Abstract
DNA-damaging agents have been reported to be associated with cardiovascular complications, however, the underlying mechanisms remain to be clarified. In the present study, the possible vascular effects of cisplatin was assessed by measuring its effects on the contractile function of thoracic aortic rings dissected from Sprague-Dawley (SD) rats. Contraction of the aortic ring was induced by 60 mM KCl or 10−6 M phenylephrine (PE) in an ex vivo perfusion system. Cisplatin (200 μM) counteracted KCl- and PE-induced contraction by 57.6 and 91.8%, respectively, in endothelium-intact aortic rings. Similar results were obtained in endothelium-denuded aortas. Electromicroscopy analysis revealed severe damage to blood vessel walls in vivo by cisplatin. In addition, cisplatin significantly inhibited adenosine triphosphate (ATP)-induced intracellular Ca2+ concentration ([Ca2+]i) increases in human umbilical vein endothelial cells (HUVECs). These results suggested that the DNA-damaging agent cisplatin can affect the contractile function of thoracic aortas. In addition, in accordance with its DNA-damaging properties, the cardiovascular toxicity of cisplatin may be the result of its direct cytotoxicity.
Keywords: DNA damage, cisplatin, cardiovascular toxicity, vascular contraction
Introduction
DNA-damaging agents, or genotoxic agents, are those chemicals that can produce alterations in the genetic material of the host. Such agents can be further subdivided into direct- and indirect-acting agents. Direct-acting agents are intrinsically reactive and do not require metabolic activation by cellular enzymes to interact with DNA. By contrast, indirect-acting agents require metabolic activation by cellular enzymes to form the DNA-reactive metabolite. DNA-damaging agents exist widely in our natural and social environment, with examples including some of the chemotherapeutic agents and environmental pollutants (1).
Cisplatin, a direct-acting agent, is one of the most widely used chemotherapeutic agents in the treatment of a wide variety of malignancies (2). Although its biochemical mechanism of action has yet to be elucidated, cisplastin is believed to exert cytotoxic effects through the interaction and formation of adducts with DNA, which then leads to apoptosis and necrosis (3,4). Despite its clinical efficacy in treating malignancies, cisplatin-based chemotherapy regimens have been reported to be associated with vascular toxicity and serious vascular complications (e.g., myocardial infarction and stroke) (5–7). Such vascular toxicity has been manifested by increased von Willebrand factor plasma levels as well as an enhanced intima-media thickness of the carotid artery (8). In addition, cisplatin has the potential to induce ototoxicity and toxicity towards renal, peripheral sensory and autonomic nervous systems, potentially attributed to cisplatin-caused microvascular damage (9–11).
Ca2+ plays an important role in the regulation of vascular tone, which is generally relatively constant. Initiation of contraction in vascular smooth muscle is due to an increase in the free cytosolic Ca2+ concentration, which is caused by Ca2+ entry via adenosine triphosphate (ATP)-dependent ion channels (12). Aortic tension also plays an important role in regulating the entire cardiovascular system. Large arteries are involved in the regulation of hydrostatic capillary pressure (13,14) and blood pressure through changing pulsatile pressure (15). Arteries have a prominent cushioning effect in addition to their conducting function and this effect is associated with the viscoelastic properties of the arterial wall (16). In particular, the vasomotor reactions have been reported to be related to plasma volume and to help maintaining cardiovascular homeostasis (13). Although in previous studies it was reported that cisplatin is associated with a higher incidence of cardiovascular mortality and morbility (17,18), the effect of cisplatin on vascular tone remains to be elucidated. Therefore, the aim of the present study was to evaluate the cardiovascular toxicity of cisplatin on the contractile function of rat thoracic aortas and to elucidate the possible underlying mechanisms.
Materials and methods
Reagents
Phenylephrine (PE), acetylcholine (ACh), cisplatin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Fluo-4/AM and ATP were purchased from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade and were purchased from Sinopharm Chemical Reagent (Shanghai, China). Cisplatin was dissolved in phosphate-buffered saline (PBS) for in vitro and in vivo experiments. Current guidelines for use of biohazardous materials in Zhejiang University were followed when using cisplatin.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were grown in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C under humidified air (5% CO2).
