Abstract
Background
Measurement of N-terminal pro brain natriuretic peptide (NT-proBNP) in the evaluation of patients with acute coronary syndrome has appeared to be a useful prognostic marker of cardiovascular risk.
Aim of the work
To assess the in-hospital prognostic value of NT-proBNP in patients with acute coronary syndrome (ACS) and its relation to the severity of coronary artery disease.
Patients and methods
This study included 132 consecutive patients with ACS, 64 patients with unstable angina (UA), 46 patients with non-ST segment elevation myocardial infarction (NSTEMI), and 22 patients with ST segment elevation myocardial infarction (STEMI). ECG, echocardiography and pre and post coronary angiography measurement of troponin I, creatine kinase (Ck), C-reactive protein (CRP) and NT-proBNP were done. Patients were divided into two groups: Group A with NT-proBNP less than 474 pg/ml and Group B with NT-proBNP equal or more than 474 pg/ml.
Results
There was a significant negative correlation between NT-proBNP and ejection fraction. Incidence of heart failure and duration of hospital stay were significantly higher in Group B (with NT-proBNP equal or more than 474 pg/ml) than Group A (with NT-proBNP less than 474 pg/ml). Moreover, there was a trend to an increased incidence of cardiogenic shock and mortality in Group B compared to Group A. The number of coronary vessels affected, severity of stenosis and proximal left anterior descending artery (LAD) disease were higher in Group B than in Group A. TIMI flow grade was significantly higher in Group A than in Group B.
Conclusion
NT-proBNP is a valuable marker for predicting prognosis and severity of coronary artery disease in patients with acute coronary syndrome.
Keywords: Brain natriuretic peptid, Coronary artery disease, Acute coronary syndrome
Abbreviations
- BMI
= body mass index
- HTN
= hypertension
- ACS
= acute coronary syndrome
- MI
= myocardial infarction
- UN
= unstable angina
- STEMI
= ST segment elevation myocardial infarction
- NSTEMI
= non-ST segment elevation myocardial infarction
- MACE
= major adverse cardiac events
- PCI
= percutaneous coronary intervention
- CABG
= coronary artery bypass graft
- EF
= ejection fraction
- NT-proBNP
= N-terminal pro brain natriuretic peptide
- BNP
= brain natriuretic peptide
- CRP
= C-reactive protein
- Ck
= creatine kinase
- GFR
= glomerular filtration rate
- LAD
= left anterior descending artery
- LCX
= left circumflex artery
- RCA
= right coronary artery
- M ± SD
= mean ± standard deviation
- CHF
= congestive heart failure
Introduction
Natriuretic peptides are vasoactive hormones secreted by the heart as part of a systemic response to cardiac stress and ventricular dysfunction. The precursor peptide of brain natriuretic peptide (BNP) is stored in granules of ventricular myocytes, where it is cleaved into an amino-terminal product (NT-proBNP) and the physiologically active BNP [1]. Release of BNP and NT-proBNP are regulated by wall stress and myocyte stretch [1–3]. Its diverse actions include natriuresis, vasodilation, inhibition of the renin-angiotensin-aldosterone system, and inhibition of sympathetic nerve activity [4]. The BNP levels trend upward to a peak between 14 and 40 h after an ischemic event [1,5,6]. Elevated BNP and NT-proBNP concentrations at admission in the setting of acute coronary syndrome (ACS) are associated with poor prognosis, including increased mortality, development of congestive heart failure (CHF), and recurrent ischemic events [1,7,8]. Specifically, when measured between one and four days after presentation with transmural infarction, an elevated plasma concentration of BNP was associated with increased mortality risk, independently of left ventricular function [9–11]. Baseline elevated BNP and NT-proBNP concentrations were predictive of adverse events at 30 and 90 days. Serial sampling did not improve the prognostic value of BNP or NT-proBNP [12]. Among patients with suspected ACS considered to be at low risk because of normal troponin values, NT-proBNP level of more than 474 pg/ml is able to identify individuals at higher risk. Because of its incremental prognostic value, NT-proBNP assessment should be considered in clinical routine for risk stratification of patients with normal troponin [13]. NT-proBNP can be a good parameter for predicting the severity of coronary vessels involvements alongside other diagnostic tools [14].
