Abstract
Coronary artery disease (CAD) has become the main cause of mortality worldwide. Lectin galactoside-binding soluble-2 (LGALS2) is involved in the cytokine lymphotoxin-α (LTA) cascade that may influence the progress of CAD. The aim of the present study was to assess the association between the LGALS2 3279C>T (rs7291467) polymorphism and CAD. A total of 562 cases and 572 controls were recruited to examine the association. A systematic meta-analysis was performed to evaluate the contribution of LGALS2 3279C>T polymorphism to the risk of CAD among 12,093 cases and 11,020 controls. There was no significant association found in the present case-control study. However, the meta-analysis showed that LGALS2 3279C>T played a protective role in CAD [P=0.008, odds ratio (OR), 0.90; 95% confidence interval (95% CI), 0.82–0.97] and particularly in the Asian population (P=0.006; OR, 0.82; 95% CI, 0.71–0.94). The present case-control study did not find a significant association between LGALS2 3279C>T and CAD in the Eastern Han Chinese population. However, the meta-analysis indicated that LGALS2 3279C>T played a protective role in CAD, suggesting an ethnic difference in the association of the locus with CAD.
Keywords: coronary artery disease, lectin galactoside-binding soluble-2 3279C>T, rs7291467, meta-analysis, polymorphism
Introduction
Severe coronary artery disease (CAD), caused by occlusive epicardial coronary artery stenosis, is one of the leading causes of mortality in the developed countries (1,2). The incidence of CAD in developing countries has increased quickly in recent years due to an increasing exposure to CAD risk factors, including diabetes, hypercholesterolemia, hypertension and smoking (3,4). CAD causes >2 million fatalities each year in China (5), resulting in extensive health problems and a burden to society.
CAD is a complex disease contributed to by environmental and genetic factors. The environmental factors may play important roles in the initiation and progression of CAD through the epigenetic modifications, including altered DNA methylation (6,7). Genetic factors are estimated to contribute to 30–60% of the CAD risk (8,9). Studies of twins and families have shown that the CAD fatalities are influenced by inherited factors (8,10). Inflammation plays an important role in the process of atherosclerosis (11,12), which is the fundamental pathological change of CAD. Recent genome-wide association studies have identified particular inflammation-related loci that are associated with CAD risk (13,14).
Lectin galactoside-binding soluble-2 (LGALS2) belongs to the galectins family that has a variety of intra- and extracellular functions. LGALS2 is located on 22q13.1, a risk locus of CAD (15,16). The LGALS2 product, galectin-2, is involved in the cytokine lymphotoxin-α (LTA) cascade that participates in multitudinous biological responses (17). Galectin-2 and LTA have been shown to be expressed in smooth muscle cells (SMCs) and macrophages in the intima of human atherosclerotic plaques, but not in quiescent or normal medial SMCs (16). Recently, one study in the Japanese population proved that the CC genotype of LGALS2 3279C>T caused high expression of galectin-2 and acted as an inhibitor of arteriogenesis in the murine hindlimb model (18). Another study in the Japanese population revealed an association between the LGALS2 polymorphism and the severity of coronary atherosclerosis through a pathological study of 1,503 consecutive autopsy cases (19). However, other associated studies could not replicate the findings in British, Germany, Japanese and Korean populations (20–23).
The inconsistent results in various studies may be due to the limited power and different ethnic background. Meta-analysis is often used to enhance the power by combining different studies and can draw a more comprehensive conclusion (24–29). In the present study, a meta-analysis was conducted to evaluate the contribution of LGALS2 3279C>T to the risk of CAD, in addition to a case-control study in the Eastern Han Chinese population.