Cytotoxicity assay
The toxicity of cisplatin was examined using the MTT assay. Briefly, cells were seeded into a 96-well culture plate at density of 1×104 cells/well. After 24 h, the medium was discarded and replaced with fresh medium containing the indicated concentrations of chemicals. The cells were treated for 6 or 12 h and by the end of each time point, 20 μl of MTT (5 mg/ml in PBS) was added to each well and further incubated for 3 h. The solution was then discarded and 150 μl of isopropanol was added. Following agitation for 10 min to dissolve the formazan, the absorbance at 570 nm was read on a microtiter plate reader (BioTek, Winooski, VT, USA). Relative survival was represented as the absorbance of the treated sample/absorbance of the control group.
Animals and treatment of cisplatin
Male Sprague-Dawley (SD) rats weighing 230–260 g were obtained from the Laboratory Animal Center of Zhejiang University (Hangzhou, China). All the procedures were approved by the Ethics Committee for the Use of Experimental Animals in Zhejiang University. Rats were housed in an air-conditioned colony room at 20±2°C and diet and water were provided ad libitum. To detect the effect of cisplatin on thoracic aortas, the rats were injected intraperitoneally with cisplatin at a dose of 5 mg/kg body weight and an equivalent volume of saline was used as control (11).
Preparation of thoracic aortic rings
Rats were anaesthetized with 10% chloral hydrate (0.4 g/kg, intraperitoneally) and sacrificed by cervical dislocation. The chest was opened and the thoracic aorta was rapidly removed and placed in a 4°C Krebs-Henseleit (K-H) solution [mM: NaCl, 120.0; KCl, 4.5; CaCl2, 1.25; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25.0; and glucose, 10.0 (pH 7.4)]. Following removal of the superficial connective tissue, the aorta was cut into rings of 3–4 mm in length and subjected to contractile functional studies and electron microscopic measurements.
Electron microscopy
Three aortic rings cut from different segments of the thoracic aorta (superior, middle and inferior segment) were fixed with 2.5% glutaraldehyde at 4°C for >24 h and then washed twice in 0.1 M PBS at 4°C for 15 min. The tissue was incubated in 1% osmic acid at 4°C for 1 h and then rinsed twice in the same buffer for 15 min. After staining in 2% uranyl acetate (en bloc) at 4°C for 30 min, the tissues were processed by graded dehydration with different concentrations of ethanol (50, 70, 90 and 100%) and 100% acetone, respectively, each for 15 min. The dehydrated thoracic aortic rings were then agitated with a mixture of embedding medium (Epon 812) to acetone (1:1) for 1 h and then transferred into pure embedding medium (Epon 812) for another 1.5–2 h. Subsequently, the aortas were embedded with the same medium at 37, 45 and 60°C, each for 24, 24 and 48 h, respectively. Sections of ~120 nm were taken from the prepared rings using an ultramicrotome (Leica Microsystems, Milton Keynes, UK). The sections were stained with 4% uranyl acetate for 20 min and lead citrate for 5 min and then observed under transmission electron microscopy (Tecnai 10, 80 kV). The number of smooth muscle cells was counted depending on the image (x970) captured from electron microscopy.
Measurement of contractile function of thoracic aortic rings
Fresh isolated rings were mounted in 5.0 ml organ baths containing the K-H solution. The bath solution was maintained at 37°C and bubbled continuously with a gas mixture of 95% O2 and 5% CO2. Aortic rings were equilibrated for 60 min at resting tensions of 2 g. Isometric tension was measured with force isometric transducers connected to a data acquisition system (MedLab; Nanjing Medease Co., Ltd., Nanjing, China). In some aortic rings, the endothelium was mechanically removed by gentle rubbing with moistened cotton. The removal of endothelial cells was confirmed by the loss of ACh-induced relaxation (19–21). After 6 h pre-incubation with cisplatin (200 μM), contraction of the endothelium-intact and endothelium-denuded aortic rings was induced by KCl (60 mM) or PE (10−6 M). The vascular response was expressed as a percentage of the ‘plateau’ contraction evoked by KCl or PE (22).