Aim of this work
The purpose of this study was to assess the short-term prognostic value of NT-proBNP and its relation to the severity of coronary artery disease in patients presenting with acute coronary syndrome.
Methodology
This study was conducted between February 2010 and August 2012 at Jeddah’s King Faisal Specialist Hospital and Research Centre, Ibn Sina Faculty of Medicine Hospital, and New Jeddah Clinic Hospital. It included 132 consecutive patients presenting with acute coronary syndrome (ACS), 64 patients with unstable angina (UA), 46 patients with non-ST segment elevation myocardial infarction (NSTEMI), and 22 patients with ST segment elevation myocardial infarction (STEMI). A written consent was taken from each patient according to the declaration of Helsinki and the ethical committee of all included hospitals. All patients were subjected to full history taking, clinical examination, ECG, echocardiography to assess ejection fraction, routine laboratory testing including serum creatinine, serial troponin I, creatine kinase (Ck), C-reactive protein (CRP) and NT-proBNP at the time of admission and post coronary angiography. Serum NT-proBNP was measured by a double antibody sandwich technique using Electrochemiluminescence as signal (Elecsys NT-proBNP, Roche Diagnostics), the intra-assay coefficient of variation was <6% on both normal and elevated levels [13]. Coronary angiography and percutaneous coronary intervention (PCI) when indicated were carried out for all patients within 48 h of admission.
Our patients were divided into two groups according to the levels of NT-proBNP: Group A with NT-proBNP less than 474 pg/ml and Group B with NT-proBNP equal or more than 474 pg/ml [13]. Both groups were compared regarding risk factors, clinical characteristics, major adverse cardiac events (MACE), ejection fraction and angiographic parameters including: site, degree of stenosis as assessed quantitatively, number of lesions, number of vessels affected, LAD versus non-LAD lesions and proximal versus distal lesions. The number of diseased coronary arteries was defined by the number of major coronary arteries with luminal diameter stenosis more than 70% or more than or equal to 50% stenosis in the left main coronary artery.
Statistical analyses were performed using Microsoft Office Excel. Continuous variables were expressed as means and standard deviation and compared by Student’s t-test while categorical variables were expressed as percentages and compared by chi square test, and results were considered significant if the P value was <0.05.
Results
There were no significant differences between Group A (NT-proBNP less than 474 pg/ml) and Group B (NT-proBNP equal or more than 474 pg/ml) regarding the base line characteristics (Table 1).
Table 1.
Baseline clinical characteristics of the studied population by NT-proBNP.
| Group A (NT pro-BNP < 474 pg/ml (N = 53) | Group B (NT pro-BNP ⩾ 474 pg/ml (N = 79) | p Value | |
|---|---|---|---|
| Median age (years) mean ± SD | 57.4 ± 9.9 | 59.8 ± 13.3 | >0.05 |
| Male gender (%) | 41 (77.4%) | 52 (65.8%) | >0.05 |
| Obesity (%) | 20 (37.7%) | 29 (36.7%) | >0.05 |
| BMI (mean ± SD) | 30.2 ± 5.99 | 30.4 ± 7.3 | >0.05 |
| Diabetes (%) | 37 (69.8%) | 54 (68.4%) | >0.05 |
| HTN (%) | 40 (75.5%) | 61 (77.2%) | >0.05 |
| Smoking (%) | 29 (54.7%) | 33 (41.8%) | >0.05 |
| Hyperlipidemia(%) | 49 (92.5%) | 68 (86.1%) | >0.05 |
| Previous MI (%) | 13 (24.5%) | 31 (39.2%) | >0.05 |
| History of angina (%) | 25 (47.2%) | 47 (59.5%) | >0.05 |
| Previous PCI(%) | 15 (28.3%) | 20 (25.3%) | >0.05 |
| History of CABG (%) | 5 (9.4%) | 7 (8.9%) | >0.05 |
There were no significant differences between Group A and Group B regarding ST segment deviation, CRP, troponin I and Ck (pre and post coronary angiography), serum creatinine and glomerular filtration rate. However, there were significant reductions in the ejection fraction and significant increase in T wave changes in Group B compared to Group A (Table 2).
