Abstract
Importance
Estrogen therapy is the gold standard treatment for hot flashes and night sweats, but some women are unable or unwilling to use it because of associated risks. The serotonin-norepinephrine reuptake inhibitor venlafaxine is used widely as a non-hormonal treatment. While clinical impression is that serotonin-norepinephrine reuptake inhibitors are less effective than estrogen, these medications have not been simultaneously evaluated in one clinical trial.
Objective
To determine the efficacy and tolerability of low-dose oral 17-beta-estradiol and low-dose venlafaxine XR in alleviating vasomotor symptoms.
Design and Participants
339 peri- and postmenopausal women with ≥2 bothersome vasomotor symptoms per day (mean 8.1, SD 5.3/day) were recruited from the community to MsFLASH (Menopause Strategies: Finding Lasting Answers for Symptoms and Health) clinical network sites November 2011—October 2012.
Interventions
Participants were randomized to double-blinded treatment with low-dose oral 17-beta-estradiol 0.5-mg/day (n=97), low-dose venlafaxine XR 75-mg/day (n=96), or placebo (n=146) for 8 weeks.
Main Outcomes
Primary outcome was the mean daily frequency of vasomotor symptoms after 8 weeks of treatment. Secondary outcomes were vasomotor symptom severity, bother and interference. Intent-to-treat analyses compared change in vasomotor symptom frequency between each active intervention and placebo and between the two active treatments.
Results
Compared to baseline, mean vasomotor symptom frequency at week 8 decreased by 53% with estradiol, 48% with venlafaxine, and 29% with placebo. Estradiol reduced the frequency of symptoms by 2.3 (95% CI 1.3–3.4) more per day than placebo (p<0.001), and venlafaxine by 1.8 (95% CI 0.8–2.7) more per day than placebo (p=0.005). Results were consistent for VMS severity, bother and interference. Low-dose estradiol reduced symptom frequency by 0.6 more per day than venlafaxine (95% CI, 1.8 more per day to 0.6 fewer per day than venlafaxine; p=0.09). Treatment satisfaction was highest (69%) on estradiol (p<0.001 versus placebo), lowest (39%) on placebo, and intermediate (52%) for venlafaxine (p=0.06 versus placebo). Both interventions were well tolerated.
Conclusions
Low-dose oral estradiol and venlafaxine are both effective treatments for vasomotor symptoms in midlife women. While efficacy of low-dose estradiol may be slightly superior to that of venlafaxine, the difference is small in magnitude and of uncertain clinical relevance.
Clinicaltrials.gov identifier
NCT01418209, http://clinicaltrials.gov/ct2/show/NCT01418209?term=NCT01418209&rank=1
BACKGROUND
Hot flashes and night sweats, together called vasomotor symptoms (VMS), are highly prevalent in women during midlife, affecting up to 80% of women.1 VMS are the primary menopause-related symptom leading peri- and postmenopausal women to seek medical attention.2 Estrogen therapy (ET) remains the gold standard treatment for VMS and was the only FDA-approved treatment for VMS until a selective serotonin reuptake inhibitor (SSRI) was recently approved.3 However, prescriptions for ET have declined markedly since findings from the Women’s Health Initiative (WHI) demonstrated associated risks in postmenopausal women.4 Because of these risks, current recommendations are that ET be used at the lowest possible dose for the shortest possible duration,5 shifting usage patterns to lower-dose preparations. Studies suggest that low-dose ET preparations diminish VMS, but to a lesser extent than standard doses and with a slower onset of action.6
Since the publication of WHI results, investigation of non-hormonal treatments for VMS has intensified. Many SSRI/SNRI have been shown to be more effective than placebo in reducing VMS,7–9 with one SSRI recently FDA-approved to treat VMS.3,9 The SNRI venlafaxine is one of the most widely studied serotonergic agents with accumulating evidence showing that low doses (75–150 mg/day) reduce VMS more than placebo.10–12 SSRI/SNRI are used widely to treat VMS, with venlafaxine a first-line treatment in women unable or unwilling to take ET.13,14
While clinical impression is that SSRI/SNRI medications are less effective than ET,8,15 trials simultaneously examining the efficacy of these agents have not been conducted. In addition, the majority of ET trials have used doses higher than currently recommended low-dose regimens.16 As a result, no data on the relative efficacy of the widely used low-dose oral ET and serotonergic agents are available to guide VMS treatment decisions.
MsFLASH (Menopause Strategies: Finding Lasting Answers for Symptoms and Health) is an NIH-funded research network designed to test treatments for menopause-related symptoms. We report here results of a 3-arm double-blinded trial randomizing healthy peri- and postmenopausal women with bothersome VMS to low-dose oral 17-beta-estradiol, low-dose venlafaxine, or placebo for 8 weeks. The primary objective of this trial was to determine the efficacy of both ET and venlafaxine relative to placebo in reducing the number of VMS reported. We hypothesized that both ET and venlafaxine would be superior to placebo for reducing VMS frequency and, secondarily, that both active agents would improve VMS severity, bother and interference more than would placebo.