Measurement of intracellular Ca2+ concentration ([Ca2+]i)
HUVECs grown on coverslips were treated with cisplatin (200 μM) for 6 h. The cells were loaded with the Ca2+ sensitive dye Fluo-4/AM (1 nM) for 30 min at 37°C in serum-free RPMI-1640 medium. After loading, the cells were washed three times with serum-free RPMI-1640 medium. The loaded cells were maintained at 37°C for 15 min before measurement of [Ca2+]i in order to allow the Fluo-4/AM in the cytosol to deesterify. The cells were subsequently placed on the stage of a Nikon inverted microscope equipped with a SFX-1 microfluorimeter (Solamere Technology Group, Salt Lake City, UT, USA). During the experiments, the cells were superfused in a 0.3-ml bath chamber at 35°C under a constant flow (1 ml/min) of HEPES-buffered saline (135 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 10 mM HEPES; pH 7.4) (23). To examine the action of cisplatin on ATP-induced increased [Ca2+]i, the cells were stimulated with ATP (10 μM) for 1 min. ATP was then washed away twice by HEPES-buffered saline. Fluo-4 fluorescence was excited at 470 nm. Emitted Fluo-4 fluorescence was measured at wavelengths of 520 nm. Changes in [Ca2+]i were reported as the Fluo-4/AM ratio ΔF/ΔF0, where ΔF is the difference between baseline fluorescence intensity and maximal fluorescence intensity after ATP stimulation, while ΔF0 is the difference between baseline fluorescence intensity and the minimal fluorescence intensity prior to ATP stimulation.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical analysis was carried out using Student’s paired t-test or one-way analysis of variance followed by Dunnett’s t test. Differences were considered statistically significant at P<0.05.
Results
Effects of cisplatin on the contractile function of thoracic aortas of SD rats
First the cytotoxicity of cisplatin was examined by MTT using HUVECs to determine the appropriate dose for subsequent experimentation. As shown in Fig. 1, cisplatin at doses ranging 5–500 μM induced cytotoxicity at 6 and 12 h in a dose-dependent manner, with maximal decreases in cell survival (20–30%) observed at the higher concentrations.
Figure 1.
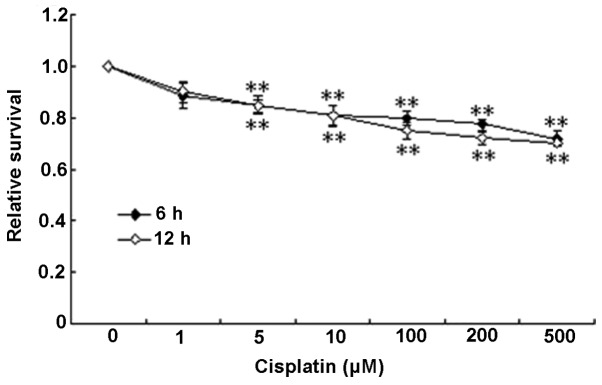
The cytotoxicity of cisplatin on human umbilical vein endothelial cells (HUVECs). HUVECs were treated with various doses of cisplatin for 6 or 12 h and cell viability was measured by the MTT test. Data are expressed as mean ± SEM. **P<0.01 compared to the control.
Using the ex vivo perfusion system, the effects of cisplatin on the contraction of aortic rings were examined. At concentrations ranging 10–500 μM, cisplatin itself had no direct effect on the baseline tension of endothelium-intact aortic rings (Table I). Combined with the cytotoxicity data, 200 μM was selected as the concentration for subsequent experimentation. KCl (60 mM) induced significant contraction with the relative tension as 1 at time 0 and gradually decreased to 0.61±0.08 after 6 h. It was found that cisplatin decreased the contraction tension provoked by KCl in a time-dependent manner, with a maximum inhibition of 57.6% (0.26±0.10, P<0.05 versus 0.61±0.08) at 6 h (Fig. 2A). PE (10−6 M) increased the contraction tension to a similar level as KCl (0.59±0.12) at 6 h and it was almost abolished by cisplatin, as shown in Fig. 2B, in which cisplatin reduced PE-induced contraction tension by 91.8% (0.049±0.01, P<0.05 versus 0.59±0.12).
Table I.
The relative baseline tension of aortic rings treated with cisplatin.
| Thoracic aortic rings | Control (n) | Cisplatin (n) | |||
|---|---|---|---|---|---|
|
| |||||
| 10 μM | 100 μM | 200 μM | 500 μM | ||
| +E | 0.018±0.036 (6) | 0.033±0.045 (5) | 0.073±0.054 (6) | 0.091±0.063 (6) | 0.059±0.034 (5) |
| −E | 0.033±0.052 (9) | 0.037±0.050 (5) | 0.056±0.058 (6) | 0.021±0.045 (7) | 0.063±0.069 (5) |
Thoracic aortic rings (+E, endothelium-intact thoracic aorta; −E, endothelium-denuded thoracic aorta) were incubated in various concentrations of cisplatin (μM) for 6 h. Relative tensions of aortic rings were evaluated by ex vivo perfusion. Data are expressed as mean ± SEM.
