Abstract
Immunoglobulin (Ig) A nephropathy (IgAN) is the most common form of glomerulonephritis. In clinical practice, it is difficult to monitor the repeating relapse in patients suffering from IgAN, which usually occurs within 10 years of end-stage renal disease. In order to identify and quantify the total protein content in the renal tissue of patients with IgAN, isobaric tags for relative and absolute quantification (iTRAQ) technology was performed. iTRAQ coupled with multiple chromatographic fractionation and tandem mass spectrometry was used to analyze the total protein of normal renal tissue in IgAN and healthy patients. The individual proteins were identified by the Mascot search engine and any that were differentially expressed were monitored. A total of 574 different proteins were identified, and 287 proteins were up- or downregulated by >1 fold alteration in levels. The results showed that iTRAQ-based quantitative proteomic technology for the identification and relative quantitation of the renal tissue proteome is efficiently applicable. The differential expression of the proteome profiles for IgAN patients was determined. Further studies using large cohorts of patient samples with long-term clinical follow-up data should be conducted to evaluate the usefulness of the pathogenesis and novel biomarker candidates of IgAN, which may develop a novel technique for the diagnosis of IgAN.
Keywords: isobaric tags for relative and absolute quantification technology, immunoglobulin A nephropathy, proteomic, tandem mass spectrometry
Introduction
Immunoglobulin (Ig) A nephropathy (IgAN), also known as Berger disease (1), is the most common form of glomerulonephritis globally and is characterized by the deposition of polymeric IgA (predominantly of the IgA1 subclass) (2). The typical symptom of IgAN is macroscopic hematuria associated with proteinuria (3). The clinical course of the disease has been established (4) and 20–40% of patients with IgAN are likely to develop an end-stage renal disease within 25 years of diagnosis. Thus far, glomerular diseases are diagnosed by clinical manifestations, urinalysis, clinical chemistry tests and renal histopathology. The diagnosis mainly depends on a kidney biopsy, which is an invasive technique that has a low risk of bleeding and complications that are not often repeated in the same patient. Therefore, the development of non-invasive diagnostic tools would be a significant progression for patients with IgAN and other glomerular diseases (5,6).
Isobaric tags for relative and absolute quantification (iTRAQ) reagents, including a peptide reactive group, and are used for reporting group analysis and a molecular mass balance (7). The technology is usually applied to the identification of protein biomarkers in glomerular diseases, including two-dimensional gel electrophoresis, two-dimensional difference gel electrophoresis, surface-enhanced laser desorption/ionization time-of-flight (TOF) mass spectrometry (MS) and capillary electrophoresis-MS. Quantitative proteomics is an important branch of proteomics research as it is used to quantify and identify all the protein expressed in a whole genome or in a complex mixture. iTRAQ was originally developed by Applied Biosystems, Inc., (Foster City, CA, USA) in 2004. The iTRAQ reagent consists of a peptide reactive group and the reporter group is used to analyze the molecular mass balance. This unique approach labels samples with eight independent isobaric tags, and the eight unique reporter ions (m/z from 113–121) provide quantitative information following integration of the peak areas, which quantifies the eight different samples (8,9).
iTRAQ quantification has been previously applied in biomarker studies of various disease states, including prostate (10), ovarian (11) and gastric cancers (12). Currently, there are limited studies on the adoption of IgAN by iTRAQ technology. In the present study, iTRAQ technology was used to analyze the total proteins of the renal tissue from patients with IgAN, which may help to improve the understanding of the pathogenesis, diagnosis and treatment for IgAN.
Materials and methods
IgAN and control groups
Between March and August 2012, renal tissue was collected from eight IgAN patients from the 181st Hospital (Guilin, China), subsequent to obtaining consent from all patients. The IgAN patients were biopsy-diagnosed and the control group consisted of four patients with no clinical evidence of IgAN. The study was performed according to the guidelines established by the 181st Hospital, which abides by the Helsinki Declaration on ethical principles for medical research involving human subjects. Written informed consent was obtained from all subjects or their guardians.
Sample preparation
Nine biopsies were collected from the IgAN patients and control group, which were immediately washed with 0.9% RNase-free NaCl and dipped briefly in RNase inhibitor (Epicentre Biotechnologies, Madison, WI, USA) according to the manufacturer’s instructions. The samples were subsequently stored at −80°C for further analysis.