Materials and methods
Sample collection
A total of 1,134 unrelated controls were recruited between May 2009 and April 2012 from Ningbo Lihuili Hospital, Ningbo Yinzhou Hospital and Second Affiliated Hospital of Zhejiang University (Zhejiang, China). All the subjects were examined by standardized coronary angiography. The results of the angiograms were independently judged by at least two physicians according to the Seldinger method (30). The patients with CAD (n=562; 400 males and 162 females; mean age, 61.96±9.49 years) should have ≥1 major coronary artery with ≥50% stenosis by the angiographic evidence (31), undergone coronary artery bypass surgery or have a history of prior angioplasty according to the classification standard (30). The remaining 572 subjects (320 males and 252 females; mean age, 58.80±9.49 years) with <50% stenosis were used as non-CAD controls (30). All the patients were Han Chinese, originating from the Zhejiang province in Eastern China, without congenital heart disease, cardiomyopathy, severe liver or kidney disease. The blood samples were collected at the same time and treated by the same investigators. The study was approved by the Ethical Committee of Ningbo Lihuili Hospital, Ningbo Yinzhou Hospital and Second Affiliated Hospital of Zhejiang University in Hangzhou. Informed written consent was obtained from all the subjects. The severity of coronary atherosclerosis was classified into one-, two- or three-vessel diseases according to the number of coronary vessels with significant stenosis (angiographic luminal stenosis ≥50%) (20).
Single-nucleotide polymorphism genotyping
Human genomic DNA was isolated from peripheral blood using a conventional phenol-chloroform extraction method. DNA was quantified using PicoGreen® double-stranded DNA quantification kit (Molecular Probes, Inc., Eugene, OR, USA). The polymerase chain reaction (PCR) primers were designed using Oligo® 6 program (Molecular Biology Insights Inc., Cascade, CO, USA), and the two specific primers were 5′-GCG GGCAGGGCGGCGCCCTGCGCACACACACG-3′; and 5′-GATTACCGGCCCTGCGCACACACACA-3′ and the reverse primer was 5′-GGAGCCATCTCCTGATGCTTGGT-3′. PCR contained 2 μl genomic DNA, 0.2 μmol/l primers, 0.4 mmol/l dNTP, 1.5–2 μmol/l magnesium ions, 0.2X SYBR-Green I, 10% DMSO, 1X Roche Taq Gold buffer, 0.6 units ABI Taq Gold polymerase, and a volume of ddH2O that created a total reaction volume of 12 μl. The PCR conditions included an initial denaturation stage at 94°C for 15 sec, followed by 30 cycles at 94°C for 20 sec, 56°C for 30 sec, 72°C for 1 min and a final extension at 72°C for 3 min. PCR was performed on the GeneAmp® PCR system 9700 Dual 384-Well Sample Block Module (Applied Biosystems, Foster City, CA, USA). The genotyping experiments were performed using a melting temperature shift on the LightCycler® 480 (Roche Diagnostics, Basel, Switzerland). The PCR products were used for genotyping on the Roche LightCycler 480 real-time PCR instrument for melting curve detection. The melting curve program was 95°C for 5 sec, 60°C for 1 min, with an increase in temperature to 95°C at 0.11°C/sec, and the fluorescence signal was collected in the process and subsequently maintained at 40°C. The melting curve data, provided by the Roche onboard software, automatically depended on the fluorescence intensity.
Meta-analysis
All the studies were selected through a systematic search in online databases (PubMed, WanFang, WeiPu and CNKI) without time and language restrictions using the following keywords: ‘Coronary heart disease LGALS2 association’ and ‘coronary heart disease LGALS2 polymorphism’. The studies that met the following criteria were included in the meta-analyses: i) An original case-control study with an assessment of the association between LGALS2 3279C>T polymorphism and CAD risk in humans; ii) it contained sufficient information to infer the odds ratios (ORs) and 95% confidence intervals (95% CIs); and iii) genotype distribution of LGALS2 3279C>T in the controls met the Hardy-Weinberg equilibrium (HWE). For each involved case-control study, the following information was extracted or calculated: First author’s name, ethnicity, year of publication, numbers of cases and controls, HWE for controls, the result of the individual study regarding the association of LGALS2 3279C>T with CAD and the power analysis for each study.