Figure 2.
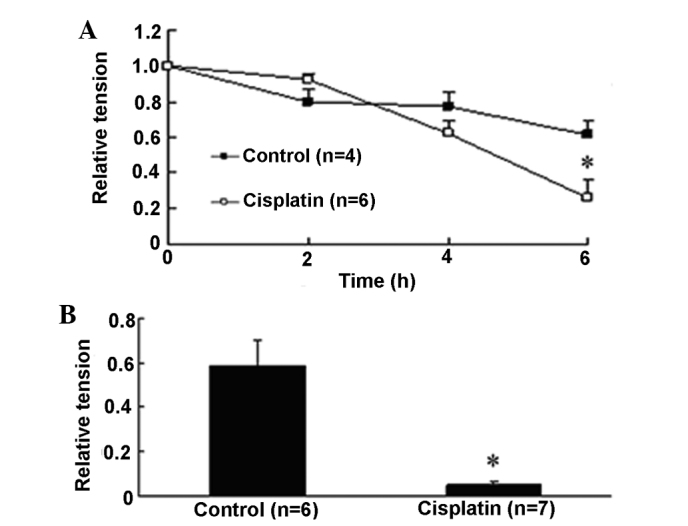
Effect of cisplatin on the relative tension of endothelium-intact thoracic aorta. (A) Cisplatin decreased the relative tension of endothelium-intact rat thoracic aortic rings precontracted with KCl at the resting tension of 2 g in an ex vivo perfusion system in a time-dependent manner. (B) Cisplatin inhibited the contraction induced by phenylephrine in endothelium-intact rat thoracic aortic rings at 6 h. Data are expressed as mean ± SEM. *P<0.05 versus control endothelium-intact aorta rings.
Effects of cisplatin on the contractile function of endothelium-denuded thoracic aortas of SD rats
Endothelium is known to play an important role in regulating the vascular tone by releasing factors involved in relaxation and contraction (24). Therefore, we also assessed the effects of cisplatin on endothelium-denuded aortic rings. In the aortic rings of endothelium denudation, KCl and PE also increased vascular tension to 0.65±0.07 and 0.36±0.03 at 6 h, respectively (Fig. 3). Similar to the endothelium-intact situation, cisplatin decreased KCl-induced contraction tension by 63.0% (0.24±0.05, P<0.05 versus 0.65±0.07) and PE-induced contraction tension by 81.3% (0.067±0.01, P<0.05 versus 0.36±0.03).
Figure 3.
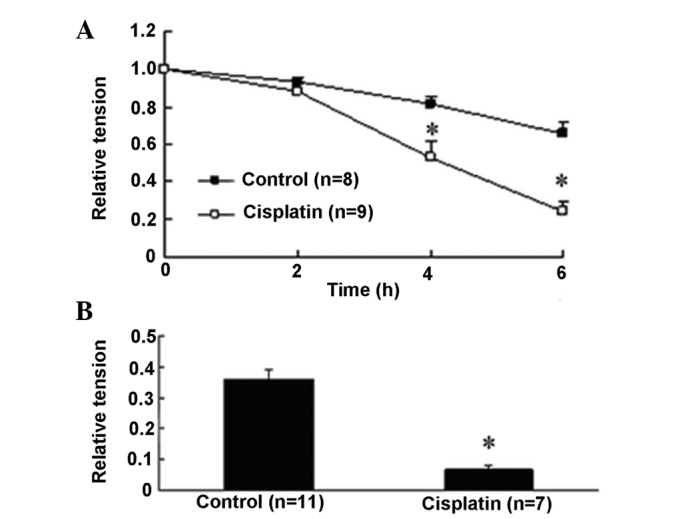
Effect of cisplatin on the relative tension of endothelium-denuded thoracic aorta. (A) Cisplatin decreased the relative tension of endothelium-denuded rat thoracic aortic rings precontracted with KCl at the resting tension of 2 g in an ex vivo perfusion system in a time-dependent manner. (B) Cisplatin inhibited the contraction induced by phenylephrine in endothelium-denuded rat thoracic aortic rings at 6 h. Data are expressed as mean ± SEM. *P<0.05 versus control endothelium-denuded aorta rings.
