Abstract
There is currently no standard first-line regimen for patients with extranodal natural killer (NK)/T-cell lymphoma (ENKTCL). In this study, we investigated the efficacy and toxicity of gemcitabine (GEM) combined with oxaliplatin (L-OHP), L-asparaginase (L-ASP) and dexamethasone (DXM) (GOLD regimen) as a systemic treatment scheme for newly-diagnosed ENKTCL cases. A total of 55 patients were recruited at the Henan Province Cancer Hospital and the Cancer Center of Sun Yat-sen University between May, 2008 and August, 2012. The GOLD regimen included a 14-day treatment cycle with GEM (1,000 mg/m2) on day 1, L-OHP (100 mg/m2) on day 1, L-ASP (10,000 U/m2) on days 1–5 and DXM (20 mg b.i.d.) on days 1–4. The response rate, survival rate and treatment toxicity were analyzed. The overall response rate was 91% (48/55) with a complete response in 62% (34/55) and a partial response in 29% (15/55) of the patients. For all patients, the 1-, 2- and 3-year progression-free survival (PFS) rate was 86, 64 and 57% and the overall survival (OS) 91, 80 and 74%, respectively. The 1-year PFS in patients with stage I/II vs. those with III/IV disease was 87 vs. 66% (P<0.001) and the 1-year OS was 98 vs. 75%, respectively (P<0.001). No chemotherapy-related mortality or severe complications were recorded. In conclusion, the GOLD regimen was found to be highly effective and safe for the treatment of patients with newly-diagnosed ENKTCL.
Keywords: extranodal natural killer/T-cell lymphoma, chemotherapy, GOLD regimen, efficacy, toxicity
Introduction
In the 2008 World Health Organization (WHO) classification system for malignant lymphoma, extranodal natural killer (NK)/T-cell lymphomas (ENKTCL) are considered to be a distinct entity. ENKTCLs are significantly more common in Asia and Latin America compared to Europe and North America (1,2). We collected a total of 4,801 cases with lymphoma in South China and 963 cases fulfilled the diagnostic criteria of mature T-cell or NK/T-cell lymphoma and accounted for 20.1% of all cases of lymphoma encountered during the same period. Of these cases, 281 (29.2%) were diagnosed as ENKTCL, nasal type (3). Radiotherapy was the treatment selection for early-stage nasal ENKTCL. However, distant and local relapse often occurred and the 5-year overall survival (OS) was low (29.8–66%) (4–7). An optimal treatment for ENKTCL has not yet been established, particularly for advanced-stage disease. The low treatment efficacy may be mainly attributed to the fact that this disease is resistant to several chemotherapeutic agents, due to the expression of P-glycoprotein (P-gp) (8,9). Therefore, investigations have been focused on improving the efficacy of chemotherapy and reducing the risk of disease recurrence. It has been reported that L-asparaginase (L-ASP) and gemcitabine (GEM) may achieve satisfactory response and survival rates, as they are not regulated by P-gp in relapsed and refractory ENKTCL (10–14). GEM combined with oxaliplatin (L-OHP) has also been verified to be an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma, with acceptable toxicity (15–17). Nowak-Göttl et al (18) reported that the use of dexamethasone (DXM) instead of prednisone significantly reduced the incidence of venous thromboembolism, which is a common adverse reaction of L-ASP. Therefore, we investigated the efficacy and toxicity of GEM combined with L-OHP, L-ASP and DXM (GOLD regimen) in patients with newly-diagnosed ENKTCL.
Patients and methods
Patient eligibility
A total of 55 patients were recruited at the Henan Province Cancer Hospital and the Cancer Center of Sun Yat-sen University between May, 2008 and August, 2012. Only patients aged >15 years were considered to be eligible for this study. Additional eligibility criteria included ENKTCL histologically confirmed by biopsy, with measurable lesions radiologically; normal hepatic and renal function; and adequate bone marrow reserve without previous treatment. Patients were excluded if they had severe organ dysfunction or concomitant malignant tumors. The diagnosis was established according to the WHO classification criteria. Aggressive NK/T-cell leukemia was excluded. The lesions were staged according to the Ann Arbor staging system. Additional examinations included complete blood count, serum biochemistry and lactate dehydrogenase (LDH) levels (19); in addition, the Eastern Cooperative Oncology Group performance status (ECOG PS) and presence of hemophagocytic syndrome (HPS) and/or B symptoms were assessed. All the patients underwent bone marrow aspiration and biopsy, computed tomographic scanning of the involved organ(s), chest and abdomen and nasal endoscopic examination.