Protein extraction and quantification
Following the collection of the renal tissue (250 mg) from the IgAN patients and control group, the tissue was ground into a fine powder in liquid nitrogen and supplemented with acetone followed by 10% trichloroacetic acid in acetone for 2 h at −20°C. Total protein was extracted with extraction buffer [8 M urea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 40 mmol/l Tris-HCl, 1 mmol/l phenylmethanesulfonyl fluoride, 2 mmol/l EDTA, 10 mmol/l dithiothreitol and 0.5–2% isotonic glucose phosphate buffer (pH 8.5)] and subjected to centrifugation at 40,000 × g for 1 h at 10°C. The protein concentration of the supernatant was determined using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA) according to the manufacturer’s instructions.
iTRAQ reagent labeling, strong cation exchange (SCX) fractionation and tandem MS (MS/MS)
The protein was pooled for each group and subsequently blocked, digested and labeled according to the iTRAQ protocol (Applied Biosystems, Inc.). The iTRAQ tags were healthy control-iTRAQ 113 and IgAN-iTRAQ 119. The labeled digests were subsequently combined into one sample mixture.
Multidimensional liquid chromatography was performed to separate the tryptic peptides prior to MS. The combined samples were separated into 10 SCX fractions using a 3.5 μm particle size coluIgAN (35×0.3 mm, 300 Å, Zorbax Bio-SCX, Santa Clara, CA, USA), with a potassium formate gradient in 25% acetonitrile. The peptides in these fractions were then separated on a Tempo™LC Nanoflow and matrix-assisted laser desorption/ionization (MALDI) spotting system equipped with a reversed-phase Magic C18AQ coluIgAN. Each chromatography run yielded ~380 MALDI spots on a stainless steel MALDI target plate Agilent 1290 Infinity (2D-LC) (Santa Clara, CA, USA) (13).
MS data were obtained using an Applied Biosystems 4800 Plus MALDI TOF/TOF. Signal-to-noise ratios of ≥40 were required for the MS/MS spectra. The mass spectra from 500 laser shots were obtained for each spot. The MS/MS data from all 10 fractions were combined and subsequently analyzed using the Paragon Algorithm search engine and Human v3.62 downloaded from the EBI website (http://www.ebi.ac) (13).
Statistical analysis and gene ontology (GO) analysis
The threshold used for protein identification was a ProtScore >1.3 (95%) with at least more than one peptide above the 95% confidence level. Proteins yielding tryptic peptides with average reporter ion ratios between ≥1.5 and ≤0.67 were classified as up- and downregulated, respectively. The GO database annotates selected proteins according to molecular function (MF), cellular component (CC) and biological process (BP). To investigate the functions of the identified proteins, the online GO tool WEGO (Web Gene Ontology Annotation Plot; http://wego.genomics.org.cn/) was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Protein identification
Using a confidence interval of >95% (P<0.05) with peptides >1 as the cutoff, a total of 1,860 proteins were identified and quantified from renal tissues. There were 24 proteins that had a fold change difference of >1.5, with 12 upregulated and 12 downregulated (Tables I and II, respectively). The MF, CC and BP of the proteins are shown in Tables I and II.
Table I.
Upregulated proteins in renal tissue of immunoglobulin A (IgA) nephropathy patients.