Effect of cisplatin on [Ca2+]i in HUVECs
As stated before, since [Ca2+]i is also an important factor in regulating vascular tone, the effect of cisplatin on [Ca2+]i was examined in HUVECs. As shown in Fig. 4, cisplatin (200 μM) significantly decreased the [Ca2+]i in HUVECs increased by ATP (2.79±0.58 versus 4.42±0.33).
Figure 4.
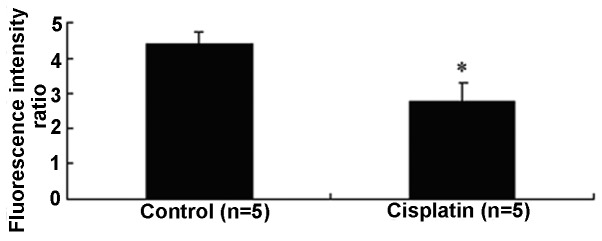
Effects of cisplatin on adenosine triphosphate-induced changes of intracellular Ca2+ concentration in human umbilical vein endothelial cells. Data are expressed as mean ± SEM. *P<0.05 versus control.
Effects of cisplatin on aortic vessel wall components in vivo
To examine the effects of cisplatin on the aortic walls, aortas from the control- and cisplatin-treated rats were isolated and processed for electron microscopic observation. As shown in Fig. 5, cisplatin caused severe damage to the smooth muscle layer. Compared to the control, the boundary of smooth muscle layers was less clearly defined and almost touching each other in the aortas from cispatin-treated rats. Cisplatin also caused significant loss of smooth muscle cells, for example, the average smooth muscle cell count was decreased by 34.7% (from 26,383±2,561 to 17,217±1,452 mm−2 with cisplatin treatment, P<0.05).
Figure 5.

Cisplatin induced damages in the smooth muscle layer in Sprague-Dawley rats. Representative images of thoracic aorta segments (superior segment of aortas) from control and cisplatin-treated rats are shown.
Discussion
In the present study, by measuring the contractile function of thoracic aortas in an ex vivo perfusion system, we evaluated the vascular effects of the DNA-damaging agent cisplatin. It has been reported that there is a high incidence of cardiovascular events in survivors of cisplatin-based chemotherapy cancer patients (25), either in acute or long-term chemotherapy (26,27). Cisplatin was reported to lead to aortic occlusion in some cases and this impairment was usually irreversible (28,29). In one case, a 36-year-old man who was treated with bleomycin-etoposide-cisplatin therapy for testicular cancer received chronic coronary artery dissection (30). A long-term follow-up in a large cohort of testicular cancer survivors showed that compared with healthy controls, cisplatin-treated patients had, at follow-up, increased systolic blood pressure, increased diastolic blood pressure and a higher prevalence of hypertension (18). In that cohort, patients in the high-dose cisplatin-treated group had increased odds for having intermediate/high risk in cardiovascular mortality, compared with the surgery group (OR=3.4, 95% CI: 1.3–8.7) (17). All of the above case reports led to the hypothesis that cisplatin may cause deleterious effects on the cardiovascular system. Thus, we undertook a first step to investigate whether cisplatin has such an effect and in particular, we focused on the contractile function of aortic rings as an indicator.
First, we demonstrated that the baseline tensions of thoracic aortas were not altered by cisplatin treatment, which means that cisplatin had no direct deleterious effect on the contractile function of aortas. However, cisplatin inhibited KCl- or PE-induced contraction in the endothelium-intact and endothelium-denuded aortas, suggesting an endothelium-independent mechanism (Figs. 2 and 3). To investigate the reason for cisplatin decreasing the tension of aortic rings, we examined the structure of the aortic walls. As shown in Fig. 5, cisplatin severely damaged the smooth muscle layer, and significantly decreased smooth muscle cells in the thoracic aortic wall. The fundamental structural and functional unit of the aortic wall is the medial lamellar unit. The media layer comprises elastic membrane layers between which are the smooth muscle layer and a small amount of collagen and elastic fibers. The integrity of the vascular smooth muscle layer is crucial in the maintenance of normal vascular morphology and tone. Changes in arterial wall composition and function underlie all forms of vascular disease (31). Therefore, the finding that cisplatin damaged the arterial wall may partially explain its inhibitory effect on the contraction of aortic rings.