Informed consent was obtained from all the subjects and the study was approved by the Medical Ethics Committee of the Henan Province Cancer Hospital.
Chemotherapeutic regimen
This treatment scheme was a 14-day cycle and included four drugs, namely GEM, L-OHP, L-ASP and DXM. The patients received GEM (1,000 mg/m2) on day 1, L-OHP (100 mg/m2) on day 1, L-ASP (Changzhou Qianhong Bio-pharma Co., Ltd, Changzhou, China) (10,000 U/m2) on days 1–5 and DXM (20 mg b.i.d.) on days 1–4. An intradermal test was required prior to the administration of L-ASP. Every patient received chemotherapy for ≥4 cycles.
All the patients with Ann Arbor stage I/II disease received involved-field radiation (IFRT) following chemotherapy. The decision was made at the discretion of the treating physician. Three-dimensional conformal radiotherapy was performed with a linear accelerator at a daily fraction of 2.0 Gy. The total dose was ≥50 Gy.
Response to treatment and toxicity assessment
Tumor response was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), according to the report of an International Workshop to standardize the response criteria for non-Hodgkin’s lymphomas. All the adverse reactions were evaluated according to the National Cancer Institute Common Toxicity Criteria, version 3 (20,21).
Statistical analysis
OS was calculated from the date of treatment initiation to the date of the last follow-up or death from any cause. Progression-free survival (PFS) was measured from the date of diagnosis to relapse, death or last follow-up visit. Survival was estimated using Kaplan-Meier curves and compared by the log-rank test. P<0.05 with two-side test was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 55 patients were recruited from two centers. The median age was 41 years (range, 15–69 years) and the patients were mainly young adults. The male:female ratio was 3:1. The majority of the patients had a good ECOG PS and international prognostic index and early-stage disease by the Ann Arbor staging system. B symptoms were present in 58% of the patients at diagnosis and HPS occurred in 18% patients. The majority of the lesions (87%) were located in the nasal cavity or nasopharynx (Table I).
Table I.
Patient characteristics (n=55).
| Characteristics | Patient no. | Percentage |
|---|---|---|
| Gender | ||
| Male | 41 | 75 |
| Female | 14 | 25 |
| Age (years) | ||
| 14–20 | 6 | 10 |
| 21–60 | 41 | 75 |
| 60–80 | 8 | 15 |
| ECOG PS | ||
| 0 | 42 | 76 |
| 1 | 9 | 16 |
| 2 | 4 | 8 |
| B symptoms | ||
| Yes | 32 | 58 |
| No | 23 | 42 |
| Ann Arbor stage | ||
| I/II | 45 | 82 |
| III/IV | 10 | 18 |
| IPI | ||
| 1 | 29 | 53 |
| 2 | 16 | 29 |
| 3 | 8 | 15 |
| 4 | 2 | 3 |
| LDH level | ||
| Elevated | 12 | 22 |
| Normal | 43 | 78 |
| HPS | ||
| Yes | 10 | 18 |
| No | 45 | 82 |
| Involved sites | ||
| Nasal cavity/nasopharynx | 48 | 87 |
| Other | 7 | 13 |
ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; LDH, lactate dehydrogenase; HPS, hemophagocytic syndrome.
Response to treatment and survival outcomes
A total of 48 patients (91%) responded to the GOLD regimen, with 34 cases (62%) achieving CR and 16 (29%) a PR, whereas 2 patients achieved SD and 3 developed PD (Table II). Of the patients with CR, 25 received radiotherapy sequentially. Of the patients with PR, 10 were treated with radiotherapy, of whom 6 achieved a CR, 3 a PR and 1 exhibited PD. Two patients with SD received radiotherapy, with one patient achieving a CR and the other SD. Three patients with PD refused the second-line treatment.
Table II.