| No. | Accession no. | Protein name | Molecular function | Cellular component | Biological process | Peptides | Ratio |
|---|---|---|---|---|---|---|---|
| 1 | sp|P09493 | Isoform TPM1κ of tropomyosin α-1 chain | Structural constituent of cytoskeleton | Intracellular organelle | Establishment of localization, transport | 31 | 3.35 |
| 2 | sp|P61769 | β-2-microglobulin | Protein binding | Organelle | Immune response | 4 | 2.99 |
| 3 | sp|P68133 | Actin, α skeletal muscle | Nucleoside binding | Intracellular organelle | Regulation of biological quality | 122 | 2.76 |
| 4 | sp|Q9NZP8 | Complement C1r subcomponent-like protein | Hydrolase activity | Extracellular region | Immune response | 1 | 2.58 |
| 5 | sp|P04083 | Annexin A1 | Lipid binding | Intracellular | Transport | 14 | 2.56 |
| 6 | sp|P04075 | Fructose- bisphosphate aldolase A | Lyase activity | Non-membrane- bound organelle | Regulation of biological quality | 36 | 2.38 |
| 7 | sp|P62328 | Thymosin β-4 | Protein binding | Intracellular organelle | Regulation of cellular component organization | 7 | 1.91 |
| 8 | sp|P09493 | Isoform TPM1κ of tropomyosin α-1 chain | Structural constituent of muscle | Organelle | Establishment of localization | 31 | 3.35 |
| 9 | sp|P01011 | α-1-antichymotrypsin | Protein binding | Intracellular | Regulation of biological quality | 12 | 1.58 |
| 10 | sp|P05156 | Complement factor I | Hydrolase activity | Extracellular region | Response to external stimulus | 3 | 1.56 |
| 11 | sp|P98160 | Basement membrane- specific heparan sulfate proteoglycan core protein | Protein binding | Extracellular region | Extracellular structure organization | 48 | 1.56 |
| 12 | sp|P08238 | HSP 90-β | Nucleotide binding | Vesicle | Response to chemical stimulus | 30 | 1.53 |
TPM1, tropomyosin 1; HSP, heat-shock protein.
Table II.
Downregulated proteins in renal tissue of immunoglobulin A (IgA) nephropathy patients.
| No. | Accession no. | Protein name | Molecular function | Cellular component | Biological process | Peptides | Ratio |
|---|---|---|---|---|---|---|---|
| 1 | sp|P09669 | Cytochrome c oxidase subunit 6C | Oxidoreductase activity | Membrane- bound organelle | Cellular metabolic process | 3 | 0.62 |
| 2 | sp|P00403 | Cytochrome c oxidase subunit 2 | Oxidoreductase activity | Organelle membrane | Oxidation reduction | 6 | 0.56 |
| 3 | sp|P51649 | Succinate- semialdehyde dehydrogenase, mitochondrial | Oxidoreductase activity | Cell fraction | Oxidation reduction | 4 | 0.55 |
| 4 | sp|P10809 | 60 kDa heat shock protein, mitochondrial | Nucleotide binding | Organelle lumen | Positive regulation of immune system process | 47 | 0.53 |
| 5 | sp|P62195 | 26S protease regulatory subunit 8 | Nucleoside binding | Protein complex | Catabolic process | 1 | 0.50 |
| 6 | sp|P05091 | Aldehyde dehydrogenase, mitochondrial | Oxidoreductase activity | Organelle lumen | Oxidation reduction | 28 | 0.47 |
| 7 | sp|P01031 | Complement C5 | Protein binding | Protein complex | Cellular metabolic process | 4 | 0.45 |
| 8 | sp|P30837 | Aldehyde dehydrogenase X, mitochondrial | Oxidoreductase activity | Intracellular | Oxidation reduction | 7 | 0.44 |
| 9 | sp|P61604 | 10 kDa heat shock protein, mitochondrial | Nucleotide binding | Intracellular organelle | Response to chemical stimulus | 11 | 0.36 |
| 10 | sp|P02753 | Retinol-binding protein 4 | Lipid binding | Extracellular region | Response to external stimulus | 11 | 0.36 |
| 11 | sp|P00966 | Argininosuccinate synthase | Nucleoside binding | Intracellular | Cellular metabolic process | 21 | 0.36 |
| 12 | sp|P05062 | Fructose- bisphosphate aldolase B | Lyase activity | Non-membrane- bound organelle | Catabolic process | 53 | 0.25 |
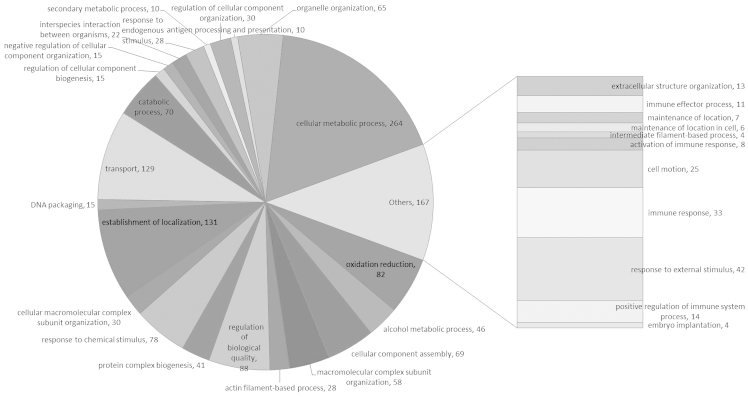
These proteins were linked to the GO MF, CC and BP categories (Figs. 1–3). According to the GO database, the differentially-expressed proteins were divided into MF, CC and BP. The top five components for MF, CC and BP of these proteins are shown in Table III. The top five components for MF were protein binding, nucleotide binding, hydrolase activity, oxidoreductase activity and nucleoside binding. For the upregulated proteins, protein binding exhibited a significant change (Table I) and in the downregulated proteins oxidoreductase activity changed significantly (Table III). Notably, oxidoreductase activity was reported for numerous proteins.