Statistical analyses
HWE was analyzed using the Arlequin program (version 3.5) (32). The comparison of the genotype distribution was performed by the Pearson’s χ2 test. The genotype and allele frequency among three degrees of coronary artery stenosis with CAD was compared using Kruskal-Wallis test. The ORs with 95% CI were calculated by an online program (http://faculty.vassar.edu/lowry/odds2x2.html) in the case-control study and by Review Manager 5 (33) in the meta-analyses. Cochran’s Q statistic and I2 test (34) were used to calculate statistical heterogeneity to decide the type of analysis. The fixed-effect model was used for the studies with minimal to moderate heterogeneity (I2<50%) and the random-effect model was used for the studies with significant heterogeneity (I2≥50%). Funnel plots were drawn to observe the potential publication bias. The power analyses were estimated by the Power and Sample Size Calculation software (v3.0.43). A two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
Associations between LGALS2 3279C>T and CAD
As shown in Table I, all the genotyping distributions of LGALS2 3279C>T met HWE in controls. No significant association was found between LGALS2 3279C>T and CAD in all the subjects (P>0.05; Table I). Subsequently, the subjects were stratified into subgroups by age and gender. No significant results were found in all the subgroup analyses (Table II), and no positive results were observed under the dominant and recessive genetic models (Table III). There was no significant association between the degree of coronary artery stenosis and genotype in the CAD patients (Table IV).
Table I.
Genotype and allele distribution between LGALS2 3279C>T and CAD.
| Individuals | Group | Genotype (CC/CT/TT) | χ2 | P-value (df=2) | HWE | Allele (C/T) | χ2 | P-value (df=2) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All | CAD cases (n=562) | 337/198/27 | 0.76 | 872/252 | |||||
| Controls (n=572) | 335/209/28 | 0.23 | 0.89 | 0.53 | 879/265 | 0.18 | 0.67 | 0.96 (0.79–1.17) | |
| Male | CAD cases (n=400) | 242/138/20 | 0.95 | 622/178 | |||||
| Controls (n=320) | 193/110/17 | 0.04 | 0.98 | 0.80 | 496/144 | 0.01 | 0.91 | 0.99 (0.77–1.26) | |
| Female | CAD cases (n=162) | 95/60/7 | 0.52 | 250/74 | |||||
| Controls (n=252) | 142/99/11 | 0.22 | 0.90 | 0.22 | 383/121 | 0.15 | 0.70 | 0.94 (0.67–1.30) |
LGALS2, lectin galactoside-binding soluble-2; CAD, coronary artery disease; df, degrees of freedom; HWE, Hardy-Weinberg equilibrium; OR, odds ratio; 95% CI, 95% confidence interval.
Table II.
Post hoc analysis of LGALS2 3279C>T with the risk of CAD in various ages and gender.
| Age, years | Gender | Group | Genotype (CC/CT/TT) | χ2 | P-value (df=2) | HWE | Allele (C/T) | χ2 | P-value (df=1) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| ≤55 | Male | CAD case (n=103) | 65/32/6 | 0.45 | 162/44 | |||||
| Controls (n=137) | 86/44/7 | 0.07 | 0.96 | 0.66 | 216/58 | 0.003 | 0.96 | 1.01 (0.65–1.57) | ||
| Female | CAD case (n=32) | 16/15/1 | 0.25 | 47/17 | ||||||
| Controls (n=65) | 40/19/6 | 3.51 | 0.17 | 0.12 | 99/31 | 0.17 | 0.68 | 1.16 (0.58–2.29) | ||
| 55–65 | Male | CAD case (n=141) | 80/57/4 | 0.10 | 217/65 | |||||
| Controls (n=98) | 63/30/5 | 2.87 | 0.24 | 0.57 | 156/40 | 0.47 | 0.49 | 1.09 (0.64–1.86) | ||
| Female | CAD case (n=51) | 27/21/3 | 0.68 | 75/27 | ||||||
| Controls (n=117) | 62/52/3 | 1.18 | 0.55 | 0.04 | 176/58 | 0.11 | 0.74 | 1.09 (0.64–1.86) | ||
| ≥65 | Male | CAD case (n=156) | 97/49/10 | 0.27 | 243/69 | |||||
| Controls (n=85) | 44/36/5 | 2.91 | 0.23 | 0.50 | 124/46 | 1.48 | 0.22 | 0.76 (0.50–1.18) | ||
| Female | CAD case (n=79) | 52/24/3 | 0.91 | 128/30 | ||||||
| Controls (n=70) | 40/28/2 | 1.53 | 0.46 | 0.26 | 108/32 | 0.67 | 0.41 | 0.79 (0.45–1.38) |
LGALS2, lectin galactoside-binding soluble-2; CAD, coronary artery disease; df, degrees of freedom; HWE, Hardy-Weinberg equilibrium; OR, odds ratio; 95% CI, 95% confidence interval.
