Abstract
Erythroblast transformation-specific-related gene (ERG) fusions, the most common and validated prostate cancer (CaP) genome alteration, result in alterations in the expression of the ERG oncoprotein. Significantly lower frequencies of ERG have been reported in tumors of African American (AA) in comparison to Caucasian American (CA) men. Building on our preliminary observations, this study has focused on the increased association of the ERG-negative status with higher-grade prostate tumors in AA men. Representative whole-mount prostate sections from a matched cohort of 63 AA and 63 CA men with Gleason scores of 4+3 and those with Gleason scores of 8–10 were analyzed for ERG oncoprotein by immunohistochemistry. The striking finding of this study was that ERG expression was 3 times more likely to be present in the higher-grade index tumors of CA men compared to AA men (31 of 63 vs. 10 of 63 patients, respectively; P<0.0001). Although the mechanisms underlying these differences have not been elucidated, the present study along with our previous observations underscores that ERG typing may enhance the understanding of ethnic differences and future targeted therapy of CaP.
Keywords: prostate cancer, erythroblast transformation-specific-related gene, transmembrane protease serine 2 gene fusion, race, ethnicity
Introduction
African American (AA) men exhibit the highest incidence and mortality from prostate cancer (CaP) compared to other races in the United States (1). While socioeconomic factors contribute to CaP outcomes among men of different ethnicities (2), it has also been recognized that AA men have more advanced CaP at diagnosis (3). Although there remains controversy over the role of biological differences between prostate tumors in AA and Caucasian American (CA) men, emerging data suggest the presence of differences in somatic and germline alterations (4,5).
One of the most common and validated CaP genome alterations represents fusion of the protein-coding sequences of erythroblast transformation-specific (ETS)-related transcription factors [predominantly ETS-related gene (ERG)] with promoter sequences of androgen-regulated genes [predominantly transmembrane protease serine 2 (TMPRSS2) gene] (6–9). The highly prevalent ERG fusions, present in over half of all CaPs in Western countries, result in androgen-dependent and prostate tumor-specific expression of the ERG fusion transcripts and a near-full-length ERG protein with a 32-amino acid deletion at the amino terminus (6–9). Evaluations of the ERG alterations at the genomic, transcriptional and protein levels have continued to suggest lower frequencies of ERG in AA CaP in comparison to CA CaP (10–13). Almost complete concordance between the detection of ERG gene fusions by fluorescence in situ hybridization and ERG protein detection by immunohistochemistry (IHC), has significantly accelerated the evaluation of the ERG protein as the surrogate of this common CaP genome alteration in pathological specimens (14–17). Studies from our and other groups indicate that the overall frequency of ERG alterations in CaP varies significantly among different ethnicities: It is highest in CA, intermediate in AA and lowest in Asian CaP patients (4,5). Our recent evaluations of representative whole-mount prostate sections from a matched cohort of 91 CA and 91 AA men demonstrated a significant difference (P<0.0001) in the prevalence of the ERG oncoprotein in index tumors of CA (63%) and AA (29%) men (13). Our preliminary data also suggested that the majority of higher-grade tumors in AA patients may be ERG-negative (13). The present study focuses on comparative evaluations of ERG in higher-grade tumors in CA and AA CaP patients.
Materials and methods
Specimens and study criteria
The Center for Prostate Disease Research database was queried to identify CaP patients who were enrolled in the Institutional Review Board-approved protocol from Walter Reed National Military Medical Center. The CaP patients underwent radical prostatectomy (RP) between 1994 and 2011. Archived clinicopathological data were evaluated for 1,304 patients who self-identified their race. The study sample was powered for ERG evaluation. A total of 63 AA and 63 CA patients matched for age at RP and Gleason scores of 8–10 and 4+3 of prostate tumors met the study inclusion criteria.
