Abstract
The aim of the present study was to investigate the association between the homocysteine (Hcy) levels and polymorphisms of the CBS844ins68 and MTHFR C677T genes in essential hypertension (EH). The effects of the MTHFR C677T and CBS844ins68 haploid genotypes and the combined genotypes on EH and levels of Hcy were further explored. The polymorphisms of CBS844ins68 and MTHFR C677T genes in 200 EH and 200 normal tensive (NT) patients were detected using polymerase chain reaction-restriction fragment length polymorphism and analysis of the distribution of genotypes. An automated biochemical analyzer was used to measure the plasma Hcy levels and the clinical biochemistry data. The plasma Hcy levels in EH were significantly higher than those of the NT group (P<0.05). There were no significant differences (P>0.05) between males and females. Two genotypes, deletion/deletion (DD) and deletion/insertion (DI), of the CBS844ins68 polymorphism were found in two groups with no clear differences in two genotypes and allele frequency distribution (P>0.05). There were significant differences in the three genotype frequencies (χ2=6.658, χ2=4.410, P<0.05) for MTHFR C677T locus genotypes CC, CT and TT. The Hcy levels in genotypes DD and DI had no significant differences (P>0.05) and the CT and TT types were significantly higher compared to the CC genotype (P<0.05). The CC/DD combined genotype in the two groups was significantly different (P<0.05), and the odds ratio (OR), 0.569 showed that the CC/DD genotype may be a protective factor of hypertension. In the two groups, the Hcy levels for combined genotypes CC/DD, CT/DD, TT/DD and TT/DI were significantly different (P<0.05). The SHEsis software analysis linkage disequilibrium coefficient=0.216, indicates that there is probably a weak linkage for MTHFR C677T and CBS844ins68. Haplotype analysis suggested that the C-D haplotype was negatively correlated with EH (OR, 0.727) and that there was a positive correlation between T-D haplotype and EH (OR, 1.376). MTHFR C677T and CBS844ins68 polymorphisms were present in the populations studied and the CBS844ins68 homozygous mutation was not present. Therefore, there is a correlation between the polymorphisms of the MTHFR C677T gene and EH, and allele T may be one of the predisposing factors. MTHFR C677T and CBS844ins68 may exist with a certain linkage and the T-D haplotype may be a risk factor for EH.
Keywords: homocysteine, CBS844ins68, MTHFR C677T, polymorphisms
Introduction
Essential hypertension (EH) is the most common cardiovascular disease, and its incidence is increasing yearly with a trend apparent in younger patients. Previously, a large number of studies have shown that hyperhomocysteinemia (HHcy) is an independent risk factor of cardiovascular and cerebrovascular diseases, and increased hypertension and plasma homocysteine (Hcy) in causing these diseases have a synergistic effect (1).
Hcy is a body of sulfur-containing amino acids, and an important intermediate product in the metabolism of methionine and cysteine. There are four key metabolic enzymes in the metabolic pathways of Hcy, and CBS and MTHFR are the key common enzymes. The thermolabile MTHFR C677T gene mutation is the most common genetic factor to cause mild to moderate HHcy change. C/T mutation resulting in encoded alanine replaced by valine, decreased its activity and produced heat, which affected the most important metabolic pathways of Hcy-remethylation (2). Studies have shown that the T allele frequencies in different ethnicities and countries vary widely. The Guangxi Yao population rate was 22.6% (3) and in Australia it was 32.2% (4). The Yang et al (5) study of 15,357 Han patients found that the mutation frequency in Hainan was the lowest with a T allele of 24.0%, and in Shandong it was the highest (63.1%). This study regarding the association between the MTHFR C677T gene polymorphism and EH is not consistent. The study by Fowdar et al (4) of a population of Caucasian Australians showed that EH was not associated with the polymorphism of MTHFR C677T. Alghasham et al (6) studied an Arab population and found that the mutation frequency in groups of hypertension and obesity, and hypertension and diabetes were significantly higher compared to the healthy group (P<0.05), indicating that the T allele may be a risk factor for EH.
CBS844ins68 is the 8th exon insertion mutation, with a size of 68 base pairs (bp). The mutation causes CBS metabolic activity to decrease and affects the metabolism of Hcy. Previous studies have shown that the CBS844ins68 gene polymorphisms exist in different populations; the frequency of the insertion (I) allele was 10% in Britian, 7% in Pakistan and 1.4% in the Henan Han population (7–9). Thus far, the correlation of the CBS844ins68 gene polymorphism and EH is rarely reported in domestic and foreign countries. Lucock et al (7) studied a British population and found that the CBS844ins68 gene polymorphism may not be an independent risk factor of EH.