Table III.
Genetic test under the dominant and recessive models.
| Individuals | Group | Dominant (CC/CT+TT) | χ2 | P (df=2) | OR (95% CI) | Recessive (CC+CT/TT) | χ2 | P-value (df=2) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All | CAD cases (n=562) | 337/225 | 535/27 | ||||||
| Controls (n=572) | 335/237 | 0.23 | 0.63 | 0.94 (0.74–1.20) | 544/28 | 0.005 | 0.94 | 0.98 (0.57–1.69) | |
| Male | CAD cases (n=400) | 242/158 | 380/20 | ||||||
| Controls (n=320) | 193/127 | 0.002 | 0.96 | 0.99 (0.73–1.34) | 303/17 | 0.04 | 0.85 | 0.93 (0.48–1.82) | |
| Female | CAD cases (n=162) | 95/67 | 155/7 | ||||||
| Controls (n=252) | 142/110 | 0.21 | 0.65 | 0.91 (0.61–1.36) | 241/11 | 0.0005 | 0.98 | 0.99 (0.38–2.61) |
df, degrees of freedom; OR, odds ratio; 95% CI, 95% confidence interval; CAD, coronary artery disease.
Table IV.
Association of LGALS2 3279C>T and the severity of CAD.
| Model | Artery lesion, n | P-value (df=2) | ||
|---|---|---|---|---|
|
| ||||
| 1 | 2 | ≥3 | ||
| Dominant | ||||
| TT+TC | 90 | 58 | 77 | |
| CC | 155 | 81 | 101 | 0.36 |
| Recessive | ||||
| TC+CC | 237 | 130 | 168 | |
| TT | 8 | 9 | 10 | 0.31 |
LGALS2, lectin galactoside-binding soluble-2; CAD, coronary artery disease; df, degrees of freedom.
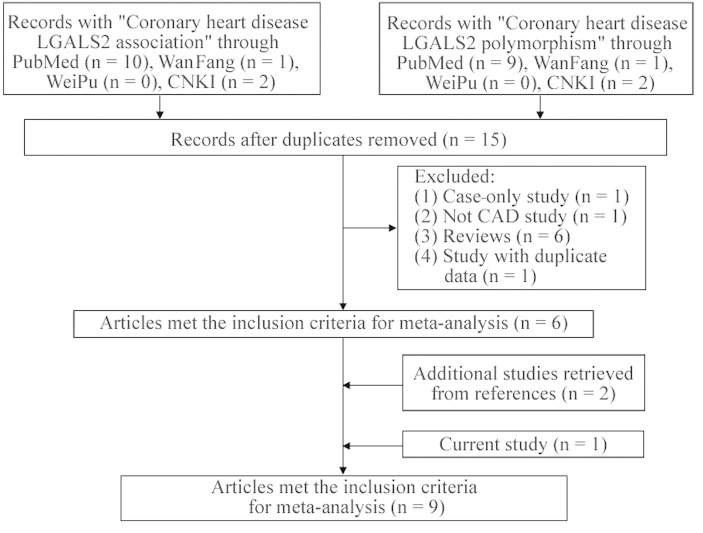
As shown in Fig. 1, following the removal of the duplicate studies, there were 15 potential studies collected from the PubMed, WanFang, WeiPu and CNKI databases. Excluded from these studies was one study that had no association data with CAD, one case-only study, six review studies and one with duplicate data. In addition, two studies were added from the references in the retrieved studies. There were nine studies (16,20–23,35–37 and the present study) included in the current meta-analysis among 12,093 CAD patients and 11,020 controls. The genotyping distribution met HWE in all the involved studies (Table V).