In addition, we have also shown that cisplatin inhibited ATP-induced Ca2+ influx in HUVECs (Fig. 4). However, whether cisplatin similarly affects Ca2+ in aortic tissues remains to be determined.
The effects of another envionmentally prevalent DNA damaging agent, benzo(a)pyrene [B(a)P] on the contractile function of thoracic aortas have also been investigated. Unlike cisplatin, B(a)P is an indrect-acting agent and has to be metabolically processed by aryl hydrocarbon receptor-induced enzymes, such as cytochrome P4501A1 (CYP1A1) and epoxide hydrolase, to form carcinogenic active form B(a)P-diol epoxides (32). Of note, B(a)P only affected the contractile function of thoracic aortas following long-term in vivo exposure (4 weeks) (data not shown).
In conclusion, in the present study, we have shown that the DNA-damaging agent cisplatin: i) decreased the contractile function of thoracic aortas; and ii) caused direct vessel well damage and cytotoxicity towards smooth muscle cells. Therefore, it seems that DNA-damaging agents may also have cardiovascular toxicity and thus, the potential cardiovascular complications associated with DNA-damaging agents should be investigated in future studies.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81373036, 81172692 and 81202241) and Zhejiang Provincial of Science and Technology Department (grant no. 2013C14016). J. Yang is a recipient of the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
References
- 1.Williams GM, Weisburger JH. Chemical carcinogenesis. In: Amdur MO, Doull J, Klaassen CD, editors. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 4th edition. Pergamon Press; New York, NY: 1991. pp. 127–132. [Google Scholar]
- 2.Abu-Surrah AS, Kettunen M. Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Curr Med Chem. 2006;13:1337–1357. doi: 10.2174/092986706776872970. [DOI] [PubMed] [Google Scholar]
- 3.Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara K, Bando T, Sasaki S, Sakakibara Y, Minoshima M, Sugiyama H. Antitumor activity of sequence-specific alkylating agents: pyrolle-imidazole CBI conjugates with indole linker. Cancer Sci. 2006;97:219–225. doi: 10.1111/j.1349-7006.2006.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll DC, List AF, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48–51. doi: 10.7326/0003-4819-105-1-48. [DOI] [PubMed] [Google Scholar]
- 6.Huddart RA, Norman A, Shahidi M, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 7.Vaughn DJ, Palmer SC, Carver JR, Jacobs LA, Mohler ER. Cardiovascular risk in long-term survivors of testicular cancer. Cancer. 2008;112:1949–1953. doi: 10.1002/cncr.23389. [DOI] [PubMed] [Google Scholar]
- 8.Nuver J, Smit AJ, van der Meer J, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23:9130–9137. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- 9.Kirchmair R, Walter DH, Ii M, et al. Antiangiogenesis mediates cisplatin-induced peripheral neuropathy: attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth. Circulation. 2005;111:2662–2670. doi: 10.1161/CIRCULATIONAHA.104.470849. [DOI] [PubMed] [Google Scholar]
- 10.Kohn S, Fradis M, Ben-David J, Zidan J, Robinson E. Nephrotoxicity of combined treatment with cisplatin and gentamicin in the guinea pig: glomerular injury findings. Ultrastruct Pathol. 2002;26:371–382. doi: 10.1080/01913120290104683. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Han J, Cui P, et al. Cisplatin up-regulates ICAM-1 expression in endothelial cell via a NF-kappaB dependent pathway. Cancer Sci. 2008;99:391–397. doi: 10.1111/j.1349-7006.2008.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aromolaran AS, Zima AV, Blatter LA. Role of glycolytically generated ATP for CaMKII-mediated regulation of intracellular Ca2+ signaling in bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C106–C118. doi: 10.1152/ajpcell.00543.2006. [DOI] [PubMed] [Google Scholar]