The prevalence of hypertension in Chinese provinces is different. Northeast and North of China is higher compared to the region of Southwest and Southeast, and the Eastern region is higher compared to the Western. A number of reasons for the differences may be associated with the population salt intake, variation in the proportion of obese subjects, ethnicity and climate. Standardized prevalence of hypertension in the same area between various ethnic groups and regions of the same nation were different. In 1991, the Third National Sample Survey of hypertension showed that the lowest prevalence was in Yi (3.28%), and was higher for the Korean (22.95%), Tibetan (21.04%) and Mongolian (20.22%) populations, and for Xinjiang Kazakh and Han it was 18.97 and 13.88%, respectively (10). Using the Xinjiang Tianshan mountains as the boundary, China is divided into North and South regions. The Northern winter is long and cold so therefore exercise outdoors is minimal, and the local residents are carnivorous, whereas in the Southern region the winter is shorter, and vegetable and fruit intake is relatively higher, which also generates certain body weight and blood lipid characteristics. Kawamura et al (11) studied the region of Balikun (Xinjiang Northern) and found that the prevalence of hypertension in the Han population was 42%, but in the Hotan prefecture (Xinjiang Southern) the Han prevalence of hypertension was 27%. The aim of the present study was to investigate the correlation between Hcy and the gene polymorphisms of its metabolic enzymes, CBS844ins68 and MTHFR C677T, and EH in patients from the North of Xinjiang.
Materials and methods
Ethics statement
Written informed consent was obtained from all the participating patients prior to enrollment in the study. The study was approved by the Institutional Ethics Committee at the First Affiliated Hospital of Shihezi University School of Medicine (Shihezi, China) and conducted in accordance with the Ethical Guidelines of the Declaration of Helsinki.
Study subjects
Between May 2012 and March 2013, 200 patients with EH (47.01±8.06 years) and 200 control subjects (45.23±9.88 years) were recruited sequentially from the Chinese Han population in Xinjiang Shihezi. All the patients fulfilled the hypertension diagnosis standard of the World Health Organization/International Society of Hypertension in 2003 and secondary hypertension (12), cardiomyopathy, diabetic and other patients were excluded. The control subjects inclusion criteria were systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg, no use of antihypertensive medication, no family history of hypertension, and a medical history of liver, kidney and thyroid problems, diabetes and others were excluded.
Specimen collection
After 12–14 h fasting, 4 ml venous blood was collected into an EDTA-tube, mixed and stored at −80°C for genomic DNA extraction. Another tube of heparin was used and plasma was centrifuged for 1 h at 3,000 × g within 10 min of collection. The plasma was separated for biochemical testing.
Detection of Hcy and biochemical analyses
Using an AU 2700 biochemical analyzer (Olympus, Tokyo, Japan), the Hcy concentration was determined by enzymatic cycling assay (Nine Strong Biological Co., Ltd., Beijing, China), and the study participated in the National ‘863’ Project quality control of Hcy. Other biochemical analyses were performed by Roche 7600 (Roche Diagnostics, Madison, WI, USA), according to the manufacturer’s instructions.
Genotype analysis
Genomic DNA was extracted from the blood samples of the study subjects using a blood genome DNA extraction kit (GeneCore BioTeke Co., Ltd., Beijing, China). The primers were designed as described previously (4).
Polymerase chain reaction (PCR) amplification of the CBS844ins68 and MTHFR C677T polymorphisms
PCR was performed in a total volume of 2 μl containing 3.0 μl DNA, 0.5 μl of each primer, 12.5 μl PCR mix (including dNTPs and MgCl2; Shanghai Sangon Biological Engineering Technology and Services Co., Ltd., Shanghai, China) and distilled water 8.5 μl. Amplification was carried out at Biometra (Göttingen, Germany) using a gradient automatic PCR amplification instrument (negative controls without DNA template). The reaction of CBS844ins68 was performed as follows: Initial denaturation was at 95°C for 3 min; denaturation at 95°C for 30 sec and annealing at 57°C for 30 sec, with extension for 60 sec at 72°C, repeated for 35 cycles, followed by a final extension at 72°C for 5 min. The CBS844ins68 PCR product was analyzed in 2% agarose gel electrophoresis directly, and the gel bands and typing were observed under an imager.
The reaction of MTHFR C677T was carried out as follows: initial denaturation was at 95°C for 5 min; denaturation at 95°C for 60 sec and annealing at 57°C for 60 sec, with extension for 60 sec at 72°C, repeated for 36 cycles, followed by a final extension at 72°C for 5 min. The MTHFR C677T product (198 bp) was digested using HinfI (Fermentas, Waltham, MA, USA), and the digested products were run on a 9% agarose gel and stained with silver nitrate 35 min, observed and genotyped in the UV gel imager.
Statistical analysis
Statistical analysis was performed with the SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Each result was calculated as mean ± standard deviation. Hardy-Weinberg equilibrium was used to confirm whether the sample had a group representation, the differences in genotype and allelic frequency between the ethnic groups were evaluated with Fisher’s exact test or χ2 tests as appropriate. The odds ratio (OR) was calculated together with its 95% confidence interval (CI). P<0.05 was considered to indicate a statistically significant difference. Linkage disequilibrium (LD) and haplotype analysis was performed using SHEsis software (13), and the LD coefficient (D′)<0.2 was considered to have no LD, D′>0.5 had a LD, D′>0.8 had a strong LD and D′=1 is in complete LD. r2 was the relevant factor.