IHC analyses of the ERG
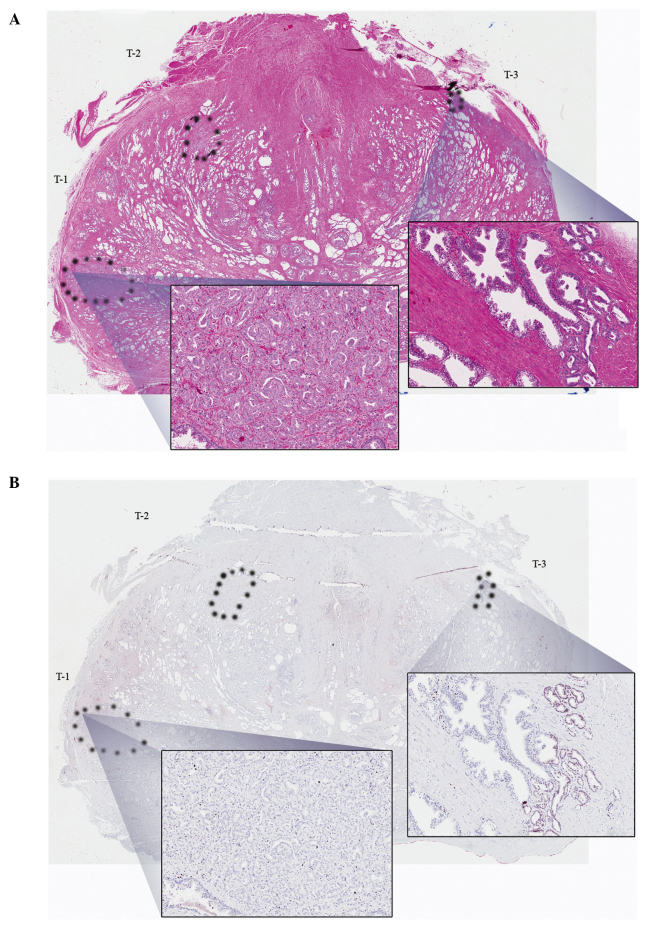
Representative whole-mount 4-μm cross-sections from each prostatectomy specimen were selected. The index tumor consisting of the largest tumor with the highest grade was identified along with all other tumor foci in each specimen. Specimens for ERG IHC were cut and stained with a highly specific anti-ERG monoclonal antibody (clone 9FY; Biocare Medical Inc., Concord, CA, USA) as previously described (13,14). The index tumor and all other tumors were classified as ERG-positive (any number of tumor cells positive) or negative (all tumor cells negative). Fig. 1 provides representative examples.
Figure 1.
Representative images of whole-mount sections analyzed by hematoxylin and eosin (H&E) staining, as well as ERG immunohistochemistry (IHC), with view fields enlarged (magnification, ×20). (A) H&E staining with tumor foci denoted by dotted outline. The higher power insert from T1 (index tumor) contains poorly differentiated (Gleason 4) disease. The T3 (tertiary tumor) insert on the right is well-differentiated (Gleason 3). (B) Analogous section with ERG IHC staining, in which the nuclear staining for ERG is negative in T1 and focally positive in T3.
Sample size and statistical analysis
Categorical patient clinicopathological data were described across race using frequencies and percentages. Continuously measured variables were compared using measures of central tendency, namely mean, median and standard deviation. The Chi-square test was used to compare the distribution of the clinicopathological characteristics between the CA and AA cohorts, as well as IHC status (positive vs. negative) for the AA vs. CA cohorts. Biochemical recurrence (BCR), was defined as 2 consecutive prostate-specific antigen (PSA) measurements of ≥0.2 ng/ml at least 8 weeks post-RP. Unadjusted Kaplan-Meier estimate curves and multivariable Cox proportion hazards analysis were used to evaluate the prognostic significance of ERG oncoprotein on BCR-free survival. The log-rank test was used to test for differences in the Kaplan-Meier curves by ERG status. P<0.05 was considered to indicate a statistically significant difference. All data analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Clinicopathological characteristics
The study cohort of 126 patients (63 CA and 63 AA) did not exhibit significant differences in clinicopathological variables across race (Table I). The majority of the tumors had Gleason scores of 8–10 and pT3 disease (Table I). This patient cohort provided an 80% power to detect a 25–30% absolute difference across race for ERG positivity (two-sided P-value=0.05).
Table I.
Clinicopathological characteristics of all patients and breakdown across racial cohorts.
| Variables | All (n=126) | AA (n=63) | CA (n=63) | P-value |
|---|---|---|---|---|
| Age at RP, years | 0.5887 | |||
| Mean (SD) | 60.4 (7.1) | 60.1 (7.2) | 60.8 (7.1) | |
| PSA at diagnosis, ng/ml | 0.2718 | |||
| Median (range) | 6.7 (0.9–5,065) | 6.9 (1–5,065) | 6.5 (0.9–23.4) | |
| Pathological T stage | 0.2008 | |||
| pT2 | 49 (38.9) | 28 (44.4) | 21 (33.3) | |
| pT3 or higher | 77 (61.1) | 35 (55.6) | 42 (66.7) | |
| Gleason sum | 0.8538 | |||
| 4+3 | 47 (37.3) | 24 (38.1) | 23 (36.5) | |
| 8–10 | 79 (62.7) | 39 (61.9) | 40 (63.5) | |
| ECE | 0.6855 | |||
| Negative | 49 (43.0) | 26 (44.8) | 23 (41.1) | |
| Positive | 65 (57.0) | 32 (55.2) | 33 (58.9) | |
| SV | 0.2496 | |||
| Negative | 91 (72.8) | 48 (77.4) | 43 (68.2) | |
| Positive | 34 (27.2) | 14 (22.6) | 20 (31.8) | |
| Margin status | 0.3230 | |||
| Negative | 83 (69.2) | 44 (73.3) | 39 (65.0) | |
| Positive | 37 (30.8) | 16 (26.7) | 21 (35.0) |
AA, African American; CA, Caucasian American; RP, radical prostatectomy; SD, standard deviation; PSA, prostate-specific antigen; ECE, extracapsular extension; and SV, seminal vesicles invasion.