Figure 1.
Flowchart of selection process in the meta-analyses. LGALS2, lectin galactoside-binding soluble-2; CAD, coronary artery disease.
Table V.
Characteristics of the case-control studies in the current meta-analyses.
| First author | Ethnicity | Year | Cases/controls | HWE | Resulta | Power | (Refs.) |
|---|---|---|---|---|---|---|---|
| Ozaki | Asians | 2004 | 2302/2038 | Yes | S | 0.980 | (16) |
| Kimura | Asians | 2007 | 967/745 | Yes | NS | 0.684 | (20) |
| Sedlacek | Europeans | 2007 | 1801/2476 | Yes | NS | 0.982 | (23) |
| Koch | Europeans | 2007 | 3657/1211 | Yes | NS | 0.971 | (21) |
| Mangino | Europeans | 2007 | 746/698 | Yes | NS | 0.675 | (22) |
| Asselbergs | Europeans | 2007 | 1266/2541 | Yes | NS | 0.958 | (36) |
| Zhao | Asians | 2008 | 284/218 | Yes | S | 0.298 | (37) |
| Tian | Asians | 2011 | 508/521 | Yes | S | 0.483 | (38) |
| Present study | Asians | 2014 | 562/572 | Yes | NS | 0.430 |
Association between lectin galactoside-binding soluble-2 3279C>T and coronary artery disease;
HWE, Hardy-Weinberg equilibrium; NS, no significant; S, significant.
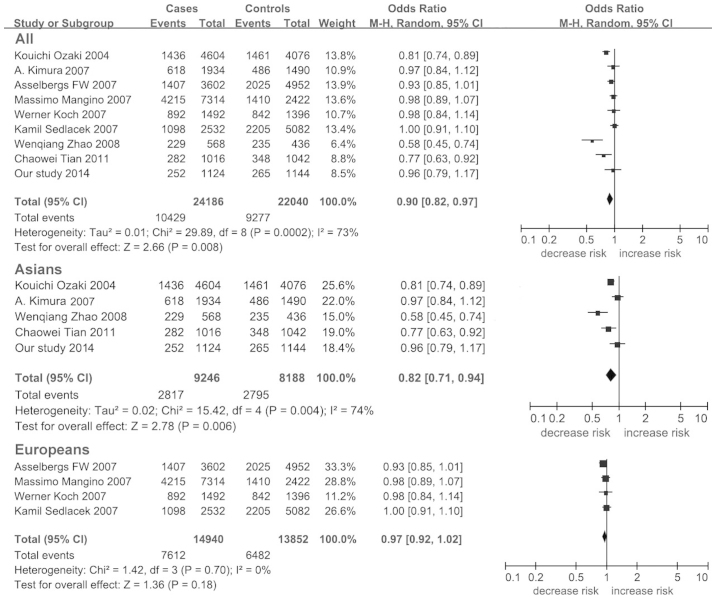
Significant heterogeneity was observed among the combined populations (I2=73%). The frequency of the LGALS2 3279C allele in Europeans (Hapmap-CEU) is 0.367, which is significantly lower compared to the Asian population (Hapmap-HCB=0.733, Hapmap-JPT=0.682). A further analysis showed an ethnic difference of LGALS2 3279C between the Asian and European populations (Fst=0.136). Therefore, a further subgroup meta-analyses was performed by ethnicity. Minimal heterogeneity was found in Europeans (I2=0%), in contrast to the high heterogeneity present in the Asian population (I2=74%, Table VI).
Table VI.