Results
Characteristics of the subjects
The analysis of the clinical data in the EH and normal tensive (NT) groups (400 cases were selected) is shown in Table I. There were no significant differences (P>0.05) between the two groups for age, urea, creatinine, uric acid, glucose, high-density lipoprotein cholesterol (HDL-C), apolipoprotein (apo) A, apo B and lipoprotein(a) (Lpa). In systolic blood pressure, diastolic blood pressure, body mass index, triglyceride (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), EH was significantly higher compared to the NT group (P<0.05; Table II). In the two groups, the level of Hcy in males was higher compared to females, but was not statistically significant (Table III).
Table I.
General clinical data analysis of EH and NT groups.
| Characteristics | EH group (n=200) | NT group (n=200) | T | P-value |
|---|---|---|---|---|
| Age, years | 47.01±8.06 | 45.23±9.88 | 1.958 | 0.051 |
| SBP, mmHg | 152.38±9.60 | 115.51±12.18 | 33.605 | 0.001a |
| DBP, mmHg | 101.82±10.15 | 76.34±8.50 | 27.213 | 0.001a |
| BMI, kg/m2 | 27.07±2.50 | 24.23±1.68 | 13.283 | 0.003a |
| BUN, mmol/l | 5.03±1.51 | 4.86±1.18 | 1.277 | 0.202 |
| CR, mmol/l | 73.13±16.22 | 72.32±13.75 | 0.538 | 0.591 |
| UA, mmol/l | 280.70±90.55 | 272.72±69.37 | 0.989 | 0.323 |
| FPG, mmol/l | 5.31±1.09 | 5.21±0.84 | 0.966 | 0.335 |
| TG, mmol/l | 1.45±0.85 | 0.97±0.46 | 6.923 | 0.001a |
| TC, mmol/l | 4.71±1.12 | 4.09±0.53 | 6.981 | 0.002a |
| HDL-C, mmol/l | 1.24±0.40 | 1.19±0.21 | 1.477 | 0.141 |
| LDL-C, mmol/l | 2.83±0.84 | 2.45±0.52 | 5.263 | 0.001a |
| Apo A, mmol/l | 1.42±0.34 | 1.37±0.27 | 1.580 | 0.115 |
| Apo B, mmol/l | 0.96±0.28 | 0.94±0.22 | 0.838 | 0.403 |
| Lpa, mmol/l | 217.07±179.23 | 199.71±75.59 | 1.262 | 0.208 |
P<0.01 compared to NT group.
EH, essential hypertension; NT, normal tensive; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; BUN, blood urea nitrogen; CR, creatinine; UA, uric acid; FPG, fasting plasma glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Apo, apolipoprotein; Lpa, lipoprotein(a).
Table II.
Hcy level in the NT and EH groups.
| Groups | Hcy, μmol/l | T | P-value |
|---|---|---|---|
| NT (n=200) | 10.33±2.04 | 0.28 | 0.001a |
| EH (n=200) | 16.05±7.59 |
P<0.01. Data are mean ± standard deviation.
Hcy, homocysteine; NT, normal tensive; EH, essential hypertension.
Table III.
Hcy level in the different genders.
| Groups | Female, μmol/l | Male, μmol/l | T | P-value |
|---|---|---|---|---|
| EH (112/88) | 15.29±7.27 | 17.01±7.93 | −1.598 | 0.112 |
| NT (107/93) | 10.13±2.12 | 10.56±1.93 | −1.497 | 0.136 |
Data are mean ± standard deviation. Hcy, homocysteine; EH, essential hypertension; NT, normal tensive.
Polymorphism detection
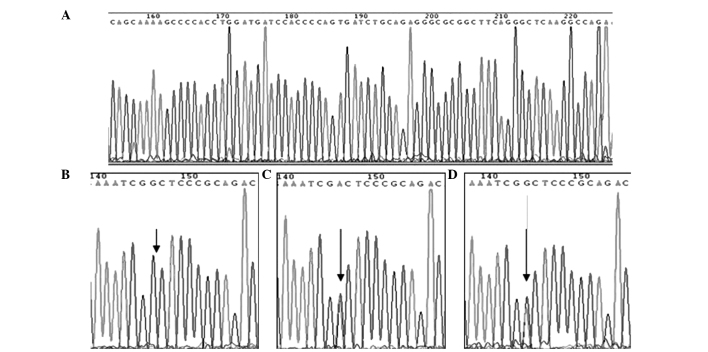
The CBS844ins68 gene polymorphism is shown in Fig. 1. The detection of the PCR products of CBS844ins68 obtained two genotypes: Homozygous wild-type deletion/deletion (DD) (184 bp) and heterozygous genotype deletion/insertion (DI) (184 and 252 bp). Through digestion, the cleavage products of MTHFR C677T detected three genotypes: Wild-type CC (198 bp), heterozygous genotype CT (198, 175 and 23 bp, but only 198 and 175 bp were visible on the imager; since the cleavage product of the gene was extremely small, 23 bp was not visible when visualized), homozygous mutant genotype TT (175 and 23 bp, but only 175 bp was visible) (Fig. 2). The sequencing results are shown in Fig. 3.