Figure 1.
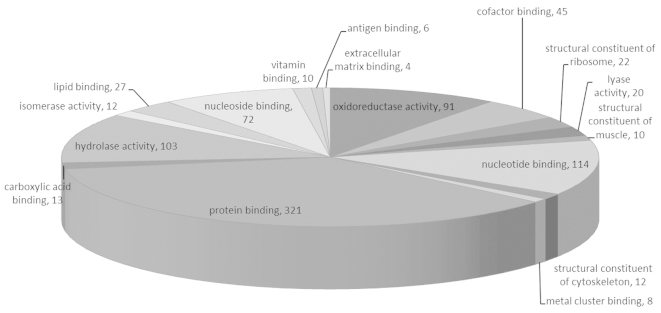
Molecular function of the IgA-nephropathy tissue proteins. IgA, immunoglobulin A.
Figure 3.
Biological process of the IgA-nephropathy tissue proteins. IgA, immunoglobulin A.
Table III.
Top five components for molecular function, cellular component and biological process.
| Molecular function | Count, n | % | Cellular component | Count, n | % | Biological process | Count, n | % |
|---|---|---|---|---|---|---|---|---|
| Protein binding | 321 | 57.12 | Intracellular | 464 | 82.56 | Cellular metabolic process | 264 | 46.98 |
| Nucleotide binding | 114 | 20.28 | Intracellular | 462 | 82.21 | Establishment of localization | 131 | 23.31 |
| Hydrolase activity | 103 | 18.33 | Intracellular organelle | 374 | 66.55 | Transport | 129 | 22.95 |
| Oxidoreductase activity | 91 | 16.19 | Membrane- bound organelle | 310 | 55.16 | Regulation of biological quality | 88 | 15.66 |
| Nucleoside binding | 72 | 12.81 | Organelle | 230 | 40.93 | Oxidation reduction | 82 | 14.59 |
Discussion
The development of quantitative proteomics has significantly improved the capacity of proteomic methods for assessing the expression, modification and function of protein markers. The iTRAQ has been indicated to be appropriate for the detection of biomarkers and it also allows the simultaneous comparison of protein abundance by measuring the peak intensities of the reporter ions that are released from the iTRAQ-tagged peptides. Therefore, it may be a potential tool for identifying biomarkers (14). Thus, iTRAQ technology was applied in the present study, as well as GO analysis, to quantitatively analyze the renal tissue proteome of IgAN patients and healthy controls. In total, 1,860 proteins were identified via GO analysis, involving MF, CC and BP (Table III). A general proteome database was constructed for the renal tissues proteome, and to the best of our knownledge, this database has not been previously reported.
The up- and downregulated proteins in the renal tissue of IgAN patients are shown in Tables I and II. Among them, there was significant deviation of five proteins [β-2-microglobulin, annexin A1, complement C5, retinol-binding protein 4 (RBP4) and argininosuccinate synthase], which are known to potentially participate in IgAN and certain glomerular diseases (15–18). The present study provides additional evidence that iTRAQ technology accurately quantifies the relative changes in the protein abundance of renal tissue. The renal tissue proteome pathology diagnosis or utility are at the initial stages of assessment and require further study.
Certain differentially expressed proteins have previously been shown to play important roles in the pathogenesis of IgAN. For example, β-2-microglobulin is a component of the class I major histocompatibility complex and it is involved in the presentation of peptide antigens to the immune system. This protein has numerous functions, including response to chemical stimulus, antigen processing and presentation, and immune response. The binding of β-2-microglobulin to the low molecular weight protein antigens on the human leukocyte antigen heavy chain has a structure that is similar to immunoglobulin. In particular, the immune reactions stimulate β-2-microglobulin release and studies have shown that the β-2-microglobulin concentration is associated with the severity of IgAN (19,20). Notably, in the present study, β-2-microglobulin was found to be highly expressed in IgAN compared to healthy controls.