Table 2.
ECG, Echocardiographic and laboratory characteristics of the studied population.
| NT pro-BNP < 474 pg/ml (N = 53) | NT pro-NP ⩾ 474 pg/ml (N = 79) | p Value | |
|---|---|---|---|
| % of patients who developed ST segment deviation at time of admission. | 20 (37.7%) | 35 (44.3%) | >0.05 |
| T wave changes % | 20 (37.7%) | 54 (68.4%) | <0.05 |
| EF % (mean ± SD) | 45.7 ± 7.8 | 36.7 ± 11.6 | 0.006 |
| CRP (mg/dl) (mean ± SD) | 18.5 ± 34.1 | 16.9 ± 34.0 | 0.79 |
| Troponin pre angio. (mean ± SD) | 0.9 ± 3.2 | 6.4 ± 28.6 | 0.166 |
| Troponin post angio. (ng/ml) (mean ± SD) | 1.34 ± 3.3 | 8.8 ± 48.7 | 0.268 |
| CK pre angio (ng/ml) (mean ± SD) | 363.3 ± 815.9 | 243.6 ± 504.4 | 0.3 |
| CK post angio. (ng/ml) (mean ± SD) | 335 ± 478.3 | 311.1 ± 515.8 | 0.639 |
| Serum creatinin (mmol/l) (mean ± SD) | 101.7 ± 68.7 | 128.3 ± 119.9 | 0.146 |
| GFR (mean ± SD) | 56.1 ± 9.7 | 53.0 ± 15.0 | 0.186 |
We found that the number of vessels affected and the percentage of stenosis were higher in Group B compared to Group A. Also, the percentage of proximal LAD stenosis was significantly higher in Group B compared to Group A. The TIMI flow was found to be significantly higher in Group A compared to Group B (Table 3).
Table 3.
Angiographic characteristics of the studied population.
| NT pro-BNP < 474 pg/ml (N = 53) | NT pro-BNP ⩾ 474 pg/ml (N = 79) | p Value | |
|---|---|---|---|
| LAD (%) | 35 (66.03%) | 65 (82.28.5%) | <0.05 |
| LCX (%) | 19 (35.8%) | 39 (49.4%) | >0.05 |
| RCA (%) | 22 (41.5%) | 46 (58.2%) | >0.05 |
| Single vessel disease % | 14 (26.4%) | 20 (25.3%) | >0.05 |
| Double vessels disease % | 22 (41.5%) | 27 (34.2%) | >0.05 |
| Three vessels disease % | 9 (16.98%) | 25 (31.6%) | >0.05 |
| Number of vessles mean ± SD | 1.68 ± 1.11 | 1.95 ± 1.07 | 0.004 |
| Degree of stenosis mean ± SD | 81 ± 25.9 | 89.1 ± 18.7 | 0.039 |
| Proximal LAD lesions (%) | 33 (6.3%) | 54 (68.4%) | 0.001 |
| Distal LAD lesions (%) | 6 (11.3%) | 5 (6.3%) | >0.05 |
| TIMI flow mean ± SD | 1.14 ± 0.95 | 0.82 ± 0.8 | 0.039 |
| Thrombus % | 8 (15.1%) | 9 (11.4%) | >0.05 |
There were significant increases in the incidence of heart failure and duration of hospital stay in Group B compared to Group A. There was a trend of increased incidence of cardiogenic shock and mortality in Group B compared to Group A, but this did not reach a significant value (Table 4).
Table 4.
Prognostic characteristics of the studied population.
| NT pro-BNP less than 474 pg/ml (N = 53) | NT pro-BNP equal or more than 474 pg/ml (N = 79) | p Value | |
|---|---|---|---|
| Heart failure | 2 (3.8%) | 12 (16.2%) | 0.03 |
| Cardiogenic shock | 1 (1.9%) | 6 (7.6%) | 0.05 |
| Mortality (%) | 0 (0%) | 4 (5.06%) | 0.06 |
| Duration of hospital stay | 4.1 ± 2.3 | 7.1 ± 4.5 | 0.005 |
When we classified patients according to their ejection fraction (EF) into three subgroups (less than 40%, from 40% to 50%, and more than 50%) we found that NT-proBNP levels were significantly higher in patients with EF less than 40% than other subgroups with EF from 40% to 50%, and more than 50% (Table 5; Fig. 1).