Figure 1.
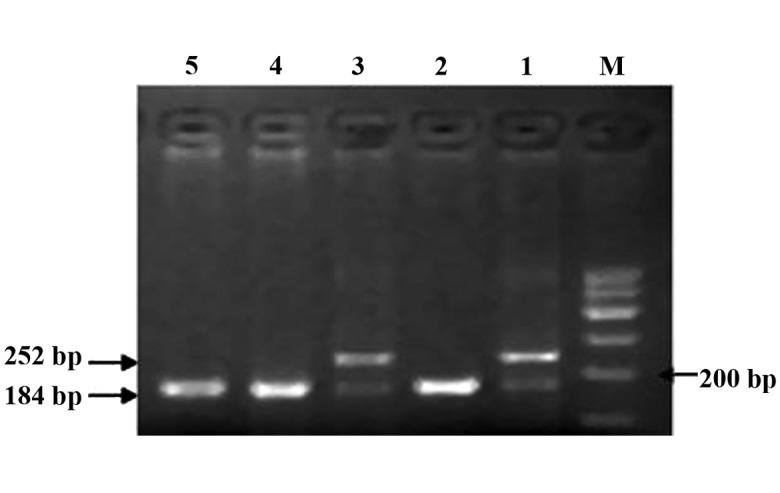
Polymerase chain reaction amplification of CBS844ins68. Lanes 2, 4 and 5, DD genotype; lanes 1 and 3, DI genotype; M, marker.
Figure 2.
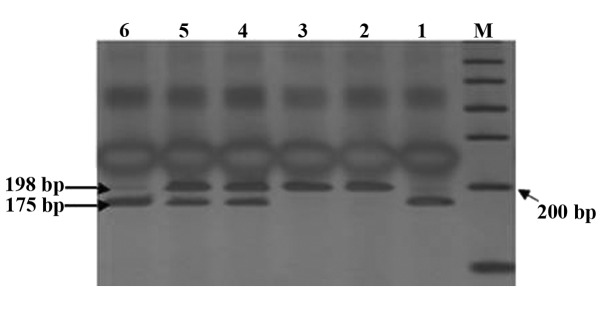
Cleavage map of MTHFR C677T. Lanes 2 and 3, CC genotype; lanes 4 and 5, CT genotype; lanes 1 and 6, TT genotype; M, marker.
Figure 3.
DNA sequencing of CBS844ins68 and MTHFR C677T polymorphisms. (A) Position 155 to 222 were CBS844ins68 insertion mutant (type DI). (B) Position 146 to G (CC genotype). (C) Position 146 to A/A (TT genotype). (D) Position 144 to G/A (CT genotype).
As shown in Table IV, there was no significant difference (P>0.05) between the CBS844ins68 genotype and allele frequency distribution. In the two groups, the MTHFR C677T gene had three genotypes; CC, CT and TT. The three genotype frequencies in the EH group were 19.5, 49.5 and 31% and in the NT group were 32.5, 44.5 and 25%, respectively. There were differences in the overall genotype and allele frequency distribution in the two groups. The risk of suffering from EH for the CT+TT genotype is 1.812 times higher than that of the CC genotype, and this was statistically significant (P<0.05), as shown in Table V. This indicates that the MTHFR C677T polymorphism is associated with the development of EH and that the T allele may be present in the genetic susceptibility genes.
Table IV.
Comparison of the CBS844ins68 genotypes and alleles in the EH and NT groups.
| Genotypes, n (%) | Alleles, n (%) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Groups | DD | DI | II | D | I |
| EH (n=200) | 191 (95.5) | 9 (4.5) | 0 (0) | 391 (97.75) | 9 (2.25) |
| NT (n=200) | 188 (94.0) | 12 (6.0) | 0 (0) | 388 (97.00) | 12 (3.00) |
| χ2 | 0.452 | 0.440 | |||
| P-value | >0.05 | 0.05 | |||
EH, essential hypertension; NT, normal tensive; D, deletion; I, insertion.
Table V.
Comparison of the MTHFR C677T genotypes and alleles in the EH and NT groups.
| Genotype, n (%) | Alleles, n (%) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Groups | CC | CT | TT | C | T |
| EH (n=200) | 39 (19.5) | 99 (49.5) | 62 (31.0) | 177 (44.25) | 223 (55.75) |
| NT (n=200) | 61 (30.5) | 89 (44.5) | 50 (25.0) | 211 (52.75) | 189 (47.25) |
| χ2 | 6.658 | 5.785 | |||
| P-value | <0.05 | <0.05 | |||
CT+TT gene, odds ratio (OR), 1.812 [95% confidence interval (CI), 1.142–2.874]. T allele, OR, 1.407 (95% CI, 1.065–1.858). EH, essential hypertension; NT, normal tensive.