Response rate after the GOLD regimen.
| Type of response | Patient no. | Percentage |
|---|---|---|
| Complete response | 34 | 62 |
| Partial response | 16 | 29 |
| Stable disease | 2 | 4 |
| Progressive disease | 3 | 5 |
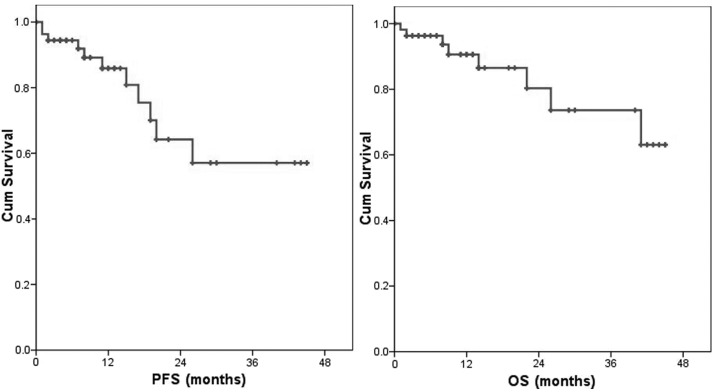
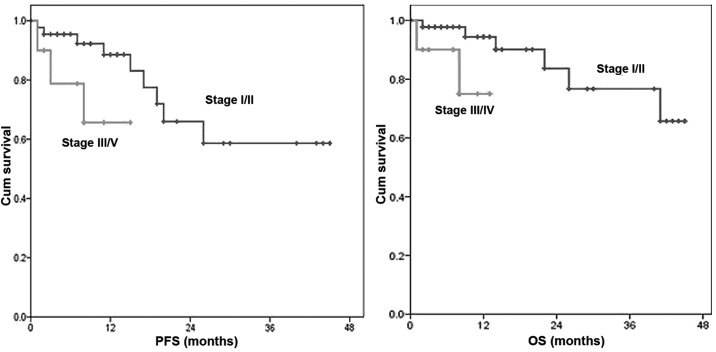
For all patients, the 1-, 2- and 3-year PFS was 86, 64 and 57% and the OS was 91, 80 and 74%, respectively (Fig. 1). Eleven patients were relapse. Eight patients presented with systemic recurrence and 3 patients with local recurrence. The 1-year PFS in patients with stage I/II vs. those with III/IV disease was 87 vs. 66% (P<0.001) and the 1-year OS was 98 vs. 75%, respectively (P<0.001) (Fig. 2).
Figure 1.
Progression-free survival (PFS) and overall survival (OS) of all patients. The 1-, 2- and 3-year PFS rate was 86, 64 and 57% and the OS rate was 91, 80 and 74%. Cum, cumulative.
Figure 2.
Comparison of progression-free survival (PFS) and overall survival (OS) between patients with stage I/II and III/IV disease. The 1-year PFS in patients with stage I/II vs. those with III/IV disease was 87 vs. 66% (P<0.001) and the 1-year OS was 98 vs. 75%, respectively (P<0.001). Cum, cumulative.
Toxicity
In our study, myelosuppression was observed in 16 patients, with grade 1/2 neutropenia and/or thrombocytopenia in 5 cases, grade 3/4 neutropenia and/or thrombocytopenia in 9 cases and febrile neutropenia in 2 cases. Hepatic dysfunction was observed in 2 patients. The levels of aminotransferases were >200 U/l and returned to normal following treatment with liver protectants. Mild coagulation abnormalities were observed in 19 patients, reflected by a prolongation of the activated partial thromboplastin time and D-dimer elevation. One patient with elevated D-dimers and decreased fibrinogen levels prior to treatment returned to normal levels after chemotherapy and treatment with low-molecular weight heparin (LMWH). Another patient developed left lower limb deep venous thromboembolism and revascularization was achieved following treatment with urokinase and LMWH. The consumption of fatty foods was prohibited to lower the risk of pancreatitis and no such events were reported. All the patients exhibited some peripheral nervous system toxicity and grade 1/2 gastrointestinal reactions, such as anorexia and vomiting. There were no hypersensitivity reactions or chemotherapy-related mortality.