Table 5.
Diagram for correlation between NT-pro BNP and ejection fraction.
| EF less than 40%N. 42 | EF 40–50 N. 70 | EF more than50 N. 20 | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| NT pro-BNP | 2569.5 ± 2270.5 | 1342.46 ± 2002.0 | 328.4 ± 46.8 | 0.001 | 0.02 | 0.05 |
Abbreviations: P1 = EF less than 40% vs. EF 40–50%. P2 = EF less than 40% vs. EF more than 50%. P3 = EF40–50% vs, EF more than 50%.
Figure 1.
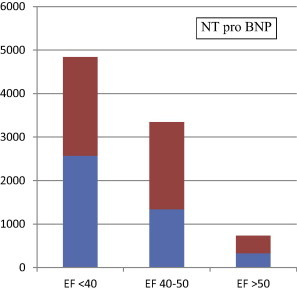
Mean and SD of NT-pro BNP according to the ejection fraction of all patients.
Patients with STEMI were found to have significantly higher levels of CRP, troponin I, NT-proBNP and CK compared to patients with NSTEMI and UA. Also, patients with NSTEMI were found to have significantly higher levels of CRP, troponin I, NT-proBNP and CK compared to patients with UA. Patients with STEMI were found to have significantly lower EF compared to patients with NSTEMI and UA (Table 6).
Table 6.
Comparison between the different presentation of ACS regarding heart failure, EF and laboratory results.
| STEMI N = 22 | NSTEMI N = 46 | UA N = 64 | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| EF % | 36.8 ± 7.9 | 38.9 ± 11.1 | 44.5 ± 11.8 | 0.006 | 0.04 | 0.01 |
| NTpro-BNP pre angio. M ± SD | 2295 ± 3215 | 1686.7 ± 1595.9 | 1317.7 ± 1891.7 | 0.001 | 0.018 | 0.284 |
| NTpro-BNP post angio. M ± SD | 3760.1 ± 3583.1 | 2410. 2 ± 374 | 1460.4 ± 2067 | 0.004 | 0.028 | 0.69 |
| CRP M ± SD | 39.8 ± 45.9 | 19.8 ± 30.3 | 8.3 ± 7.5 | 0.036 | 0.004 | 0.001 |
| Troponin pre angio. M ± SD | 8.01 ± 15.4 | 7.97 ± 26.0 | 0.20 ± 1.25 | 0.99 | 0.005 | 0.019 |
| Troponin post angio. M ± SD | 31.8 ± 89.2 | 1.1 ± 1.2 | 0.11 ± 0.32 | 0.022 | 0.005 | 0.001 |
| CK pre angio. M ± SD | 1140 ± 1281 | 183.4 ± 151.8 | 77.8 ± 45.8 | 0.003 | 0.002 | 0.001 |
| CK post angio. M ± SD | 891.9 ± 699 | 318.8 ± 358 | 125.8 ± 365.4 | 0.002 | 0.004 | 0.007 |
P1 = STENI vs. NSTEMI. P2 = STEMI vs. UA. P3 NSTEMI vs. UA.
Discussion
Brain natriuretic peptide is a neurohormone synthesized in ventricular myocardium and released in response to cardiac stretch. NT-proBNP is the N-terminal fragment of the prohormone BNP. These natriuretic peptides have prognostic value across the full spectrum of acute coronary syndrome patients. Patients with elevated BNP or NT-proBNP are at significantly increased risk for subsequently developing heart failure and death, in both short and long-term outcomes. This is seen regardless of their troponin levels and even when there is no clinical evidence of heart failure [15,16]. The prognostic value of these peptides is over conventional risk factors like age, Killip class, and left ventricular ejection fraction. Studies have shown that BNP can predict high risk features in ACS, such as more severe underlying atherosclerosis, left ventricular dysfunction, left ventricular hypertrophy, and the burden of the ischemic insult [17]. In patients with ACS, the higher the BNP, the more severe the hemodynamic insult due to ischemia and the worse the prognosis [18].