Associations between the two genotype and Hcy level
The level of Hcy had no significant difference in CBS844ins68 DD and DI type (P>0.05) and there was a significant difference in the MTHFR C677T CC, CT and TT-types (P<0.05), as shown in Table VI.
Table VI.
Comparison of the two genotypes and Hcy levels.
| Genotype | No. | Hcy, μmol/l | P-value | |
|---|---|---|---|---|
| CBS844ins68 | DD | 379 | 13.20±6.32 | >0.05 |
| DI | 21 | 12.98±4.85 | ||
| MTHFR C677T | CC | 100 | 11.54±4.63 | |
| CT | 188 | 13.86±7.04 | <0.05 | |
| TT | 112 | 13.55±5.87 | <0.05 |
Comparison of CC and CT, TT types, P<0.05. Comparison of DI and DD type, P>0.05. Data are mean ± standard deviation. Hcy, homocysteine; D, deletion; I, insertion.
Analysis of MTHFR C677T, CBS844ins68 combined genotype
The two genes were composed of six types of joint genotypes: CC/DD, CC/DI, CT/DD, CT/DI, TT/DD and TT/DI. As CBS844ins68 type II was not detected, the CC/II, CT/II and TT/II combined genotype were not found. Through the analysis, the frequency distribution of the CC/DD gene type in EH was lower than that of the NT group, and the difference was statistically significant (P<0.05, Table VII). OR, 0.569 showed that the CC/DD gene type was a protective factor of hypertension, and the frequency distribution of the remaining joint genotype between the two groups showed no significant difference (P>0.05).
Table VII.
Distribution of MTHFR C677T and CBS844ins68 combined genotype frequency.
| MTHFR C677T | CBS844ins68 | EH (n=200) (%) | NT (n=200) (%) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| CC | DD | 37 (18.5) | 57 (28.5) | 5.563 | 0.018a | 0.569 (0.356–0.912) |
| CC | DI | 2 (1.0) | 4 (2.0) | 0.677 | 0.411 | 0.495 (0.090–2.733) |
| CT | DD | 95 (47.5) | 85 (42.5) | 1.010 | 0.315 | 1.224 (0.825–1.816) |
| CT | DI | 4 (2.0) | 4 (2.0) | 0.000 | 1.000 | 1.000 (0.247–4.055) |
| TT | DD | 59 (29.5) | 46 (23.0) | 2.182 | 0.140 | 1.401 (0.895–2.193) |
| TT | DI | 3 (1.5) | 4 (2.0) | 0.145 | 0.703 | 0.746 (0.165–3.378) |
P<0.05.
EH, essential hypertension; NT, normal tensive; OR, odds ratio; CI, confidence interval; D, deletion; I, insertion.
For the T-test statistical analysis, the joint genotype CC/DD, CT/DD, TT/DD and TT/DI of Hcy levels were significantly different in the two groups (P<0.05; Table VIII) and Hcy levels in CC/DI and CT/DI genotypes showed no statistically significant difference (P>0.05).
Table VIII.
Level of plasma Hcy in MTHFR C677T and CBS844ins68 combined genotype.
| MTHFR C677T | CBS844ins68 | EH-Hcy (μmol/l) | NT-Hcy (μmol/l) | T | P-value |
|---|---|---|---|---|---|
| CC | DD | 13.28±6.67 | 10.44±2.37 | 2.94 | 0.004a |
| CT | DD | 17.14±8.33 | 10.16±1.87 | 7.55 | 0.003a |
| TT | DD | 16.04±6.84 | 10.43±1.84 | 5.40 | 0.002a |
| CC | DI | 10.20±1.98 | 11.83±2.74 | −0.73 | 0.506 |
| CT | DI | 17.80±7.10 | 10.60±2.24 | 1.93 | 0.101 |
| TT | DI | 17.73±2.45 | 9.53±2.07 | 4.82 | 0.005a |
P<0.01.
EH, essential hypertension; Hcy, homocysteine; NT, normal tensive; D, deletion; I, insertion.
MTHFR C677T, CBS844ins68 polymorphism in LD, analysis of LD
Use of the SHEsis software analysis showed D′=0.216 and r2=0.02. D′<0.2 was considered to have no LD and D′>0.5 with LD, where r2 represents the correlation coefficient. The results indicate that MTHFR C677T and CBS844ins68 have a weak linkage.