ERG status by race and grade
Overall, 46% of the patients had ≥1 ERG-positive tumor foci. The index tumor was ERG-positive in 41 of the 126 patients. In CA men, the index tumor was ERG-positive in 31 of 63 patients (49%), which was significantly higher compared to 10 of 63 patients (16%) in AA men (P<0.0001) (Table II). CA men were also significantly more likely to have any tumor focus positive for ERG compared to AA men (59 vs. 41%, P=0.0042, data not shown). ERG-positive status was significantly lower in higher-grade (16%) compared to lower-grade (34%) index tumors of AA men (P=0.04), which was not the case in CA men (Table II).
Table II.
Prevalence of ERG positivity across race in high-grade (Gleason score, 8–10 and 4+3) index tumors (upper lane, present study) and in low-grade (Gleason score, 6) index tumors (lower lane).
| ERG status/grade | Total | CA | AA | P-value |
|---|---|---|---|---|
| ERG+/high-grade | 33% (41/126) | 49% (31/63) | 16% (10/63) | <0.0001 |
| ERG+/low-gradea | 52% (35/67) | 69% (24/35) | 34% (11/32) | 0.0051 |
| P-value | 0.0642 | 0.0400 |
Data obtained from Rosen et al (17).
ERG, erythroblast transformation-specific-related gene; CA, Caucasian American; AA, African American.
ERG as a predictor of recurrence
ERG was not found to be an independent predictor of BCR in this cohort (Table III). Pathological stage was an independent predictor of BCR [hazard ratio (HR)=5.749, P=0.0043] and there was a trend towards higher serum PSA levels at diagnosis (HR=1.289, P=0.0564) (Table III).
Table III.
Univariable and multivariable Cox proportional hazard models for the prediction of biochemical recurrence by using ERG IHC status and clinicopathological variables.
| Univariable Cox models | Multivariable Cox model | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at RP | 1.011 (0.965–1.059) | 0.6481 | ||
| Log PSA | 1.352 (1.062–1.723) | 0.0145 | 1.289 (0.993–1.674) | 0.0564 |
| Race/ethnicity | ||||
| CA | 1 | |||
| AA | 0.705 (0.371–1.340) | 0.2866 | ||
| Pathological T stage | ||||
| pT2 | 1 | 1 | ||
| pT3 or higher | 4.737 (1.972–11.379) | 0.0005 | 5.749 (1.729–19.115) | 0.0043 |
| Gleason sum | ||||
| 4+3 | 1 | 1 | ||
| 8–10 | 1.858 (0.879–3.928) | 0.1048 | 1.272 (0.545–2.968) | 0.5777 |
| SV | ||||
| Negative | 1 | 1 | ||
| Positive | 2.240 (1.183–4.241) | 0.0133 | 1.159 (0.571–2.354) | 0.6827 |
| Margin status | ||||
| Negative | 1 | 1 | ||
| Positive | 2.276 (1.193–4.342) | 0.0126 | 0.890 (0.427–1.855) | 0.7562 |
| ERG IHC status | ||||
| ERG− | 1 | |||
| ERG+ | 1.366 (0.704–2.652) | 0.3564 | ||
ERG, erythroblast transformation-specific-related gene; IHC, immunohistochemistry HR, hazard ratio; CI, confidence interval; RP, radical prostatectomy; PSA, prostate-specific antigen; CA, Caucasian American; AA, African American; SV, seminal vesicles. P-values in bold print denote statistically significant differences (<0,05).
Discussion
CaP is a multifocal, heterogeneous disease with a variable clinical course. Two cancers of the same grade and stage do not necessarily exhibit similar progression characteristics and CaP does not behave equally across age groups or ethnicities (1–5,18). Molecular alterations are likely involved in the ethnic differences of CaP and we sought to describe the prevalence of ERG in higher-grade disease in AA and CA men with a focus on index tumors. High Gleason scores are recognized as surrogates of aggressive disease and are independently predictive of BCR (19).