Discussion
It was reported that the median OS and PFS for patients with ENKTCL was only 7.8 and 5.8 months, respectively. According to the International Peripheral T-cell Lymphoma Project (22), ENKTCL exhibited the worst survival time among all peripheral T-cell lymphoma categories (19). The authors of that study concluded that radiotherapy was efficient for localized nasal disease, whereas extranasal disease appeared to be less amenable. For patients with advanced-stage nasal disease and those with extranasal disease, the survival was dismal (<10%). In our study, we investigated the efficacy and safety of the GOLD regimen for patients with ENKTCL. The overall response rate (ORR) was 91% (CR, 62%; and PR, 29%). The 1-, 2- and 3-year PFS and OS were 86, 64 and 57% and 91, 80 and 74%, respectively. The median follow-up for all the patients was 22 months and the median PFS and OS were not attained. Wang et al (8) reported that 67% of the patients were positive for P-gp expression and the CR rate achieved in P-gp-positive patients was significantly lower compared to that in P-gp-negative patients (20 vs. 60%, respectively; P=0.045) when treated with a cyclophosphamide, adriamycin, vincristine and prednisone (CHOP)-like regimen. As adriamycin and vincristine are substrates for P-gp, Yong et al (23) reported that ENKTCL patients treated with CHOP regimen followed by IFRT exhibited a CR rate of only 27%. It is crucial to attain CR in patients with aggressive lymphoma. Several studies demonstrated that a CR with induction chemotherapy followed by radiotherapy may prolong survival in ENKTCL cases (24–26). Therefore, efforts have been focusing on designing novel chemotherapeutic regimens, effective in achieving a high CR rate. Nagafuji et al (12) first reported the case of a 60-year-old Japanese woman with stage IV ENKTCL who was treated with L-ASP and achieved a CR without disease progression for 18 months. Yamaguchi et al (27) conducted a phase II study of SMILE chemotherapy for ENKTCL and reported an ORR and CR of 79 and 45%, respectively, with a 1-year survival rate of only 55%. These results were inferior to those achieved with our regimen, possibly due to the fact that all the patients enrolled in the Yamaguchi et al study had relapsed or refractory disease. Of note, the SMILE regimen comprised the steroid dexamethasone, the multidrug resistance-unrelated agents methotrexate, ifosfamide and L-asparaginase, and etoposide, which was found to be efficient in vitro and in vivo against Epstein-Barr virus-associated lymphoproliferative disorders. Further analysis of that study found that the treatment-related toxicity was severe, with grade 4 neutropenia and infection in 92 and 61% of the cases, respectively (27). Considering the highly aggressive nature of ENKTCL, we designed a scheme of 14 days per cycle. We found that toxicity was alleviated when not using GEM on the eighth day. The safety of the GOLD regimen was also satisfactory. Grade 3/4 hematological toxicity was observed in only 16% of the cases (9/55). Leucopenia and thrombocytopenia were the major manifestations and liver injury occurred in only 2 patients. All the adverse reactions were manageable with symptomatic treatment and there were no severe complications. In the 2013 ASCO Annual Meeting, Lin et al (24) reported the results of a phase II/III study on ENKTCL treated with CID-ATT, with a 1- and 3-year OS of 80.2 and 68% and a PFS of 74.9 and 60.5%, respectively. The survival results of that study were similar to ours. The CID-ATT regimen includes administration of CHOP-B, ifosfamide + methotraxate + etoposide + dexamethasone (IMVD), and dexamethasone + cytarabine + cisplatin, in an alternating sequence, for a total of 6 courses (2 cycles). Further analysis in our study revealed significant differences in OS and PFS between early and advanced stages. Several previous studies had reported that Ann Arbor stage was a prognostic factor for OS and/or PFS (24,28,29), although there remain controversies regarding the Ann Arbor staging system.
In conclusion, our study demonstrated that the GOLD regimen was highly effective and safe for patients with newly-diagnosed ENKTCL. However, the short follow-up and the retrospective design of the study may have affected the results to a certain extent. Therefore, a prospective trial should be conducted to further evaluate the efficacy and safety of the GOLD regimen for the treatment of ENKTCL patients.
Acknowledgements
This study was supported by grants from the National Natural Foundation of China (no. 81101797) and the Project of Henan Health Department (no. 2011020158).