MTHFR C677T and CBS844ins68 gene polymorphism in different countries and regions
In this study, the CBS844ins68 genotype and allele frequencies of the North Xinjiang Han population were compared to various countries. There were a significant difference in the CBS844ins68 genotype and allele frequencies of the North Xinjiang Han population and Italy, Japan, Australia, Beijing of China (P<0.05, Table IX), but no significant difference was apparent for Germany and the Czech Republic (P>0.05). As shown in Table X, a comparison of the MTHFR C677T polymorphism in populations of various countries and regions was performed. There were a significant difference in the MTHFR C677T polymorphism and Australia, the United Kingdom, India, Turkey (P<0.05), but there was no statistically significant difference in South Korea (P>0.05). When compared to domestic Tianjin, Hebei, Yunnan, Shandong and Hainan, there was a statistical significance (P<0.05), and the difference was more clear in Northern compared to Southern China.
Table IX.
CBS844ins68 gene polymorphism in different countries and regions.
| Genotype, n (%) | Allele, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Countries | No. | DD | DI | II | D | I | χ2 | P-value |
| Germany | 229 | 207 (90.4) | 22 (9.6) | 0 (0.0) | 436 (95.2) | 22 (4.8) | 1.825 | 0.177 |
| Pakistan | 872 | 754 (86.5) | 115 (13.2) | 3 (3.4) | 1623 (93.1) | 121 (6.9) | 8.672 | 0.003a |
| Italy | 102 | 88 (86.3) | 14 (13.7) | 0 (0.0) | 190 (93.1) | 14 (6.9) | 4.893 | 0.027b |
| Czech | 400 | 356 (89.0) | 44 (11.0) | 0 (0.0) | 756 (94.5) | 44 (5.5) | 3.746 | 0.053 |
| Japan | 393 | 393 (100.0) | 0 (0.0) | 0 (0.0) | 786 (100.0) | 0 (0.0) | 23.800 | 0.001a |
| Australia | 186 | 150 (80.6) | 34 (18.3) | 2 (1.1) | 334 (89.8) | 38 (10.2) | 16.570 | 0.002a |
| China | ||||||||
| Beijing | 375 | 366 (97.6) | 9 (2.4) | 0 (0.0) | 741 (98.8) | 9 (1.2) | 4.715 | 0.030b |
| Han in Xinjiang | 200 | 188 (94.0) | 12 (6.0) | 0 (0.0) | 388 (97.0) | 12 (3.0) | ||
P<0.01;
P<0.05.
Table X.
MTHFR C677T polymorphism in different countries and regions.
| Genotype, n (%) | Allele, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Countries | No. | CC | CT | TT | G | T | χ2 | P-value |
| Australia | 393 | 175 (44.5) | 183 (46.6) | 35 (8.9) | 533 (67.8) | 253 (32.2) | 25.720 | 0.001a |
| America | 898 | 402 (44.8) | 407 (45.3) | 89 (9.9) | 1211 (67.4) | 585 (32.6) | 30.880 | 0.003a |
| Korea | 1700 | 540 (31.8) | 863 (50.8) | 297 (17.5) | 1943 (57.1) | 1457 (42.9) | 2.818 | 0.093 |
| India | 103 | 85 (82.5) | 18 (17.5) | 0 (0.0) | 188 (91.3) | 18 (8.7) | 89.670 | 0.003a |
| Turkey | 45 | 5 (11.1) | 16 (35.6) | 24 (53.3) | 26 (28.9) | 64 (71.1) | 16.750 | 0.002a |
| China | ||||||||
| Tinjing | 932 | 199 (21.4) | 450 (48.3) | 283 (30.4) | 848 (45.5) | 1016 (54.5) | 6.970 | 0.008a |
| Hebei | 475 | 172 (36.2) | 223 (46.9) | 80 (16.8) | 567 (59.7) | 383 (40.3) | 5.543 | 0.019 |
| Yunnan | 124 | 53 (42.7) | 52 (41.9) | 19 (15.3) | 158 (63.7) | 90 (36.3) | 7.500 | 0.006a |
| Shangdong | 1052 | 154 (14.6) | 469 (44.6) | 429 (40.8) | 777 (36.9) | 1327 (63.1) | 35.200 | 0.004a |
| Hainan | 3016 | 1763 (58.5) | 1061 (35.2) | 192 (6.4) | 4587 (76.0) | 1445 (24.0) | 107.400 | 0.003a |
| Han in Xinjiang | 200 | 61 (30.5) | 89 (44.5) | 50 (25.0) | 211 (52.8) | 189 (47.3) | ||
P<0.01.
Discussion
Using the Hardy-Weinberg equilibrium, the MTHFR C677T and CBS844ins68 genotypes were shown to achieve a genetic balance in the Han population with regard to the distribution frequency, which had a group representation. Analysis of the clinical data in the EH and NT groups (400 cases were selected) is shown in Table I. There were no significant differences (P>0.05) between the two groups for age, urea, creatinine, uric acid, glucose, HDL-C, apo A, apo B and Lpa, but for systolic blood pressure, diastolic blood pressure, body mass index, TG, TC and LDL-C, EH was significantly higher compared to the NT group (P<0.05). The plasma Hcy levels in EH were significantly higher compared to those in the NT group (P<0.05) (Table II) and between males and females there were no significant differences (P>0.05) (Table III).