- 13.Mellander S. Contribution of small vessel tone to the regulation of blood volume and formation of oedema. Proc R Soc Med. 1968;61:55–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Suga H. Physiological interpretation of negative circumferential tension in vascular walls. Jpn Heart J. 1991;32:473–480. doi: 10.1536/ihj.32.473. [DOI] [PubMed] [Google Scholar]
- 15.Nichols WV, O’Rourke MF, editors. McDonald’s Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. 5th edition. Hodder Arnold; London: 2005. pp. 344–347. [Google Scholar]
- 16.Struijker Boudier HAJ. Vascular growth and hypertension. In: Swales JD, editor. Textbook of Hypertension. Blackwell Scientific; Oxford: 1994. pp. 200–213. [Google Scholar]
- 17.Haugnes HS, Aass N, Fossa SD, et al. Predicted cardiovascular mortality and reported cardiovascular morbidity in testicular cancer survivors. J Cancer Surviv. 2008;2:128–137. doi: 10.1007/s11764-008-0054-1. [DOI] [PubMed] [Google Scholar]
- 18.Sagstuen H, Aass N, Fossa SD, et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. 2005;23:4980–4990. doi: 10.1200/JCO.2005.06.882. [DOI] [PubMed] [Google Scholar]
- 19.Gong Z, Yang J, Yang M, et al. Benzo(a)pyrene inhibits expression of inducible heat shock protein 70 in vascular endothelial cells. Toxicol Lett. 2006;166:229–236. doi: 10.1016/j.toxlet.2006.07.307. [DOI] [PubMed] [Google Scholar]
- 20.Kamata K, Kojima S. Characteristics of contractile responses of aorta to norepinephrine in db/db mice. Res Commun Mol Pathol Pharmacol. 1997;96:319–328. [PubMed] [Google Scholar]
- 21.Qian LB, Wang HP, Qiu WL, Huang H, Bruce IC, Xia Q. Interleukin-2 protects against endothelial dysfunction induced by high glucose levels in rats. Vascul Pharmacol. 2006;45:374–382. doi: 10.1016/j.vph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Ru XC, Qian LB, Gao Q, Li YF, Bruce IC, Xia Q. Alcohol induces relaxation of rat thoracic aorta and mesenteric arterial bed. Alcohol Alcohol. 2008;43:537–543. doi: 10.1093/alcalc/agn042. [DOI] [PubMed] [Google Scholar]
- 23.Sheng JZ, Wang D, Braun AP. DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate detects impairment of agonist-stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: reversal by vitamin C and L-sepiapterin. J Pharmacol Exp Ther. 2005;315:931–940. doi: 10.1124/jpet.105.087932. [DOI] [PubMed] [Google Scholar]
- 24.Luscher TF, Tanner FC. Endothelial regulation of vascular tone and growth. Am J Hypertens. 1993;6:283S–293S. doi: 10.1093/ajh/6.7.283s. [DOI] [PubMed] [Google Scholar]
- 25.Ishioka J, Fujii Y, Kageyama Y, Fukuda H, Higashi Y, Kihara K. Cardiovascular events in survivors of high-dose chemotherapy for germ cell tumors. Int J Urol. 2008;15:642–645. doi: 10.1111/j.1442-2042.2008.02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga T, Soejima H, Sugamura K, et al. Acute myocardial infarction induced by cisplatin-based combination chemotherapy for malignant melanoma: a case report. J Cardiol. 2006;47:191–195. [PubMed] [Google Scholar]
- 27.Oh JH, Baum DD, Pham S, et al. Long-term complications of platinum-based chemotherapy in testicular cancer survivors. Med Oncol. 2007;24:175–181. doi: 10.1007/BF02698037. [DOI] [PubMed] [Google Scholar]
- 28.Etgen T, Weidenhofer G, Kubin T. Cisplatin-associated occlusion of the internal carotid artery. Onkologie. 2009;32:754–757. doi: 10.1159/000252798. [DOI] [PubMed] [Google Scholar]
- 29.Grenader T, Shavit L, Ospovat I, Gutfeld O, Peretz T. Aortic occlusion in patients treated with cisplatin-based chemotherapy. Mt Sinai J Med. 2006;73:810–812. [PubMed] [Google Scholar]
- 30.Ghosh N, Chow CM, Korley V, Chisholm R. An unusual case of chronic coronary artery dissection: did cisplatin play a role? Can J Cardiol. 2008;24:795–797. doi: 10.1016/s0828-282x(08)70688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabagh M, Jalali P, Konttinen YT, Sarkomaa P. Distribution of shear stress over smooth muscle cells in deformable arterial wall. Med Biol Eng Comput. 2008;46:649–657. doi: 10.1007/s11517-008-0338-7. [DOI] [PubMed] [Google Scholar]
- 32.Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–316. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