METHODS
Trial design
This was a multi-site, randomized placebo-controlled 8-week trial of oral 17-β-estradiol 0.5-mg/day, venlafaxine XR 75-mg/day, or placebo. Those randomized to venlafaxine were titrated from 37.5-mg/day up to 75-mg/day over a one-week period. Details about the MsFLASH Research Network and study designs are published elsewhere.17,18 The study was approved by the each site’s Institutional Review Board. All participants provided written informed consent.
Eligible women were randomly assigned to treatment in a 2:2:3 ratio (ET:venlafaxine:placebo) to increase the efficiency of the trial design for the two primary comparisons between each active treatment and placebo. Allowing for a 10% loss to follow-up, a sample size of 304 (339 enrolled) provided >90% power to detect a 0.52 standard deviation unit difference in change in VMS frequency between placebo and active groups using a 2-sided 2.5% type I error for each comparison.
Participant selection
Participants at MsFLASH sites in Boston, Philadelphia, and Seattle were recruited from November 2011—October 2012 by mass mailings to age-eligible women using purchased mailing lists and health-plan enrollment files.
Eligible participants were healthy women ages 40–62 years, in the menopause transition (amenorrhea ≥60 days in past year), or postmenopausal (≥12 months since last menstrual period or bilateral oöphorectomy), or FSH >20 mIU/mL and estradiol ≤50 pg/mL in the absence of a reliable menstrual marker (hysterectomy with ovarian preservation, progesterone- releasing intra-uterine device, endometrial ablation). Participants were required to have ≥14 VMS/week, at least some of which were considered bothersome or severe, recorded prospectively on daily diaries for 2 weeks. A third week of VMS diaries was collected to ensure that VMS ratings did not decline by more than 50% from the first 2 screening weeks.17,18
Exclusion criteria included hypersensitivity, contraindication to study medications; recent or current use of hormone therapy, hormonal contraceptives, selective estrogen receptor modulators, or aromatase inhibitors (past 2 months); psychotropic medications or treatments for VMS (past month); pregnancy or breastfeeding; major depressive episode, drug/alcohol abuse in past year; suicide attempt in past 3 years; diagnosis of bipolar disorder or psychosis; or history of uncontrolled hypertension, cardiovascular, thrombotic, or endometrial disease, pre-breast cancer conditions, breast or gynecologic cancer, or unstable medical illness.
Data collection
The trial included a telephone screen, 3 clinic-based study visits (screening, randomization, 8-weeks) and 2 telephone assessments (1- and 4-weeks). Participants completed questionnaires at baseline and 8 weeks, and recorded VMS and vaginal bleeding pattern diaries twice daily for 3 weeks before randomization to establish baseline VMS and then throughout the 8-week trial.18
Treatment
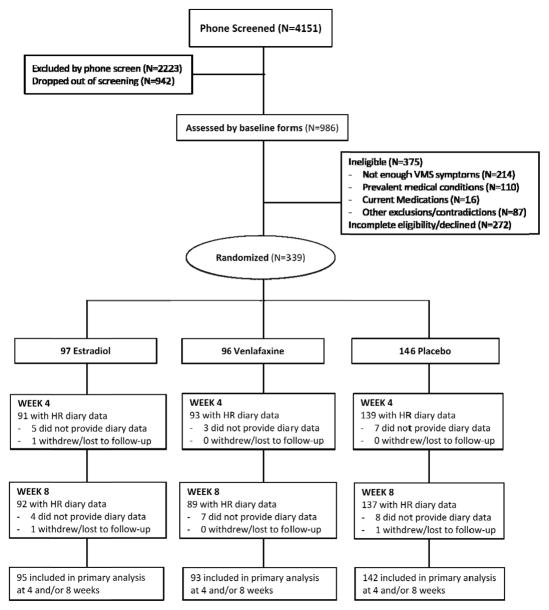
All participants took one identical appearing pill orally each day. Using a dynamic randomization algorithm,19 participants were randomized to one of three arms: ET, venlafaxine or placebo (Figure 1), stratified on clinical site. Participants and clinical site personnel were blinded to treatment assignment until all 8-week data were collected, after which assignment was unblinded so that specific post-treatment medications could be administered. Study pills were counted at week 8 to estimate adherence.
Figure 1.
CONSORT Diagram
ET was administered as 17-beta-estradiol 0.5-mg/day for 8 weeks; after unblinding, medroxyprogesterone 10-mg/day orally for 14 days was given for endometrial protection. Those assigned to venlafaxine received 37.5-mg/day for one week then 75-mg/day for 7 weeks; after unblinding, they were tapered to 37.5-mg/day for another 14 days to minimize potential SNRI withdrawal effects.
Measurements
VMS frequency, bother, and severity were recorded daily in the morning and evening. The primary outcome was VMS frequency. Secondary outcomes were VMS bother (rated from 1–4: none, a little, moderately, a lot), VMS severity (rated from 1–3: mild, moderate, severe), and perceived VMS interference (Hot Flash Related Daily Interference Scale, HFRDIS,20 evaluated at baseline, 4 and 8 weeks).
Adverse events (AEs) were assessed at each contact using open-ended questions and a self-administered questionnaire listing specific expected side effects for ET and venlafaxine. Newly emergent AEs were identified by comparing AE reports during treatment to each subject’s baseline report.