The results of the χ2 test and correlation analysis showed that gene polymorphisms of the Hcy metabolism enzymes MTHFR C677T and CBS844ins68 were present in the North Xinjiang Han population. This has relevance in MTHFR C677T polymorphism and susceptibility of EH, and significant differences were found in the allele and genotype frequencies between the two groups (P<0.05), as shown in Table V. The relative risk of suffering from EH in the CT+TT genotype was 1.812-fold higher than that of the CC genotype (OR, 1.812; 95% CI, 1.142–2.874), and the results of the C allele relative risk analysis were OR, 1.407 and 95% CI, 1.065–1.858. These results indicate that the changes observed for the MTHFR C677T gene polymorphism are one of the genetic factors of EH, thus the T allele may be a risk factor. No correlation was found between the CBS844ins68 gene polymorphism and alleles in the Xinjiang Han patients (Table IV). Thus, I allele is probably not a predisposing factor. Analysis of the single-nucleotide polymorphism using factors including sample selection, sample size and ethnicity showed that a larger cohort of samples and the application of different ethnicities in case-control studies are required to improve the understanding of EH pathogenesis.
In the present study, the Hcy levels in two points genotypes were analyzed. The results showed that the level of Hcy had no significant difference in CBS844ins68 DD and DI types (P>0.05), and there was a significant difference in the MTHFR C677T CC, CT and TT-types (P<0.05), as shown in Table VI.
The two points genotypes were composed of six kinds of joint genotypes: CC/DD, CC/DI, CT/DD, CT/DI, TT/DD and TT/DI; and as CBS844ins68 type II was not detected, the CC/II, CT/II and TT/II combined genotype were not found. Through the analysis, the frequency distribution of the CC/DD gene type in EH was lower than that of the NT group, the difference was statistically significant (P<0.05) and OR, 0.569 showed that the CC/DD gene type was a protective factor of hypertension (Table VII). The frequency distribution of the remaining joint genotype between the two groups showed no significant difference (P>0.05). Joint genotype CC/DD, CT/DD, TT/DD and TT/DI of the Hcy levels were significantly different in the two groups (P<0.05) and Hcy levels in the CC/DI and CT/DI genotypes showed no statistically significant difference (P>0.05) (Table VIII). This indicates that the combined genotypes, CC/DD, CT/DD, TT/DD and TT/DI, may affect Hcy levels and further affect the occurrence and development of EH.
In addition, the present study compared the CBS844ins68 polymorphisms and allele frequencies in the populations of various countries and regions. Table IX shows that the CBS844ins68 genotype and allele frequencies of the North Xinjiang Han population were significantly different compared to Italy (14), Japan (15), Australia (7), Beijing of China (16) (P<0.05); and no significant difference (P>0.05) was apparent for Germany (17) and the Czech Republic (18). A comparison of the MTHFR C677T polymorphism in populations of various countries and regions was performed (Table X). In comparison to Australia (7), the United Kingdom (19), India (20) and Turkey (21) the difference was statistically significant (P<0.05), but there was no statistically significant difference in South Korea (22) (P>0.05). When compared to domestic Tianjin, Hebei, Yunnan, Shandong and Hainan (5) there was a statistical significance (P<0.05), and the difference was more clear in Northern compared to Southern China. Therefore, the gene polymorphisms of the Hcy metabolism enzymes, MTHFR and CBS, and allele frequencies exist in different ethnicities and nationalities. This difference may affect certain MTHFR C677T and CBS844ins68 gene polymorphism-associated diseases.
In conclusion, the preliminary results of the present study show the single-nucleotide polymorphisms of the Hcy metabolism enzymes, MTHFR C677T and CBS844ins68, exist in the Han population. The CBS844ins68 gene polymorphism may not be associated with the development of EH, however the MTHFR C677T gene polymorphism may influence the level of Hcy and is closely associated with the occurrence of EH. The T allele may be a genetic susceptible gene of EH. However, the exact mechanism of EH in the Xinjiang Han and other populations remains to be determined in future studies.
Acknowledgements
The present study was supported by the National High Technology Research and Development Program (‘863’ Program; grant no. 2011AA02A111).