Meta-analyses of the LGALS2 3279C>T with CAD.
| Ethnicity | Stagesa | Cases/controls | OR (95% CI) | P-value | I2, % | Power |
|---|---|---|---|---|---|---|
| Overall | 9 | 12093/11020 | 0.90 (0.82–0.97) | 0.008b | 73.00 | 1.000 |
| Europeans | 4 | 7470/6926 | 0.97 (0.92–1.02) | 0.180 | 0.00 | 1.000 |
| Asians | 5 | 4623/4094 | 0.82 (0.71–0.94) | 0.006b | 74.00 | 1.000 |
Amount of stages.
P≤0.05.
LGALS2, lectin galactoside-binding soluble-2; CAD, coronary artery disease; OR, odds ratio; 95% CI, 95% confidence interval.
A significant association was found between LGALS2 3279C>T and CAD in allelic analysis for the combined populations (P=0.008; OR, 0.90; 95% CI, 0.82–0.97; Table VI; Fig. 2) and particularly in the Asian population (P=0.006; OR, 0.82; 95% CI, 0.71–0.94; Table VI; Fig. 2). The power analyses showed a strong power in all the meta-analyses (power>0.960). No publication bias was found in the present meta-analysis as the shape of the funnel plot was symmetrical (Fig. 3).
Figure 2.
Forest plots of lectin galactoside-binding soluble-2 3279C>T with coronary artery disease. 95% CI, 95% confidence interval.
Figure 3.
Funnel plot of lectin galactoside-binding soluble-2 3279C>T with coronary artery disease. OR, odd ratio; SE, standard error.
Discussion
As the fundamental cause of CAD, atherosclerosis is considered to be induced by chronic excessive inflammatory processes (38). LTA is a significant proinflammatory cytokine that has been shown to be associated with atherosclerotic lesion and CAD (16,39). LTA secretion was regulated by LGALS2 3279C>T polymorphism in vitro (16).
In the present study, there was no significant association found between the LGALS2 3279C>T polymorphism and the risk of CAD in the Han Chinese population. As genetic differences exist in different populations and as different populations are affected by environmental factors (40), the patients and controls were carefully selected so that each subject was independently evaluated by at least two experienced investigators according to the Seldinger method (30). The power of the current study was 0.430, which is significantly lower than Ozaki et al (16) (power=0.980). The moderate power in the sample of the present study may influence the result of the study.
As there were inconsistent results in the previous case-control studies regarding the association between LGALS2 3279C>T and CAD, meta-analyses were performed to summarize the overall contribution of this polymorphism. The present meta-analysis suggested that LGALS2 3279C>T played a protective role for CAD in the Asian population (P=0.006), but not in Europeans (P=0.180). A previous meta-analysis showed no association between LGALS2 3279C>T and CAD (41). The previous meta-analysis included a study on rheumatoid arthritis (42). In the present meta-analysis, this study was removed and three more independent studies were included. In addition, stricter criteria were performed in the literature selection and every involved study met the HWE. The power was >0.960 in the combined and subgroup meta-analyses. With the aforementioned advantages, the present meta-analysis may provide a more reliable and comprehensive conclusion.
There were certain limitations in the study that are noteworthy. Firstly, the power of the case-control study was too small (power=0.430), which may cause difficulty in confirming the observed results. Larger scale sample size studies are required in the future. Secondly, the meta-analysis only included the studies investigating European and Asian populations, and future studies in other populations may help to clarify whether there are ethnic differences in LGALS2 locus. Thirdly, there are 1,557 polymorphisms in LGALS2 and the present study only focused on one polymorphism, which may not completely represent the association between LGALS2 and CAD.