Statistical analysis
Baseline characteristics were compared between treatment groups using t-tests or chi-square tests. Baseline VMS frequency was calculated as the mean of daily reported VMS in the first two screening weeks. The primary hypotheses for this trial were that low-dose oral ET and venlafaxine would each be superior to placebo in treating VMS frequency. Intent-to-treat analyses included all randomized participants who provided follow-up VMS diary data at week 4 and/or week 8 (n=330, 97%), regardless of treatment adherence. The primary outcome was the mean daily VMS frequency for the week prior to the week 4 and 8 study assessments. VMS severity and bother were similarly defined.
Treatment contrasts between placebo and each active group were computed as Wald-statistics from linear regression models summarizing VMS frequency, severity, bother, and HFRDIS at weeks 4 and 8 as a function of randomization assignment, clinical site, visit, and the baseline value of the outcome. Natural logarithm transformations were applied to VMS frequencies to accommodate modeling assumptions. Robust standard errors were calculated via generalized estimating equations to account for within-woman correlations between repeated measures. A post-hoc analysis of the relative efficacy of estradiol and venlafaxine for VMS frequency was conducted using the methods applied to the active vs. placebo comparisons.
Baseline menopausal symptoms and demographic characteristics hypothesized a priori to modify treatment response relative to placebo included: age, race, body mass index (BMI), menopausal status, smoking, VMS frequency, VMS duration, insomnia symptoms, sleep quality, depressive symptoms, anxiety symptoms, sexual function, and perceived stress. Tests for interaction between these variables and treatment assignment were performed within the linear regression models, using continuous values of variables where possible, for each active vs. placebo comparison.
VMS clinical improvement (≥50% VMS frequency reduction), participant satisfaction, and adverse events were compared between each active treatment and placebo using chi-square or Fisher’s exact test. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc.). All statistical tests were 2-sided. Primary analyses were considered statistically significant at p<0.025. Secondary analyses are exploratory and considered nominally statistically significant at p<0.05.
RESULTS
Among 339 women randomized, 96 (28.3%) received estradiol, 97 (28.6%) received venlafaxine, and 146 (43.1%) received placebo (Figure 1). There were no significant differences in baseline characteristics between groups (Table 1). 319 (94%) participants were adherent to study medication (taking ≥80% of dispensed pills), and 318 (94%) women provided completed diaries at 8 weeks of follow-up. Diary adherence was high: over 95% of analyzed weekly diaries were completed on at least 6 days at week 4, and similarly at week 8.
Table 1.
Demographic and Clinical Characteristics by Treatment Arm at Baseline1
| All Participants | Estradiol | Venlafaxine | Placebo | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total Participants | 339 | 97 | 96 | 146 | ||||
|
| ||||||||
| Baseline Characteristic | N | % | N | % | N | % | N | % |
| Age (years), mean (SD) | 54.6 (3.8) | 54.9 (4.1) | 54.8 (3.7) | 54.3 (3.8) | ||||
|
| ||||||||
| Race | ||||||||
| White | 203 | 59.9 | 60 | 61.9 | 53 | 55.2 | 90 | 61.6 |