References
- 1.Petramala L, Acca M, Francucci CM, D’Erasmo E. Hyperhomocysteinemia: a biochemical link between bone and cardiovascular system diseases? J Endocrinol Invest. 2009;32(Suppl 4):10–14. [PubMed] [Google Scholar]
- 2.Lu D, Lu XC. Research progress of homocysteine and its metabolic enzyme gene polymorphisms and cardiovascular disease. Lab Med Clin. 2012;9:844–845. (In Chinese) [Google Scholar]
- 3.Zhang L, Yin RX, Liu WY, Miao L, et al. Association of methylenetetrahydrofolate reductase C677T polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 2010;9:123. doi: 10.1186/1476-511X-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowdar JY, Lason MV, Szvetko AL, Lea RA, et al. Investigation of homocysteine-pathway-related variants in essential hypertension. Int J Hypertens 2012. 2012;1909:23. doi: 10.1155/2012/190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang B, Liu Y, Li Y, Fan S, et al. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS One. 2013;8:e57917. doi: 10.1371/journal.pone.0057917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alghasham A, Settin AA, Ali A, Dowaidar M, et al. Association of MTHFR C677T and A1298C gene polymorphisms with hypertension. Int J Health Sci (Qassim) 2012;6:3–11. doi: 10.12816/0005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucock M, Yates Z, Martin C, Choi JH, et al. Hydrogen sulphide-related thiol metabolism and nutrigenetics in relation to hypertension in an elderly population. Genes Nutr. 2013;8:221–229. doi: 10.1007/s12263-012-0317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakub M, Moti N, Parveen S, Chaudhry B, et al. Polymorphisms in MTHFR, MS and CBS genes and homocysteine levels in a Pakistani population. PLoS One. 2012;7:e33222. doi: 10.1371/journal.pone.0033222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li AF, Zheng H, Xu YM, Zhao XJ, et al. The association between gene polymorphisms of homocysteine metabolism-related enzymes and ischemic cerebrovascular diseases in Chinese Henan Han population. Life Sci J. 2012;9:403–408. [Google Scholar]
- 10.The National Blood Pressure Survey Cooperative Group. Prevalence and variation trends of hypertension in China. J Hypertens. 1995;23(S1) (In Chinese) [Google Scholar]
- 11.Kawamura H, Jumabay M, Mitsubayashi H, Izumi Y, et al. 24-hour blood pressure in Uygur, Kazakh and Han elderly subjects in China. Hypertens Res. 2000;23:177–185. doi: 10.1291/hypres.23.177. [DOI] [PubMed] [Google Scholar]
- 12.Whitworth JA World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Tang K, Fu DJ, Julien D, Braun A, et al. Chip-based genotyping by mass spectrometry. Proc Natl Acad Sci USA. 1999;96:10016–10020. doi: 10.1073/pnas.96.18.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivieri O, Friso S, Trabetti E, Girelli D, et al. Homocysteine and atheromatous renal artery stenosis. Clin Exp Med. 2001;1:211–218. doi: 10.1007/s102380100005. [DOI] [PubMed] [Google Scholar]
- 15.Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, Seifried A. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States) Cancer Causes Control. 2002;13:239–248. doi: 10.1023/a:1015057614870. [DOI] [PubMed] [Google Scholar]
- 16.Bi XH, Zhao HL, Zhang ZX, Liu Q, et al. Association analysis of CbetaS 844ins68 and MTHFD1 G1958A polymorphisms with Alzheimer’s disease in Chinese. J Neural Transm. 2010;117:499–503. doi: 10.1007/s00702-010-0383-x. [DOI] [PubMed] [Google Scholar]
- 17.Ott N, Geddert H, Sarbia M. Polymorphisms in methionine synthase (A2756G) and cystathionine beta-synthase (844ins68) and susceptibility to carcinomas of the upper gastrointestinal tract. J Cancer Res Clin Oncol. 2008;134:405–410. doi: 10.1007/s00432-007-0301-2. [DOI] [PubMed] [Google Scholar]
- 18.Orendác M, Musková B, Richterová E, Zvárová J, et al. Is the common 844ins68 polymorphism in the cystathionine beta-synthase gene associated with atherosclerosis? J Inherit Metab Dis. 1999;22:674–675. doi: 10.1023/a:1005554702861. [DOI] [PubMed] [Google Scholar]
- 19.Mitrou PN, Watson MA, Loktionov AS, et al. MTHFR (C677T and A1298C) polymorphisms and risk of sporadic distal colorectal adenoma in the UK Flexible Sigmoidoscopy Screening Trial (United Kingdom) Cancer Causes Control. 2006;17:793–801. doi: 10.1007/s10552-006-0016-8. [DOI] [PubMed] [Google Scholar]
- 20.Poduri A, Mukherjee D, Sud K, Kohli HS, et al. MTHFR A1298C polymorphism is associated with cardiovascular risk in end stage renal disease in North Indians. Mol Cell Biochem. 2008;308:43–50. doi: 10.1007/s11010-007-9610-7. [DOI] [PubMed] [Google Scholar]
- 21.Onrat ST, Akci O, Söylemez Z, Onrat E, Avşar A. Prevalence of myocardial infarction polymorphisms in Afyonkarahisar, Western Turkey. Mol Biol Rep. 2012;39:9257–9264. doi: 10.1007/s11033-012-1799-1. [DOI] [PubMed] [Google Scholar]
- 22.Cui LH, Shin MH, Kweon SS, Kim HN, Song HR, et al. Methylenetetrahydrofolate reductase C677T polymorphism in patients with gastric and colorectal cancer in a Korean population. BMC Cancer. 2010;10:236. doi: 10.1186/1471-2407-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]