Abbreviations
- ENKTCL
extranodal NK/T-cell lymphoma
- GEM
gemcitabine
- L-OHP
oxaliplatin
- L-ASP
L-asparaginase
- DXM
dexamethasone
- LDH
lactate dehydrogenase
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HPS
hemophagocytic syndrome
- IFRT
involved-field radiation therapy
- LMWH
low-molecular weight heparin
References
- 1.Suzuki R, Takeuchi K, Ohshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol. 2008;26:66–72. doi: 10.1002/hon.847. [DOI] [PubMed] [Google Scholar]
- 2.Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007;139:532–544. doi: 10.1111/j.1365-2141.2007.06835.x. [DOI] [PubMed] [Google Scholar]
- 3.Liang Q, Ye ZY, Su ZL, et al. Clinicopathologic study of 963 cases of mature T-cell and natural killer/T-cell lymphoma with respect to 2008 WHO classification of lymphoid neoplasms. Chin J Pathol. 2010;39:291–295. (In Chinese) [PubMed] [Google Scholar]
- 4.Li YX, Wang H, Jin J, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1809–1815. doi: 10.1016/j.ijrobp.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Li YX, Yao B, Jin J, Wang WH, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 6.Isobe K, Uno T, Tamaru J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106:609–615. doi: 10.1002/cncr.21656. [DOI] [PubMed] [Google Scholar]
- 7.Koom WS, Chung EJ, Yang WI, et al. Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys. 2004;59:1127–1137. doi: 10.1016/j.ijrobp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83:795–799. doi: 10.1002/ajh.21256. [DOI] [PubMed] [Google Scholar]
- 9.Drénou B, Lamy T, Amiot L, et al. CD3− CD56+ non-Hodgkin’s lymphomas with an aggressive behavior related to multidrug resistance. Blood. 1997;89:2966–2974. [PubMed] [Google Scholar]
- 10.Jaccard A, Petit B, Girault S, et al. L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol. 2009;20:110–116. doi: 10.1093/annonc/mdn542. [DOI] [PubMed] [Google Scholar]
- 11.Obama K, Tara M, Niina K. L-asparaginase-based induction therapy for advanced extranodal NK/T-cell lymphoma. Int J Hematol. 2003;78:248–250. doi: 10.1007/BF02983802. [DOI] [PubMed] [Google Scholar]
- 12.Nagafuji K, Fujisaki T, Arima F, Ohshima K. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol. 2001;74:447–450. doi: 10.1007/BF02982090. [DOI] [PubMed] [Google Scholar]
- 13.Yong W, Zheng W, Zhu J, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009;88:647–652. doi: 10.1007/s00277-008-0669-3. [DOI] [PubMed] [Google Scholar]
- 14.Ahn HK, Kim SJ, Hwang DW, Ko YH, Tang T, Lim ST, Kim WS. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Invest New Drugs. 2013;31:469–472. doi: 10.1007/s10637-012-9889-4. [DOI] [PubMed] [Google Scholar]
- 15.Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013;98:1726–1731. doi: 10.3324/haematol.2013.090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López A, Gutiérrez A, Palacios A, et al. GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: a phase II study. Eur J Haematol. 2008;80:127–132. doi: 10.1111/j.1600-0609.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 17.El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18:1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 18.Nowak-Göttl U, Ahlke E, Fleischhack G, et al. Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101:2529–2533. doi: 10.1182/blood-2002-06-1901. [DOI] [PubMed] [Google Scholar]
- 19.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 22.Au WY, Weisenburger DD, Intragumtornchai T, et al. International Peripheral T-Cell Lymphoma Project. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 23.Yong W, Zheng W, Zhang Y. Clinical characteristics and treatment of midline nasal and nasal type NK/T cell lymphoma. Zhonghua Yi Xue Za Zhi. 2001;81:773–775. (In Chinese) [PubMed] [Google Scholar]
- 24.Lin N, Song Y, Zheng W, et al. A prospective phase II study of L-asparaginase- CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol. 2013;6:44. doi: 10.1186/1756-8722-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au WY, Lie AK, Liang R, et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: a progress report on its value. Ann Oncol. 2003;14:1673–1676. doi: 10.1093/annonc/mdg458. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Bang SM, Lee J, et al. High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant. 2006;37:819–824. doi: 10.1038/sj.bmt.1705349. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Li P, Zhao J, et al. A clinical study of 115 patients with extranodal natural killer/T-cell lymphoma, nasal type. Clin Oncol (R Coll Radiol) 2008;20:619–625. doi: 10.1016/j.clon.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 2008;112:3057–3064. doi: 10.1182/blood-2008-05-160176. [DOI] [PubMed] [Google Scholar]