| African American | 116 | 34.2 | 32 | 33.0 | 38 | 39.6 | 46 | 31.5 |
| Other/Unknown | 20 | 5.9 | 5 | 5.2 | 5 | 5.2 | 10 | 6.8 |
|
| ||||||||
| BMI (kg/m2), mean (SD) | 28.3 (6.8) | 28.5 (6.5) | 29.3 (6.9) | 27.6 (6.8) | ||||
| <30 | 225 | 66.4 | 66 | 68.0 | 59 | 61.5 | 100 | 68.5 |
| ≥ 30 | 107 | 31.6 | 30 | 30.9 | 34 | 35.4 | 43 | 29.5 |
|
| ||||||||
| Smoking | ||||||||
| Never | 174 | 51.3 | 50 | 51.5 | 54 | 56.3 | 70 | 47.9 |
| Past | 107 | 31.6 | 30 | 30.9 | 27 | 28.1 | 50 | 34.2 |
| Current | 55 | 16.2 | 17 | 17.5 | 14 | 14.6 | 24 | 16.4 |
|
| ||||||||
| Alcohol use (drinks/week) | ||||||||
| <7 | 265 | 78.2 | 71 | 73.2 | 77 | 80.2 | 117 | 80.1 |
| ≥ 7 | 61 | 18.0 | 21 | 21.6 | 13 | 13.5 | 27 | 18.5 |
|
| ||||||||
| Marital status | ||||||||
| Never married/Divorced/Widowed | 127 | 37.5 | 38 | 39.2 | 40 | 41.7 | 49 | 33.6 |
| Married/living with partner | 210 | 61.9 | 58 | 59.8 | 56 | 58.3 | 96 | 65.8 |
|
| ||||||||
| Education | ||||||||
| < College graduate | 166 | 49.0 | 48 | 49.5 | 48 | 50.0 | 70 | 47.9 |
| College graduate | 172 | 50.7 | 49 | 50.5 | 48 | 50.0 | 75 | 51.4 |
|
| ||||||||
| Menopause status1 | ||||||||
| Perimenopausal | 52 | 15.3 | 14 | 14.4 | 16 | 16.7 | 22 | 15.1 |
| Postmenopausal | 256 | 75.5 | 74 | 76.3 | 72 | 75.0 | 110 | 75.3 |
| Indeterminate | 31 | 9.1 | 9 | 9.3 | 8 | 8.3 | 14 | 9.6 |
|
| ||||||||
| Years since final menstrual period (postmenopausal only) | ||||||||
| 0 - 5 | 135 | 52.7 | 32 | 43.2 | 37 | 51.4 | 66 | 60.0 |
| 6 - 10 | 72 | 28.1 | 23 | 31.1 | 20 | 27.8 | 29 | 26.4 |
| > 10 | 42 | 16.4 | 16 | 21.6 | 13 | 18.1 | 13 | 11.8 |
|
| ||||||||
| VMS frequency/day | 8.1 (5.3) | 8.5 (5.7) | 8.2 (5.5) | 7.7 (4.9) | ||||
| <6 | 139 | 41.0 | 36 | 37.1 | 36 | 37.5 | 67 | 45.9 |
| 6 - <9 | 102 | 30.1 | 32 | 33.0 | 31 | 32.3 | 39 | 26.7 |
| 9 - <12 | 45 | 13.3 | 11 | 11.3 | 15 | 15.6 | 19 | 13.0 |
| ≥ 12 | 53 | 15.6 | 18 | 18.6 | 14 | 14.6 | 21 | 14.4 |
|
| ||||||||
| Age at starting VMS (years) | ||||||||
| <50 | 171 | 50.4 | 47 | 48.5 | 51 | 53.1 | 73 | 50.0 |
| ≥ 50 | 163 | 48.1 | 48 | 49.5 | 44 | 45.8 | 71 | 48.6 |
|
| ||||||||
| ISI, mean (SD) | 11.0 (6.0) | 11.0 (6.3) | 11.7 (6.0) | 10.4 (5.8) | ||||
| No clinically significant insomnia (≤7) | 106 | 31.3 | 28 | 28.9 | 26 | 27.1 | 52 | 35.6 |
| Subthreshold insomnia (8–14) | 133 | 39.2 | 40 | 41.2 | 39 | 40.6 | 54 | 37.0 |
| Clinical insomnia (moderate, 15–21) | 78 | 23.0 | 21 | 21.6 | 25 | 26.0 | 32 | 21.9 |
| Clinical insomnia (severe, ≥22) | 14 | 4.1 | 5 | 5.2 | 5 | 5.2 | 4 | 2.7 |
|
| ||||||||
| PSQI, mean (SD) | 7.5 (3.4) | 7.6 (3.6) | 7.6 (3.2) | 7.3 (3.5) | ||||
| Good sleep quality (<5) | 67 | 19.8 | 23 | 23.7 | 14 | 14.6 | 30 | 20.5 |
| Moderate sleep quality (5 - <8) | 102 | 30.1 | 21 | 21.6 | 35 | 36.5 | 46 | 31.5 |
| Poor sleep quality (≥8) | 151 | 44.5 | 46 | 47.4 | 40 | 41.7 | 65 | 44.5 |
|
| ||||||||
| PHQ-9 Depression, mean (SD) | 3.4 (3.7) | 3.9 (4.4) | 3.0 (2.9) | 3.4 (3.7) | ||||
| No depression (0–4) | 246 | 72.6 | 68 | 70.1 | 70 | 72.9 | 108 | 74.0 |
| Mild depression (5–9) | 64 | 18.9 | 18 | 18.6 | 23 | 24.0 | 23 | 15.8 |
| Moderate depression (≥10) | 29 | 8.6 | 11 | 11.3 | 3 | 3.1 | 15 | 10.3 |
|
| ||||||||
| GAD-7 Anxiety, mean (SD) | 2.5 (3.6) | 3.0 (4.3) | 2.2 (3.0) | 2.4 (3.4) | ||||
| No anxiety (0–4) | 265 | 78.2 | 73 | 75.3 | 76 | 79.2 | 116 | 79.5 |
| Mild anxiety (5–9) | 51 | 15.0 | 15 | 15.5 | 17 | 17.7 | 19 | 13.0 |
| Moderate anxiety (≥10) | 23 | 6.8 | 9 | 9.3 | 3 | 3.1 | 11 | 7.5 |
VMS frequency