In the present study, we found that the ejection fraction was significantly reduced in patients with NT-proBNP equal to or more than 474 pg/ml compared to patients with NT-proBNP less than 474 pg/ml. This result was comparable to that reported by Shahabi et al. [14]. Furthermore, we found highly significant negative correlation between NT-proBNP and ejection fraction (r = 0.234, p = 0.0063). This result was concordant with Shahabi et al. [14] and was also supported by Emdin et al. [19] who found that NT-proBNP had acceptable accuracy for identifying heart failure due to left ventricular dysfunction [19]. Additionally, in the Grewal study, BNP was identified as the strongest predictor of diastolic dysfunction as determined by Doppler-echocardiography [20].
We found that in patients with acute coronary syndrome, the number of vessels affected and percentage of stenosis were significantly higher statistically in those with high NT-proBNP (equal to or more than 474 pg/ml) compared to those with low NT-proBNP (less than 474 pg/ml). These results were concordant with other studies that focused on the association between the severity of CAD and NT-pro-BNP level [21–23]. Weber et al. [21] found that serum BNP level could effectively predict coronary involvement based on the number of affected coronary vessels in patients with angina pectoris. Palazzuoli et al. [22] found that BNP was associated with a larger extent and greater severity of myocardial ischemia in patients with non-ST segment elevation myocardial infarction. Also, Sadanandan et al. [23] showed that patients with BNP more than 80 pg/ml had tighter culprit vessel stenosis and a higher number of culprit vessels compared to cases with lower plasma BNP level. Furthermore, Hamishayev et al. [24] found a significant correlation between NT-proBNP levels and the number of affected vessels in patients with unstable angina and ST segment elevation MI [24]. Also, Niizuma et al. found that plasma B-type natriuretic peptide levels reflect the presence and severity of stable coronary artery disease in chronic hemodialysis patients [25].
In contrast, in a logistic regression analysis by Nishikimi et al. it was revealed that N-terminal pro ANP, but not BNP, was independently associated with coronary artery stenosis after adjusting for clinical and demographic variables. However, the sensitivity, specificity, and positive and negative predictive values of each peptide were not sufficiently high to be used for prediction. In their study, patients with no evidence of CAD were compared to those with CAD, and the severity of coronary defects was not quantitatively determined [26].
In our study, we found that patients with elevated NT-proBNP more than 474 pc/ml had a significantly lower TIMI flow grade compared to patients with NT-proBNP less than 474 pc/ml. This result was concordant with Mega et al. [27] who found that elevated BNP at presentation was associated with evidence of subsequent impaired epicardial and myocardial reperfusion. Specifically, patients with BNP more than 80 pg/ml were more likely to have incomplete reperfusion of the infarct-related artery. In addition, elevated BNP was associated with incomplete (less than 70%) resolution of ST-segment elevation, which is an indicator of impaired myocardial tissue-level reperfusion [28].
In our study, we found a significantly higher incidence of heart failure and longer duration of hospital stay among patients with high NT-proBNP compared to those with low NT-proBNP. Also, there was a trend toward higher incidence of cardiogenic shock and mortality among patients with high NT-proBNP.
Patients with higher BNP had an 11-fold higher risk of death compared to those with lower BNP. A trend toward more frequent new or worsening CHF was also apparent among those with BNP more than 80 pg/ml [27]. These results were consistent with many studies of patients with STEMI [1,5,9–11,29]. Together, these studies provide consistent evidence for the additive prognostic value of BNP. Moreover, whether measured at presentation or later during recovery, BNP is one of the most robust indicators of mortality risk [27]. This strong prognostic association in STEMI parallels that among patients with non-ST segment elevation acute coronary syndromes [30].
Conclusion
In general, according to the results of this study and previous similar studies, it seems that the NT-proBNP in acute coronary syndrome is an appropriate marker associated with more coronary artery involvements regarding number of vessels affected and severity of stenosis. In addition, it is a valuable marker for predicting higher incidence of heart failure and lower ejection fraction.