Studies from our and other groups have demonstrated significantly lower frequencies of ERG in CaP of AA men in comparison to that of CA men (5,12,13). Our previous preliminary observation indicated more significant differences in ERG in high-grade tumors of AA compared to those of CA men. This adequately powered study addressed this issue by using matched cohorts of CA and AA CaP specimens. A striking finding of this study was that ERG was significantly (3 times) more likely to be present in the higher-grade index tumors of CA men compared to those of AA men (31 of 63 vs. 10 of 63 patients, respectively; P<0.0001). Thus, although ERG may be the most common oncogenic alteration in CA men, it does not appear to be the case in AA men, particularly not in those with higher-grade CaP. The biological basis underlying this observation remains to be elucidated; these results nonetheless support the association of an ERG-negative status with more aggressive disease in AA men. These data also suggest that ERG may not be the primary driver of higher-grade CaP in AA men.
While there is a general agreement that ERG is a highly prevalent and early oncogenic alteration in CaP and it defines a large subtype of prostate tumors, it is also important to recognize that there are significant proportions of ERG-negative prostate tumors for which a common driver gene alteration is not known. Emerging data from the present and other studies underscore the higher prevalence of the ERG-negative subtype of CaP in AA and Asian men (4,5). The higher frequency of high-grade ERG-negative tumors in AA men likely reflects the presence of distinct genomic alterations associated with the initiation and progression of this subtype of CaP.
The utility of ERG detection in CaP is apparent in the diagnostic setting and ERG typing of tumors may also be of significant value for biological classification and future targeted therapy. However, the utility of ERG in assessing CaP progression remains controversial, which may be attributed to multifactorial causes, including specific patient cohort, disease stage and assay type (8,17). In this high-grade cohort, the ERG protein status was not found to be correlated with disease progression.
In summary, this study provides important observations on the predominance of ERG-negative high-grade CaP in AA men. The biological implications of these observations are far-reaching, particularly in delineating biological typing and future treatment of CaP tumors in men of different ethnicities.
Acknowledgements
The views expressed in this review do not reflect any official policy of the Department of the Army, Department of Defense, or the US Government. We wish to thank Mr. Stephen Doyle for the artwork.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz K, Powell IJ, Underwood W, III, et al. Interplay of race, socioeconomic status and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–1302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams H, Powell IJ. Epidemiology, pathology and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol Biol. 2009;472:439–453. doi: 10.1007/978-1-60327-492-0_21. [DOI] [PubMed] [Google Scholar]
- 4.Martin DN, Starks AM, Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25:235–241. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell J, Petrovics G, McLeod DG, Srivastava S. Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. Int J Mol Sci. 2013;14:15510–15531. doi: 10.3390/ijms140815510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 7.Petrovics G, Liu A, Shaheduzzaman S, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–3852. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 8.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbieri CE, Bangma CH, Bjartell A, et al. The mutational landscape of prostate cancer. Eur Urol. 2013;64:567–576. doi: 10.1016/j.eururo.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Dobi A, Sreenath T, et al. Delineation of TMPRSS2-ERG splice variants in prostate cancer. Clin Cancer Res. 2008;14:4719–4725. doi: 10.1158/1078-0432.CCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 11.Rice KR, Chen Y, Ali A, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–1576. doi: 10.1158/1078-0432.CCR-09-2191. [DOI] [PubMed] [Google Scholar]
- 12.Magi-Galluzzi C, Tsusuki T, Elson P, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 13.Rosen P, Pfister D, Young D, et al. Differences in frequency of ERG oncoprotein expression between index tumors of Caucasian and African American patients with prostate cancer. Urology. 2012;80:749–753. doi: 10.1016/j.urology.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furusato B, Tan SH, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13:228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun M, Goltz D, Shaikhibrahim Z, et al. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer - a comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis. 2012;15:165–169. doi: 10.1038/pcan.2011.67. [DOI] [PubMed] [Google Scholar]
- 17.Rosen P, Sesterhenn IA, Brassell SA, et al. Clinical potential of the ERG oncoprotein in prostate cancer. Nat Rev Urol. 2012;9:131–137. doi: 10.1038/nrurol.2012.10. [DOI] [PubMed] [Google Scholar]
- 18.Brassell SA, Rice KR, Parker PM, et al. Prostate cancer in men 70 years old or older, indolent or aggressive: clinicopathological analysis and outcomes. J Urol. 2011;185:132–137. doi: 10.1016/j.juro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Brimo F, Montironi R, Egevad L, et al. Contemporary grading for prostate cancer: implications for patient care. Eur Urol. 2013;63:892–901. doi: 10.1016/j.eururo.2012.10.015. [DOI] [PubMed] [Google Scholar]