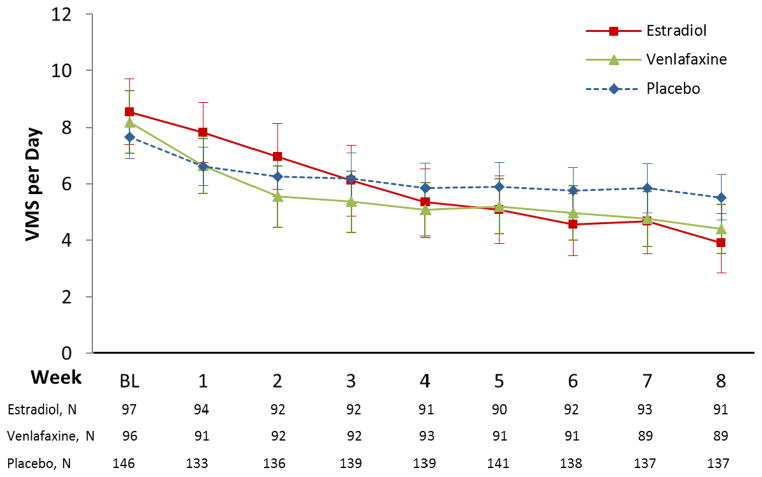
The mean VMS frequency at baseline was 8.1 (SD 5.3)/day. Treatment with ET or venlafaxine was associated with a significant reduction in VMS frequency relative to placebo (Table 2). Mean VMS frequency at week 8 decreased to 3.9 (95% CI 2.9–4.9) VMS/day (53% reduction) in the estradiol group, to 4.4 (95% CI 3.5–5.3) VMS/day (48% reduction) in the venlafaxine group, and to 5.5 (95% CI 4.7–6.3) VMS/day (29% reduction) in the placebo group (Table 2 and Figure 2). Linear model estimates can be expressed as a 32% (95% CI 20–43%) decrease in mean VMS frequency through week 8 in the estradiol group relative to placebo, and a 20% (95% CI 7–31%) decrease in the venlafaxine group relative to placebo. Low-dose ET reduced VMS frequency by an additional 0.6 VMS/day relative to venlafaxine (95% CI ranges from 1.8 VMS/day greater reduction on ET relative to venlafaxine to 0.6 VMS/day greater reduction on venlafaxine relative to ET, p=0.09). This difference translates into a 15% greater decrease (95% CI ranging from a 30% greater reduction on ET relative to venlafaxine to a 2% greater reduction on venlafaxine relative to ET) based on model estimates.
Table 2.
Daily Vasomotor Symptom (VMS) Frequency at Weeks 4 and 8 by Treatment Arm
| Estradiol | Placebo | Difference | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | p-value1 | |
|
| ||||||
| VMS Frequency2 | <0.001 | |||||
| Baseline | 97 | 8.5 (7.4, 9.7) | 146 | 7.7 (6.9, 8.5) | 0.9 (−0.5, 2.2) | |
| Week 4 – baseline | 91 | −3.1 (−4.0, −2.3) | 139 | −1.9 (−2.5, −1.3) | −1.2 (−2.2, −0.2) | |
| Week 8 – baseline | 92 | −4.5 (−5.4, −3.6) | 137 | −2.2 (−2.8, −1.6) | −2.3 (−3.4, −1.3) | |
| Venlafaxine | Placebo | Difference | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | p-value1 | |
|
| ||||||
| VMS Frequency2 | 0.005 | |||||
| Baseline | 96 | 8.2 (7.1, 9.3) | 146 | 7.7 (6.9, 8.5) | 0.5 (−0.8, 1.8) | |
| Week 4 – baseline | 93 | −3.3 (−4.0, −2.5) | 139 | −1.9 (−2.5, −1.3) | −1.4 (−2.3, −0.4) | |
| Week 8 – baseline | 89 | −3.9 (−4.7, −3.1) | 137 | −2.2 (−2.8, −1.6) | −1.8 (−2.7, −0.8) | |
CI = confidence interval; VMS = vasomotor symptoms
p-values from active treatment vs. placebo contrasts in a repeated measures linear model of outcome as a function of treatment arm, clinical site, week (4 or 8), and baseline outcome
VMS frequency values were log transformed for modeling
Figure 2.
Vasomotor Symptom (VMS) Frequency by Treatment Arm
There were no statistically significant interactions of treatment effects with age, race, BMI, smoking, menopause status, VMS duration, or baseline symptom levels (VMS frequency, insomnia symptoms, sleep quality, depressive symptoms, anxiety symptoms, sexual function, and perceived stress) for either venlafaxine or ET vs. placebo (all p>0.05, see eTables 1 and 2).
Secondary outcomes
Both ET and venlafaxine reduced VMS severity more than did placebo (Table 3). For VMS bother, the magnitude of each active intervention effect was similar, but the reduction was statistically significant for ET relative to placebo, and not for venlafaxine. ET and venlafaxine each reduced HFRDIS scores more than did placebo. Clinical improvement at week 8 was significantly more common in both the ET and venlafaxine groups relative to placebo (56%, 51%, and 31%, p<0.001 and p=0.003, respectively). Results of analyses restricted to treatment-adherent participants were consistent with those of the intent-to-treat analyses.
Table 3.