In conclusion, the present meta-analyses indicated that LGALS2 3279C>T is a protective factor of CAD, although the case-control study did not find a significant association between LGALS2 3279C>T and CAD in the Han Chinese population. As a significant heterogeneity of LGALS2 3279C exists in different populations, further studies with larger subjects and different populations are required to confirm the association between LGALS2 3279C>T and CAD.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 31100919 and 81371469), Natural Science Foundation of Zhejiang Province (grant nos. LR13H020003 and LY13H020008), K.C. Wong Magna Fund in Ningbo University, Advanced Key Scientific and Technological Programs of Ningbo (grant no. 2011C51001), Fund of Ningbo Science and Technology Innovation Team (grant no. 2011B82015), Natural Science Foundation of Ningbo City (grant no. 2011A610037), Ningbo Social Development Research Projects (grant no. 2012C50032), Ningbo Personnel Training Project (first level) and The Project of Ningbo Medicine and Science (grant no. 2009A02).
References
- 1.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 3.Foody J, Huo Y, Ji L, et al. Unique and varied contributions of traditional CVD risk factors: A systematic literature review of CAD risk factors in China. Clin Med Insights Cardiol. 2013;7:59–86. doi: 10.4137/CMC.S10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148:7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Ding H, Xu Y, Wang X, et al. 9p21 is a shared susceptibility locus strongly for coronary artery disease and weakly for ischemic stroke in Chinese Han population. Circ Cardiovasc Genet. 2009;2:338–346. doi: 10.1161/CIRCGENETICS.108.810226. [DOI] [PubMed] [Google Scholar]
- 6.Jiang D, Zheng D, Wang L, et al. Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PLoS One. 2013;8:e59752. doi: 10.1371/journal.pone.0059752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L. ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics. 2012;7:464–472. doi: 10.4161/epi.19633. [DOI] [PubMed] [Google Scholar]
- 8.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 9.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20,966 Swedish twins. J Intern Med. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 10.Murabito JM, Pencina MJ, Nam BH, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 12.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson R, Davies RW. Inflammation and coronary artery disease: insights from genetic studies. Can J Cardiol. 2012;28:662–666. doi: 10.1016/j.cjca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Duan S, Luo X, Dong C. Identification of susceptibility modules for coronary artery disease using a genome wide integrated network analysis. Gene. 2013;531:347–354. doi: 10.1016/j.gene.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 15.Iida A, Ozaki K, Tanaka T, Nakamura Y. Fine-scale SNP map of an 11-kb genomic region at 22q13.1 containing the galectin-1 gene. J Hum Genet. 2005;50:42–45. doi: 10.1007/s10038-004-0218-4. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki K, Inoue K, Sato H, et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature. 2004;429:72–75. doi: 10.1038/nature02502. [DOI] [PubMed] [Google Scholar]
- 17.Ginsburg GS, Shah SH, McCarthy JJ. Taking cardiovascular genetic association studies to the next level. J Am Coll Cardiol. 2007;50:930–932. doi: 10.1016/j.jacc.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 18.van der Laan AM, Schirmer SH, de Vries MR, et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur Heart J. 2012;33:1076–1084. doi: 10.1093/eurheartj/ehr220. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda S, Tanaka N, Arai T, Chida K, Muramatsu M, Sawabe M. Polymorphisms of LTA, LGALS2, and PSMA6 genes and coronary atherosclerosis: a pathological study of 1503 consecutive autopsy cases. Atherosclerosis. 2012;221:458–460. doi: 10.1016/j.atherosclerosis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Kimura A, Takahashi M, Choi BY, et al. Lack of association between LTA and LGALS2 polymorphisms and myocardial infarction in Japanese and Korean populations. Tissue Antigens. 2007;69:265–269. doi: 10.1111/j.1399-0039.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 21.Koch W, Hoppmann P, Michou E, et al. Association of variants in the BAT1-NFKBIL1-LTA genomic region with protection against myocardial infarction in Europeans. Hum Mol Genet. 2007;16:1821–1827. doi: 10.1093/hmg/ddm130. [DOI] [PubMed] [Google Scholar]