RBP4 from the liver is stored in the peripheral tissues. Loss of the RBP-retinol complex by filtration through the kidney glomeruli can be inhibited by its interaction with transthyretin (21). A previous study has reported that the presence of RBP4 in the early diagnosis of injury in glomerular disease is more sensitive than β-2-microglobulin and microalbumin (22). RBP4, as a rather sensitive indicator, also exists in the urine of patients and predates the emergence of microalbumin (23). In the present study, significant differentially expressed RBP4 is expected to be a potential marker of IgAN.
Certain novel candidates, including annexin A1, aldehyde dehydrogenase and complement C5, have been confirmed. These candidates are relevant to other associated diseases, the apoptotic pathway and synthetic substances in tissues (24–26). However, the development of these in the process of IgAN are completely undetermined, and therefore, these novel candidates require further investigation.
In conclusion, iTRAQ is a novel strategy for proteomic analysis. The aim of this initial study focuses on the comparison of the protein of IgAN patients and healthy controls using iTRAQ technology. A total of 1,860 proteins were differentially expressed in the kidney tissue of IgAN patients compared to the control group. However, the study did not discuss each of the candidate proteins in detail and only assessed certain IgAN-biomarker candidates that were notable. Two proteins (β-2-microglobulin and RBP4) were identified as potential biomarkers, but they require verification in future studies, which may develop a novel technique for the diagnosis of IgAN.
Figure 2.
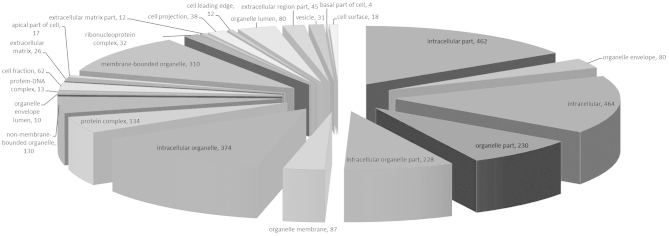
Cellular component of the IgA-nephropathy tissue proteins. IgA; immunoglobulin A.
Acknowledgements
The authors would like to thank the patients and healthy volunteers who participated in the present study. The study was supported by the Guangxi Key Laboratory of Construction Project Plan (grant no. 13-051-31), the Guangxi Science and Technology Plan (Department of Guangxi; grant no. 10124001B-27) and the Guilin City Science and Technology Plan of Science and Technology Innovation Ability and Condition Construction (grant no. 20130121-6).
References
- 1.Matousovic K, Konecný K, Mĕstecký J, et al. IgA nephropathy. Significance of immunoglobulin A glycosylation in pathogenesis and clinical presentation. Cas Lek Cesk. 2002;141:729–734. (In Czech) [PubMed] [Google Scholar]
- 2.Barratt J, Smith AC, Molyneux K, Feehally J. Immunopathogenesis of IgAN. Semin Immunopathol. 2007;29:427–443. doi: 10.1007/s00281-007-0089-9. [DOI] [PubMed] [Google Scholar]
- 3.Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN) Kidney Int. 2004;65:1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 4.Galla JH. IgA nephropathy. Kidney Int. 1995;47:377–387. doi: 10.1038/ki.1995.50. [DOI] [PubMed] [Google Scholar]
- 5.Yu HH, Chiang BL. Diagnosis and classification of IgA nephropathy. Autoimmun Rev. 2014;13:556–559. doi: 10.1016/j.autrev.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 6.He L, Liu H, Peng Y. Immune pathogenesis of IgA nephropathy and its drugable targets. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:96–101. doi: 10.11817/j.issn.1672-7347.2014.01.016. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 7.Jones AM, Nühse TS. Phosphoproteomics using iTRAQ. Methods Mol Biol. 2011;779:287–302. doi: 10.1007/978-1-61779-264-9_17. [DOI] [PubMed] [Google Scholar]
- 8.Desouza LV, Voisin SN, Siu KW. iTRAQ-labeling for biomarker discovery. Methods Mol Biol. 2013;1002:105–114. doi: 10.1007/978-1-62703-360-2_9. [DOI] [PubMed] [Google Scholar]