Secondary Outcomes of Vasomotor Symptom (VMS) Severity, Bother, and Interference, at Week 8 by Treatment Group
| Intervention | Estradiol | Placebo | Difference1 | Venlafaxine | Placebo | Difference1 | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | N | Mean (95% CI) | p-value2 | N | N | Mean (95% CI) | p-value2 | |
| VMS Severity | 0.02 | 0.02 | ||||||
| Baseline | 97 | 146 | 0.1 (0.0, 0.2) | 96 | 146 | 0.0 (−0.1, 0.1) | ||
| Week 8 – baseline | 73 | 133 | −0.3 (−0.4, −0.1) | 86 | 133 | −0.2 (−0.3, 0.0) | ||
|
| ||||||||
| VMS Bother | 0.01 | 0.07 | ||||||
| Baseline | 97 | 146 | 0.1 (0.0, 0.2) | 96 | 146 | 0.0 (−0.2, 0.1) | ||
| Week 8 – baseline | 73 | 133 | −0.3 (−0.5, −0.1) | 86 | 133 | −0.2 (−0.3, 0.0) | ||
|
| ||||||||
| HFRDIS | <0.001 | 0.03 | ||||||
| Baseline | 90 | 141 | 4.2 (−1.7, 10.1) | 92 | 141 | 4.0 (−1.8, 9.8) | ||
| Week 8 – baseline | 86 | 132 | −9.3 (−15.3, −3.4) | 84 | 132 | −6.4 (−12.7, −0.1) | ||
CI = confidence interval; VMS = vasomotor symptoms; HFRDIS =Hot Flash-Related Daily Interference Scale20
Outcome differences between active treatment and placebo
P-values from active treatment vs. placebo contrasts in a repeated measures linear model of outcome as a function of treatment arm, clinical site, week (4 or 8), and baseline outcome
Adverse events
Tolerability of treatment was high. Only 11 (3.2%) subjects stopped treatment due to AEs (4 ET, 5 venlafaxine, 2 placebo). Newly-emergent AEs were reported by 56%, 69%, and 62% of the ET, venlafaxine, and placebo groups, respectively (no significant differences from placebo; eTable 3). The most frequently reported AEs were insomnia on ET and fatigue on venlafaxine and placebo. Three participants reported suicidal ideation while on study medication (2.5% on ET; 0.7% on placebo; none on venlafaxine). Mean changes in systolic and diastolic blood pressure (SBP, DBP) were small, with SBP and DBP declining by 6.0 (SD 16.0) and 0.9 (SD 9.4) mmHg on ET, by 5.6 (SD 15.4) and 1.4 (SD 9.5) mmHg on placebo, and increasing by 0.5 (SD 14.5) and 2.1 (SD 8.7) mmHg on venlafaxine. Twelve women developed SBP >165 mmHg or DBP >95 mmHg (2.1% on ET, 10.4% on venlafaxine, 0 on placebo), all of whom had baseline SBP or DBP above the study population mean (SBP 123.4, SD 13.5 mmHg; DBP 76.2, SD 9.4 mmHg); the majority also had a baseline BMI above the population mean (28.3, SD 6.8 kg/m2). Among women with a uterus, 6/73 (8.2%) on ET, 2/124 (1.6%) on placebo, and none on venlafaxine developed abnormal vaginal bleeding (any bleeding in postmenopausal women; 2+ cycles <21 days in perimenopausal women) on treatment, which was evaluated with a transvaginal ultrasound. Three of 6 estradiol-treated participants with abnormal bleeding had an endometrial echo complex >5 mm and underwent an endometrial biopsy, all of which revealed no evidence of hyperplasia or malignancy.
Participant satisfaction
Of 319 responding, treatment ssatisfaction was highest on ET (70%, p<0.001 compared to placebo), lowest on placebo (39%), and intermediate for venlafaxine (51%, p=0.06 compared to placebo). 23% on ET, 28% on venlafaxine, and 40% on placebo group guessed their treatment assignment correctly.
DISCUSSION
This is the first randomized controlled trial designed to simultaneously investigate the efficacy of low-dose oral estradiol and the SNRI venlafaxine in the treatment of menopause-related vasomotor symptoms. Results of this study show that during an 8-week treatment period, ET and venlafaxine were each more effective than placebo in reducing VMS frequency. We observed a 53% reduction with ET, 48% with venlafaxine, and 29% with placebo, translating into a 32% and 20% greater reduction with ET and venlafaxine relative to placebo, respectively. Consistent with the effect of each treatment on VMS frequency, ET and venlafaxine both improved the severity of VMS and the interference of VMS with daily life; the small reduction in VMS bother was significant only for ET. No demographic, menopause, or symptom characteristics predicted differential response to either intervention. Both treatments were well tolerated, with newly emergent AEs consistent with their known side effects. These findings provide critical data for clinicians and midlife women making treatment decisions for VMS by showing that first-line hormonal and non-hormonal pharmacologic treatments for VMS are both well-tolerated and effective options for alleviating VMS.
The magnitude of reduction in VMS frequency we observed with each treatment is consistent with RCTs for low-dose estradiol,21–23 venlafaxine,11,12 and other serotonergic agents.7,9 However, eligibility criteria in previous trials varied widely and doses of ET differed, precluding direct comparison. In contrast to previous ET trial eligibility criteria of 7+ moderate-to-severe VMS/day,6,22,23 we required fewer VMS (2+/day) and not all VMS were required to be moderate or severe. The mean frequency of VMS at baseline was therefore lower than seen in previous ET trials,6,22,23 and consistent with the majority of SSRI/SNRI trials targeting VMS.7,11,12,24,25 Because stringent screening procedures established that participants had stable levels of VMS,18 we were able to minimize our placebo response relative to those in other trials.26,27
We observed that low-dose ET reduced VMS frequency by an additional 0.6 VMS per day than did venlafaxine, with a 95% confidence interval ranging from a larger improvement with ET relative to venlafaxine to a small advantage of venlafaxine relative to ET. These data suggest that, if a difference in efficacy between the two active interventions exists, it is small and the magnitude is of uncertain clinical relevance. However, the sample size was not large enough to provide adequate power for a direct non-inferiority comparison between the two active treatments.
