the importance of hydrophobic interactions in the uptake of a drug by the polymer. Our result is also in line with the findings. The sustained release of aceclofenac from the zein microspheres confirmed the proper encapsulation of hydrophobic drug in to the polymer matrix whereas in the case of metformin the release was faster than aceclofenac. The result obtained is in accordance with the findings of FTIR and DSC and further confirmed the poor inclusion of hydrophilic drug in the polymer matrix. The large quantity of drug release from the Pmt-Zn-Ms at the initial hours suggested that more amount of drug was present on the surface of the microspheres and only a small quantity got properly encapsulated in to it. The findings were in line with the results of electron microscopy and differential scanning calorimetry and further, confirmed the poor encapsulation of amphiphilic drug, promethazine in to zein microspheres using solvent evaporation method.
In Silico Analysis
Five different 3D structures for zein were obtained as the output of “Bhageerath” tool. The best one was selected and used as an input for further analysis. Docking analysis of these three drug compounds with the receptor zein was carried out to study the binding affinity and mode of molecular interaction among them. Ranking of ligand binding affinity was based on the Glide score, viz., lesser the value greater the binding affinity. The docking result also demonstrated that, during the process, all the drug molecules have been interacted and shared the same binding site and thus, it confirmed the predicted binding site was reliable. The resulting docking complexes of these drug molecules with receptor were further taken for the interaction analysis to study the interaction mode and stability.
Interaction Analysis of All Three Drug Compounds with the Receptor Protein
The carbonyl group of acetic acid and acetoxy moiety of aceclofenac formed a well-built hydrogen bond interaction with the NH group of Lys37 and Asn222, respectively. This drug molecule was also involved in other significant interactions such as hydrophobic, electrostatic, and polar interactions with the target. The CH group of acetic acid, phenyl, and dichlorophenyl moieties showed a strong hydrophobic interaction with carbon group of Pro223 and Leu39 and the CH group of Ile264, respectively. Electrostatic interaction was established between the carbonyl group of acetic acid and the NH group of Lys37. Polar interaction was formed between the CH group of dichlorophenyl and Gln219 of zein. These findings clearly indicated that aceclofenac has very strong hydrogen bonding and more number (three) of hydrophobic interactions with zein and, hence, has a good compatibility with it. The CH group of the phenothiazine ring and the NH group of the propanamine of promethazine showed a strong hydrophobic interaction with the CH group of Ile264 and Phe265, respectively. One of the methyl groups of dimethyl propanamine of this drug molecule showed a polar interaction with the CH group of Gln219 of the target protein. The absence of hydrogen bonding between this drug and polymer could be attributed to the poor inclusion and thus, larger quantity of drug was released in the first 2 h. Even though it has greater encapsulation efficiency and better secondary interactions, the absence of hydrogen bonding resulted in the poor sustainability of this drug from the microspheres. The amino groups of diaminomethylene of metfromin formed hydrogen bonds with carbonyl group of Ile264 and Phe265. Methyl groups of dimethylguanidine formed hydrophobic and electrostatic interactions with the carbonyl group of Pro223 and Arg255, respectively. Thus metformin has lesser hydrophobic interaction (one) than aceclofenac and had a better hydrogen bonding with zein when compared to promethazine. Hence, its binding was a little stronger, and sustainable release was achieved for a period of 8 h. This interaction analysis revealed that aceclofenac had the strongest interaction with zein protein compared to other drug molecules and ensured a stable formulation.
CONCLUSION
In conclusion, we found that aceclofenac had a better Glide score with stronger hydrophobic, electrostatic, and hydrogen bonding interactions thereby formed a stable complex with the receptor protein when compared to other drugs. This finding was further ascertained by physical and pharmaceutical characterization of the developed microspheres. Thus, even though zein is an amphiphilic polymer comprising both hydrophilic and lipophilic amino acid residues, it is more suitable for hydrophobic drugs in the development of sustained drug delivery systems using solvent evaporation technique. The study therefore envisages a scope for identifying the most suitable polymer for a sustained drug delivery system in accordance with the nature of the drug.
ACKNOWLEDGMENTS
The first author is extremely grateful to the Council of Scientific and Industrial Research, New Delhi, for the research fellowship. We are grateful to Dr A.B.. Mandal, Director, CSIR - Central Leather Research Institute for his encouragement and providing facilities to carry out this work. Vijayalakshmi is grateful to Pondicherry University, India, for the pre-doctoral fellowship and express her sincere gratitude to Centre for Excellence in Bioinformatics, Pondicherry University, funded by the Department of Information Technology (DIT) and the Department of Biotechnology (DBT).