- 9.Unwin RD. Quantification of proteins by iTRAQ. Methods Mol Biol. 2010;658:205–215. doi: 10.1007/978-1-60761-780-8_12. [DOI] [PubMed] [Google Scholar]
- 10.Glen A, Gan CS, Hamdy FC, et al. iTRAQ-facilitated proteomic analysis of human prostate cancer cells identifies proteins associated with progression. J Proteome Res. 2008;7:897–907. doi: 10.1021/pr070378x. [DOI] [PubMed] [Google Scholar]
- 11.Wang LN, Tong SW, Hu HD, et al. Quantitative proteome analysis of ovarian cancer tissues using a iTRAQ approach. J Cell Biochem. 2012;113:3762–3772. doi: 10.1002/jcb.24250. [DOI] [PubMed] [Google Scholar]
- 12.Loei H, Tan HT, Lim TK, et al. Mining the gastric cancer secretome: identification of GRN as a potential diagnostic marker for early gastric cancer. J Proteome Res. 2012;11:1759–1772. doi: 10.1021/pr201014h. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Dai Y, Qi S, Sun B, Wen J, Zhang L, Tu Z. Comparative proteome analysis of peripheral blood mononuclear cells in systemic lupus erythematosus with iTRAQ quantitative proteomics. Rheumatol Int. 2012;32:585–593. doi: 10.1007/s00296-010-1625-9. [DOI] [PubMed] [Google Scholar]
- 14.Chong PK, Lee H, Zhou J, et al. ITIH3 is a potential biomarker for early detection of gastric cancer. J Proteome Res. 2010;9:3671–3679. doi: 10.1021/pr100192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiela-Hojeńska A, Hurkacz M. The significance of beta 2-microglobulin in diagnosis and therapy. Postepy Hig Med Dosw. 1998;52:507–514. (In Polish) [PubMed] [Google Scholar]
- 16.Sui W, Tang D, Zou T, et al. Differential proteomic analysis of renal tissue in mesangial proliferative glomerulonephritis using iTRAQ technology. J Nephrol. 2013;26:191–198. doi: 10.5301/jn.5000124. [DOI] [PubMed] [Google Scholar]
- 17.Puri TS, Quigg RJ. The many effects of complement C3- and C5-binding proteins in renal injury. Semin Nephrol. 2007;27:321–337. doi: 10.1016/j.semnephrol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Cabré A, Lázaro I, Girona J, et al. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. 2007;262:496–503. doi: 10.1111/j.1365-2796.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 19.Peters HP, van den Brand JA, Wetzels JF. Urinary excretion of low-molecular-weight proteins as prognostic markers in IgA nephropathy. Neth J Med. 2009;67:54–61. [PubMed] [Google Scholar]
- 20.Nitta K, Tsutsui T, Ozu H, et al. Beta 2-microglobulin as an indicator of interstitial cell infiltration in IgA nephropathy. Nephron. 1996;74:219–220. doi: 10.1159/000189307. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Qi Q, Zong G, et al. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr. 2014;144:722–728. doi: 10.3945/jn.113.189860. [DOI] [PubMed] [Google Scholar]
- 22.Baboolal K, Meyer TW. The effect of acute angiotensin II blockade on renal function in rats with reduced renal mass. Kidney Int. 1994;46:980–985. doi: 10.1038/ki.1994.357. [DOI] [PubMed] [Google Scholar]
- 23.Koch A, Weiskirchen R, Sanson E, et al. Circulating retinol binding protein 4 in critically ill patients before specific treatment: prognostic impact and correlation with organ function, metabolism and inflammation. Crit Care. 2010;14:R179. doi: 10.1186/cc9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bizzarro V, Fontanella B, Franceschelli S, et al. Role of Annexin A1 in mouse myoblast cell differentiation. J Cell Physiol. 2010;224:757–765. doi: 10.1002/jcp.22178. [DOI] [PubMed] [Google Scholar]
- 25.Feng T, Cong Y, Qin H, et al. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onda K, Ohi H, Tamano M, et al. Hypercomplementemia in adult patients with IgA nephropathy. J Clin Lab Anal. 2007;21:77–84. doi: 10.1002/jcla.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]