Results of this trial provide clinically relevant data about the magnitude of the effect of low-dose oral ET and an SNRI in improving VMS frequency, severity, bother and interference in the same population of symptomatic women, enabling standardization of the baseline symptom profile of treated participants for the first time. Our findings extend results of previous placebo-controlled trials of these individual treatments alone by demonstrating that SNRI have a meaningful therapeutic effect on VMS which is in the range of low-dose ET. Such validation supports a serotonergic mechanism of action for VMS reduction.
Our findings may be specific to the particular dose of each agent used, as well as the preparation and oral administration of ET. Previous studies have highlighted the dose-dependent efficacy of low-dose oral ET and conjugated equine estradiol (CEE).6,21–23,28 In a 12-week trial, 17-beta-estradiol 0.5-mg reduced VMS by 65.5% (SD 34.0%) whereas 1.0-mg reduced VMS by 83.2% (SD 25.6%) and placebo reduced VMS by 47.5% (SD 37.2%).23 Because of endometrial stimulation risks with prolonged us of unopposed ET,23 our trial was restricted to 8 weeks of treatment. While our study is limited by the short-term duration of treatment and the relative efficacy beyond 8 weeks was not investigated, previous studies have shown limited additional improvement in VMS after 8 weeks of treatment with ET,6,22,23 or venlafaxine.11 In the current trial, we used low-dose ET because of recommendations to use the lowest effective ET dose.
An important strength of this trial is our racially diverse study population (one-third African-American) midlife cohort of both peri- and postmenopausal women. Our results show that, while VMS are reported more commonly by African-American women1, there is no difference in their VMS frequency response to these treatments. Other patient-level characteristics, including baseline menopause status, VMS burden, sleep and mood symptoms, also did not identify subgroups with differential response to treatment.
Tolerability of both active treatments was high. Discontinuation due to adverse events was uncommon and did not differ significantly between treatments. While previous SSRI/SNRI studies in young adults with depression suggest treatment-emergent suicidal ideation occurs rarely, we did not observe this AE on the SNRI or in a prior SSRI trial conducted in non-depressed midlife women.7 As expected, higher rates of abnormal vaginal bleeding warranting investigation occurred with ET, while increases in SBP and DBP to clinically significant thresholds occurred more commonly on venlafaxine. Elevated blood pressure on venlafaxine is well-described, with monitoring recommended, especially in those at greater baseline risk for hypertension.29 The profile of women developing high blood pressure suggests that those at risk were disproportionately overweight or obese and had higher baseline SBP/DBP. Taken together, these data suggest that the active agents investigated were well tolerated but had distinct adverse effect profiles consistent with their respective known effects.
Overall, the results of this trial provide robust evidence of the efficacy of low-dose oral 17-beta estradiol and the non-hormonal SNRI venlafaxine for treatment of VMS associated with menopause. Low-dose oral estradiol and venlafaxine were both effective and well-tolerated treatments for peri- and postmenopausal women with bothersome vasomotor symptoms. Treatment decisions should weigh the risk profile of each agent for each individual woman, taking into account her risk factor status and personal preferences regarding treatment options.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Thania Galvan, BA, Semmie Kim, BS, and Marsha Barsky, BA, for administrative assistance.
Funding Source: All financial and material support for this study was provided by the National Institutes of Health as a cooperative agreement issued by the National Institute of Aging: #U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700.
Footnotes
Author Contributions: Katherine Guthrie, PhD, has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Katherine Guthrie, PhD, and Joseph C. Larson, MS, both at the MsFLASH Data Coordinating Center, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, conducted and are responsible for the data analysis.
Design and conduct of the study: Joffe, Guthrie, LaCroix, Reed, Ensrud, Manson, Newton, Freeman, Anderson, Larson, Hunt, Shifren, Rexrode, Caan, Sternfeld, Carpenter, Cohen
Collection, management, analysis, and interpretation of data: Joffe, Guthrie, LaCroix, Reed, Ensrud, Manson, Newton, Freeman, Anderson, Larson, Hunt, Shifren, Rexrode, Caan, Sternfeld, Carpenter, Cohen
Preparation, review, or approval of the manuscript: Joffe, Guthrie, LaCroix, Reed, Ensrud, Manson, Newton, Freeman, Anderson, Shifren, Rexrode, Caan, Sternfeld, Carpenter, Cohen
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure. Dr. Joffe currently receives research support from NIH and Cephalon/Teva, and consults for Sunovion and Noven. Dr. Reed reports research support from NIH. Dr. Ensrud reported receiving financial compensation for consultancy for being on the Data Monitoring Committee at Merck Sharpe & Dohme. Dr. Newton reports receiving research support from Otsuka Pharmaceuticals Inc., LTD. Dr. Shifren reports receiving financial compensation from New England Research Institutes for consulting work related to a research study of vulvovaginal atrophy. Dr. Rexrode reports receiving financial compensation from Pfizer advisory board for consultancy. Dr. Rexrode also receives research support from NIH grants, and royalties for being the textbook editor of “Women and Health-Second Edition”. Dr. Cohen reported receiving financial compensation from Noven Pharmaceuticals, PamLab LLC for consultancy work. Additionally, Dr. Cohen receives research support from Astra-Zeneca Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Inc, Forest Laboratories, Inc, GlaxoSmithKline, Ortho-McNeil Janssen, Pfizer Inc, Sunovion Pharmaceuticals Inc, NIH, NIMH, and NIA. Dr. LaCroix reports serving as a consultant to Amgen and Bayer and to receiving research grants from the University of Massachusetts, Center for Outcomes Research and NIH. Dr. Freeman reports receiving research support from Forest Laboratories, Inc., Bionovo, and Xanodyne Pharmaceuticals, Inc. Drs. Anderson, Guthrie, Manson, Larson, Hunt, Caan, Carpenter and Sternfeld did not disclose any financial conflicts. Teva, Noven, Pfizer, and Bayer manufacture estrogen products for the treatment of menopausal symptoms. Pfizer and Teva manufacture venlafaxine. All financial activities were reported to fall outside of this study.