REFERENCES
- 1.Hurtado L, Murdan S. Formulation and characterization of zein microspheres as delivery vehicles. J Drug Deliv Sci Technol. 2005;15:267–72. [Google Scholar]
- 2.Liu XM, Sun QS, Wang HJ, Zhang L, Wang JY. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2006;51:39–43. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Muthuselvi L, Aruna D. Simple coacervates of zein to encapsulate Gitoxin. Colloid Surf B. 2006;51:39–43. doi: 10.1016/j.colsurfb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang HJ, Lin ZX, Liu XM, Sheng SY, Wang JY. Heparin-loaded zein microspheres film and hemocompatibility. J Control Release. 2005;105:120–31. doi: 10.1016/j.jconrel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Hurtado L, Murdan S. Zein microspheres as drug/antigen carriers: a study of their degradation and erosion in the presence and absence of enzymes. J Microencapsul. 2006;23:303–14. doi: 10.1080/02652040500444149. [DOI] [PubMed] [Google Scholar]
- 6.Gianazza E, Viglienghia V, Giorgio PR, Salaminib F, Soave C. Amino acid composition of zein molecular components. Phytochemistry. 1977;16:315–7. doi: 10.1016/0031-9422(77)80054-X. [DOI] [Google Scholar]
- 7.Matsushima N, Dannob G, Takezawac H, Izumic Y. Three-dimensional structure of maize α-zein proteins studied by small-angle X-ray scattering. Biochim Biophys Acta. 1997;1339:14–22. doi: 10.1016/S0167-4838(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 8.Johansen P, Merkle HP, Gander B. Technological considerations related to the up-scaling of protein microencapsulation by spray-drying. Eur J Pharm Biopharm. 2000;50:413–7. doi: 10.1016/S0939-6411(00)00123-5. [DOI] [PubMed] [Google Scholar]
- 9.Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J Control Release. 2005;102:313–32. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Yin L, Padua GW. Effect of hydrophilic and lipophilic compounds on zein microstructures. Food Biophys. 2008;3:174–81. doi: 10.1007/s11483-008-9080-9. [DOI] [Google Scholar]
- 11.Subashini M, Padma VD, Ganeshchandra SS, Mukesh D. Molecular dynamics simulation of drug uptake by polymer. J Mol Model. 2011;17:1141–7. doi: 10.1007/s00894-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 12.Samina A, Blair FJ, Simon PM, Andreas GS, Paul G, Durba S, et al. In silico modelling of drug-polymer interactions for pharmaceutical formulations. J R Soc Interface. 2010;7:423–33. doi: 10.1098/rsif.2009.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karthikeyan K, Rachita L, Rama R, Purna KS. Development and characterization of zein-based micro carrier system for sustained delivery of aceclofenac sodium. AAPS PharmSciTech. 2012;13:143–9. doi: 10.1208/s12249-011-9731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivedi P, Verma AML, Garud N. Preparation and characterization of aceclofenac microspheres. Asian J Pharm. 2008;2:110–5. doi: 10.4103/0973-8398.42498. [DOI] [Google Scholar]
- 15.Jayaram B, Bhushan K, Shenoy SR, Narang P, Bose S, Agrawal P, et al. Bhageerath: an energy based web enabled computer software suite for limiting the search space of tertiary structures of small globular proteins. Nucleic Acids Res. 2006;34:6195–204. doi: 10.1093/nar/gkl789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 17.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–96. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 18.Hu N-T, Mark AP, Gisela H, Joachim M, Irwin R. Primary structure of a genomic zein sequence of maize. EMBO J. 1982;1:1337–42. doi: 10.1002/j.1460-2075.1982.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng J, Xu X, Chen X, Liang Q, Bian XC, Yang L, et al. Biodegradable electrospun fibers for drug delivery. J Control Release. 2003;92:227–31. doi: 10.1016/S0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Mo X, He C, Wang H. Intermolecular interactions in electrospun collagen-chitosan complex nanofibers. Carbohydr Polym. 2008;72:410–8. doi: 10.1016/j.carbpol.2007.09.018. [DOI] [Google Scholar]
- 21.Duncan R. The drawing era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]