- 22.Mangino M, Braund P, Singh R, et al. LGALS2 functional variant rs7291467 is not associated with susceptibility to myocardial infarction in Caucasians. Atherosclerosis. 2007;194:112–115. doi: 10.1016/j.atherosclerosis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Sedlacek K, Neureuther K, Mueller JC, et al. Lymphotoxin-alpha and galectin-2 SNPs are not associated with myocardial infarction in two different German populations. J Mol Med (Berl) 2007;85:997–1004. doi: 10.1007/s00109-007-0211-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Yu X, Wang L, et al. Four genetic polymorphisms of lymphotoxin-alpha gene and cancer risk: a systematic review and meta-analysis. PLoS One. 2013;8:e82519. doi: 10.1371/journal.pone.0082519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Huang Y, Li C, Yang H, Lu C, Duan S. Positive association between lymphotoxin-alpha variation rs909253 and cancer risk: a meta-analysis based on 36 case-control studies. Tumour Biol. 2014;35:1973–1983. doi: 10.1007/s13277-013-1263-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Wang Y, Wang L, et al. Meta-analyses of 8 polymorphisms associated with the risk of the Alzheimer’s disease. PLoS One. 2013;8:e73129. doi: 10.1371/journal.pone.0073129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng P, Lian J, Huang RS, et al. Meta-analyses of KIF6 Trp719Arg in coronary heart disease and statin therapeutic effect. PLoS One. 2012;7:e50126. doi: 10.1371/journal.pone.0050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L, Wang L, Liao Q, et al. Genetic associations with diabetes: meta-analyses of 10 candidate polymorphisms. PLoS One. 2013;8:e70301. doi: 10.1371/journal.pone.0070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye H, Li X, Wang L, et al. Genetic associations with coronary heart disease: meta-analyses of 12 candidate genetic variants. Gene. 2013;531:71–77. doi: 10.1016/j.gene.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366:1407–1409. doi: 10.1016/S0140-6736(05)66878-X. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowski M, Szwed H, Kowalik I. Searching for the optimal strategy for the diagnosis of stable coronary artery disease. Cost-effectiveness of the new algorithm. Cardiol J. 2007;14:544–551. [PubMed] [Google Scholar]
- 32.Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online. 2007;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Kawalec P, Mikrut A, Wiśniewska N, Pilc A. The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2013;32:1415–1424. doi: 10.1007/s10067-013-2329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coory MD. Comment on: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39:932–933. doi: 10.1093/ije/dyp157. [DOI] [PubMed] [Google Scholar]
- 35.Asselbergs FW, Pai JK, Rexrode KM, Hunter DJ, Rimm EB. Effects of lymphotoxin-alpha gene and galectin-2 gene polymorphisms on inflammatory biomarkers, cellular adhesion molecules and risk of coronary heart disease. Clin Sci (Lond) 2007;112:291–298. doi: 10.1042/CS20060200. [DOI] [PubMed] [Google Scholar]
- 36.Zhao WQ, Wang J, Xie HZ, et al. Association between genetic variation of lymphotoxin-alpha/LGALS2 and risk of coronary arteriosclerosis disease and molecule makers. Chin J Clin Med. 2008;15:593–596. [Google Scholar]
- 37.Tian C. WanFang Date. Cardiovascular Sciences Guangzhou Medical University; 2011. The association between coronary artery disease and gene polymorphism in Chinese Han population; p. 95. [Google Scholar]
- 38.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 39.Laxton R, Pearce E, Kyriakou T, Ye S. Association of the lymphotoxin-alpha gene Thr26Asn polymorphism with severity of coronary atherosclerosis. Genes Immun. 2005;6:539–541. doi: 10.1038/sj.gene.6364236. [DOI] [PubMed] [Google Scholar]
- 40.Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361:865–872. doi: 10.1016/s0140-6736(03)12715-8. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Xu J, Wang X, et al. Lack of association between lymphotoxin-alpha, galectin-2 polymorphisms and coronary artery disease: a meta-analysis. Atherosclerosis. 2010;208:433–436. doi: 10.1016/j.atherosclerosis.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Panoulas VF, Nikas SN, Smith JP, et al. Lymphotoxin 252A>G polymorphism is common and associates with myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1550–1556. doi: 10.1136/ard.2007.082594. [DOI] [PubMed] [Google Scholar]