References
- 1.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health. 2006 Jul;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie JR, Dennerstein L, Taffe JR, Donnelly V. Health care-seeking for menopausal problems. Climacteric. 2003 Jun;6(2):112–117. [PubMed] [Google Scholar]
- 3.Administration UFaD. [Accessed June 28, 2013];FDA approves the first non-hormonal treatment for hot flashes associated with menopause. 2013 http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm359030.htm.
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Position Statement: The 2012 Hormone Therapy Position Statement of The North American Menopause Society. Menopause: The Journal of The North American Menopause Society. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001 Jun;75(6):1065–1079. doi: 10.1016/s0015-0282(01)01791-5. [DOI] [PubMed] [Google Scholar]
- 7.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011 Jan 19;305(3):267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006 May 3;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 9.Simon JAPD, Kaunitz AM, Mekonnen H, Kazempour K, Bhaskar S, Lippman J. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1035. doi: 10.1097/GME.0b013e3182a66aa7. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter JS, Storniolo AM, Johns S, et al. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007 Jan;12(1):124–135. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 11.Evans ML, Pritts E, Vittinghoff E, McClish K, Morgan KS, Jaffe RB. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol. 2005 Jan;105(1):161–166. doi: 10.1097/01.AOG.0000147840.06947.46. [DOI] [PubMed] [Google Scholar]
- 12.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 13.Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. The Cochrane database of systematic reviews. 2010;(9):CD004923. doi: 10.1002/14651858.CD004923.pub2. [DOI] [PubMed] [Google Scholar]
- 14.L’Esperance S, Frenette S, Dionne A, Dionne JY. Pharmacological and non-hormonal treatment of hot flashes in breast cancer survivors: CEPO review and recommendations. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2013 May;21(5):1461–1474. doi: 10.1007/s00520-013-1732-8. [DOI] [PubMed] [Google Scholar]
- 15.Sassarini J, Lumsden MA. Hot flushes: are there effective alternatives to estrogen? Menopause international. 2010 Jun;16(2):81–88. doi: 10.1258/mi.2010.010007. [DOI] [PubMed] [Google Scholar]
- 16.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. The Cochrane database of systematic reviews. 2004;(4):CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternfeld B, Lacroix A, Caan BJ, et al. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: The MsFLASH experience. Contemp Clin Trials. 2013 May;35(1):25–34. doi: 10.1016/j.cct.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton KN, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975 Mar;31(1):103–115. [PubMed] [Google Scholar]
- 20.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001 Dec;22(6):979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 21.Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004 Apr 7;291(13):1610–1620. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- 22.Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol. 2000 May;95(5):726–731. doi: 10.1016/s0029-7844(99)00643-2. [DOI] [PubMed] [Google Scholar]
- 23.Notelovitz M, Mattox JH. Suppression of vasomotor and vulvovaginal symptoms with continuous oral 17beta-estradiol. Menopause. 2000 Sep-Oct;7(5):310–317. doi: 10.1097/00042192-200007050-00005. [DOI] [PubMed] [Google Scholar]
- 24.Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007 Apr;109(4):823–830. doi: 10.1097/01.AOG.0000258278.73505.fa. [DOI] [PubMed] [Google Scholar]
- 25.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003 Jun 4;289(21):2827–2834. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 26.Archer DF, Dupont CM, Constantine GD, Pickar JH, Olivier S. Desvenlafaxine for the treatment of vasomotor symptoms associated with menopause: a double-blind, randomized, placebo-controlled trial of efficacy and safety. American journal of obstetrics and gynecology. 2009 Mar;200(3):238.e231–238 e210. doi: 10.1016/j.ajog.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006 Jul-Aug;13(4):568–575. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger B. Vasomotor symptom relief versus unwanted effects: role of estrogen dosage. The American journal of medicine. 2005 Dec 19;118(Suppl 12B):74–78. doi: 10.1016/j.amjmed.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998 Oct;59(10):502–508. doi: 10.4088/jcp.v59n1002. [DOI] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CFd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007 Nov;22(11):1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000 Apr-Jun;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–396. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.