Limitations of the study
(1) The number of patients in this study was relatively small and the duration of follow-up was short (hospital stay only) so mortality and cardiogenic shock did not reach statistical significance. (2) The study excluded patients with hepatitis C but did not exclude patients with mixed cryoglobulinemia where many studies revealed high levels of circulating NT-proBNP in patients with mixed cryoglobulinemia and hepatitis C. Antonelli et al. [31] however, concluded that increase in NT-proBNP could indicate the presence of a subclinical cardiac dysfunction.
Recommendations
Measurements of the NT-proBNP in patients with acute coronary syndrome is a useful marker for risk stratification of these patients.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Morita E., Yasue H., Yoshimura M., Ogawa H., Jougasaki M., Matsumura T. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993;88(1):82–91. doi: 10.1161/01.cir.88.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Motwani J.G., McAlpine H., Kennedy N., Struthers A.D. Plasma brain natriuretic peptide as an indicator for angiotensin-converting-enzyme inhibition after myocardial infarction. Lancet. 1993;341(8853):1109–1113. doi: 10.1016/0140-6736(93)93126-l. [DOI] [PubMed] [Google Scholar]
- 3.Hama N., Itoh H., Shirakami G., Nakagawa O., Suga S., Ogawa Y. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation. 1995;92(6):1558–1564. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 4.Stein B.C., Levin R.I. Natriuretic peptides: physiology, therapeutic potential, and risk stratification in ischemic heart disease. Am Heart J. 1998;135(5 Pt 1):914–923. doi: 10.1016/s0002-8703(98)70054-7. [DOI] [PubMed] [Google Scholar]
- 5.Talwar S., Squire I.B., Downie P.F., Mccullough A.M., Campton M.C., Davies J.E. Profile of plasma N-terminal proBNP following acute myocardial infarction; correlation with left ventricular systolic dysfunction. Eur Heart J. 2000;21(18):1514–1521. doi: 10.1053/euhj.1999.2045. [DOI] [PubMed] [Google Scholar]
- 6.Wiese S., Breyer T., Dragu A., Wakili R., Burkard T., Schmidt-Schweda S. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation. 2000;102(25):3074–3079. doi: 10.1161/01.cir.102.25.3074. [DOI] [PubMed] [Google Scholar]
- 7.Hall C. Essential biochemistry and physiology of (NT-pro) BNP. Eur J Heart Fail. 2004;6(3):257–260. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Morrow D.A., de Lemos J.A., Blazing M.A., Sabatine M.S., Murphy S.A., Jarolim P. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294(22):2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 9.Omland T., Aakvaag A., Bonarjee V.V., Caidahl K., Lie R.T., Nilsen D.W. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93(11):1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa N., Nakamura M., Aoki H., Hiramori K. Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol. 1996;27(7):1656–1661. doi: 10.1016/0735-1097(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 11.Richards A.M., Nicholls M.G., Yandle T.G., Frampton C., Espiner E.A., Turner J.G. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97(19):1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 12.Kwan G., Isakson S.R., Beede J., Clopton P., Maisel A.S., Fitzgerald R.L. Short-term serial sampling of natriuretic peptides in patients presenting with chest pain. J Am Coll Cardiol. 2007;49(11):1186–1192. doi: 10.1016/j.jacc.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Weber M., Bazzino O., Navarro Estrada J.L., Fuselli J.J., Botto F., Perez de Arenaza D. N-terminal B-type natriuretic peptide assessment provides incremental prognostic information in patients with acute coronary syndromes and normal troponin T values upon admission. J Am Coll Cardiol. 2008;51(12) doi: 10.1016/j.jacc.2007.11.054. 1188–95. [DOI] [PubMed] [Google Scholar]
- 14.Shahabi V., Moazenzadeh M., Azimzadeh B.S., Nasri H., Afshar R.M., Shahesmaili A. Relationship between serum N-terminal pro brain natriuretic peptide (NT-proBNP) level and the severity of coronary artery involvements. J Res Med Sci. 2011;16(2):143–148. [PMC free article] [PubMed] [Google Scholar]
- 15.de Lemos J.A., Morrow D.A., Bentley J.H., Omland T., Sabatine M.S., McCabe C.H. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345(14):1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 16.Omland T., Persson A., Ng L., O’Brien R., Karlsson T., Herlitz J. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106(23):2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 17.Christenson R.H., Duh S.H., Apple F.S., Bodor G.S., Bunk D.M., Dalluge J. Standardization of cardiac troponin I assays: round Robin of ten candidate reference materials. Clin Chem. 2001;47(3):431–437. [PubMed] [Google Scholar]
- 18.Nagesh C.M., Roy A. Role of biomarkers in risk stratification of acute coronary syndrome. Indian J Med Res. 2010;132(5):627–633. doi: 10.4103/0971-5916.73419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emdin M., Passino C., Prontera C., Fontana M., Poletti R., Gabutti A. Comparison of brain natriuretic peptide (BNP) and amino-terminal ProBNP for early diagnosis of heart failure. Clin Chem. 2007;53(7):1289–1297. doi: 10.1373/clinchem.2006.080234. [DOI] [PubMed] [Google Scholar]
- 20.Grewal J., McKelvie R., Lonn E., Tait P., Carlsson J., Gianni M. BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail. 2008;10(3):252–259. doi: 10.1016/j.ejheart.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Weber M., Dill T., Arnold R., Rau M., Ekinci O., Müller K.D. N-terminal B-type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J. 2004;148(4):612–620. doi: 10.1016/j.ahj.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Palazzuoli A., Gennari L., Calabria P., Quatrini I., Vecchiato L., De Paola V. Relation of plasma brain natriuretic peptide levels in non-ST-elevation coronary disease and preserved systolic function to number of narrowed coronary arteries. Am J Cardiol. 2005;96(12):1705–1710. doi: 10.1016/j.amjcard.2005.07.094. [DOI] [PubMed] [Google Scholar]
- 23.Sadanandan S., Cannon C.P., Chekuri K., Murphy S.A., Dibattiste P.M., Morrow D.A. Association of elevated B-type natriuretic peptide levels with angiographic findings among patients with unstable angina and non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44(3):564–568. doi: 10.1016/j.jacc.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Hamishayev J.Z. Can NT-proBNP together with Doppler tissue imaging predict severity of coronary artery disease in patients with acute coronary syndrome? Atherosclerosis Suppl. 2008;9(1):174–175. [Google Scholar]
- 25.Niizuma S., Iwanaga Y., Yahata T., Goto Y., Kita T., Miyazaki S. Plasma B-type natriuretic peptide levels reflect the presence and severity of stable coronary artery disease in chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24(2):597–603. doi: 10.1093/ndt/gfn491. [DOI] [PubMed] [Google Scholar]
- 26.Nishikimi T., Mori Y., Ishimura K., Tadokoro K., Yagi H., Yabe A. Association of plasma atrial natriuretic peptide, N-terminal proatrial natriuretic peptide, and brain natriuretic peptide levels with coronary artery stenosis in patients with normal left ventricular systolic function. Am J Med. 2004;116(8):517–523. doi: 10.1016/j.amjmed.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Mega J.L., Morrow D.A., de Lemos J.A., Sabatine M.S., Murphy S.A., Rifai N. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE–TIMI-23 substudy. J Am Coll Cardiol. 2004;44(2):335–339. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 28.de Lemos J.A., Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol. 2001;38(5):1283–1294. doi: 10.1016/s0735-1097(01)01550-9. [DOI] [PubMed] [Google Scholar]
- 29.de Lemos J.A., McGuire D.K., Drazner M.H. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362(9380):316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 30.Morrow D.A., de Lemos J.A., Sabatine M.S., Murphy S.A., Demopoulos L.A., DiBattiste P.M. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41(8):1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli A., Ferri C., Ferrari S.M., Ghiri E., Galetta F., Franzoni F. High circulating levels of N-terminal pro-brain natriuretic peptide and interleukin 6 in patients with mixed cryoglobulinemia. J Med Virol. 2010;82(2):297–303. doi: 10.1002/jmv.21636. [DOI] [PubMed] [Google Scholar]



