Abstract
Background
Alcohol use and abuse patterns have created a need for novel treatment models. Current research has turned its focus on reward pathways associated with intrinsic necessities, such as feeding. Theories suggest that drugs of abuse seize control of natural reward pathways and disregulate normal function, leading to chronic addiction. One such pathway involving the hunger stimulating peptide, ghrelin, is the focus of our study.
Methods
Male C57BL/6 mice were randomly assigned to groups and treated with vehicle or a ghrelin antagonist, either [D-Lys3]-GHRP-6 (DLys) or JMV2959. Three experiments tested ghrelin antagonism using different doses; Experiment 1 – 12mg/kg JMV2959; Experiment 2 – 15mg/kg DLys; Experiment 3 – 9mg/kg JMV2959. Using a two-bottle choice 24-hour access paradigm, data was collected for ethanol intake, preference, water intake, and food intake at 4- and 24-hours after injection.
Results
Experiment 1 showed that 12mg/kg of JMV2959 decreased ethanol, water, and food intake, without affecting preference. Experiment 2 showed that 15mg/kg of DLys decreased ethanol intake, preference, and water intake only on the first day of treatment. Experiment 3 showed that 9mg/kg of JMV2959 decreased only ethanol and food intake. No change was seen during deprivation and JMV2959 was still effective at reducing ethanol intake upon reintroduction. Despite the change in food intake, there were no differences in body weight throughout the experiments. It should be noted that the majority of significant effects were only found 4-hours post injection.
Conclusion
The results show that compounds that block ghrelin receptor activity are effective at decreasing ethanol intake. However, DLys was only effective at reducing intake and preference on the first day, suggesting a quick tolerance and selectivity for ethanol. JMV2959 consistently reduced ethanol intake, but at the higher dose also reduced all other consummatory behaviors. Thus, ghrelin antagonists provide a viable potential for treatment of alcohol abuse disorders, but further research is needed to determine an appropriate dose and administration paradigm.
Keywords: Ethanol, Ghrelin Antagonists, Self-Administration, Two-Bottle Choice
Introduction
Alcoholism is a chronic disorder that takes a toll on the individual and society resulting in an estimated 2.5 million deaths per year (World Health Organization, 2011). Current treatments for alcohol abuse disorders involve both behavioral and pharmacological methods and ongoing research is aimed at understanding this disease at the neurological level (Spoth and Willenbring, 2010). Research has focused on reward pathways as targets for pharmacological modification in an effort to curb addictive behaviors (Wise, 1987; Koob, 2006). In an effort to expand the understanding of addictive disorders, researchers have turned focus to systems regulating rewards associated with food consumption (review DiLeone et al, 2012). In agreement with this idea, studies have shown that appetitive hormones affect alcohol use and abuse related behaviors in humans (review, Kenna et al, 2012).
Of particular interest to research in this area is the gut derived hormone, ghrelin. Ghrelin is a peptide secreted primarily by the stomach during times of hunger and acting on growth hormone secretagogue receptors (GHSRs) (Kojima et al, 1999). GHSRs are G-protein coupled receptors that regulate energy via release of growth hormone from the pituitary (NCBI, 2013). Alcohol dependence dysregulates the ghrelin system and a positive correlation has been found between ghrelin levels and craving in actively drinking patients diagnosed with alcohol dependence (Addolorato et al, 2006; Leggio et al, 2012). This correlation has been further tested to show that intravenous ghrelin administration causes an increased craving score on the Alcohol Visual Analog Scale in alcoholics (Leggio et al, 2014). Abstinence from alcohol increases ghrelin levels and these levels are directly correlated with the amount of time abstinent (Kraus et al, 2005; Kim et al, 2005). In congruence, consumption of oral alcohol lowers ghrelin levels in healthy humans, suggesting a possible driving mechanism for drinking alcohol in response to hunger (Calissendorff et al, 2005, 2012; Zimmermann et al, 2007). However, it should be noted that route of administration is important, as Leggio et al. (2013) has shown that intravenous administration of alcohol leads to minimal change in ghrelin levels.
In contrast, recent studies have shown that pharmacologically or genetically blocking ghrelin receptor activity can reduce self-administration of drugs of abuse. Using animal models, studies have shown that administration of ghrelin can increase ethanol intake, while ghrelin antagonism reduces ethanol intake (Jerlhag et al, 2009). In addition, ghrelin antagonists have been shown to significantly reduce the behavioral and physiological effects of cocaine (Clifford et al, 2012), amphetamine (Jerlhag et al, 2010), and nicotine (Wellman et al, 2011; Jerlhag and Engel, 2011). In agreement with pharmacological studies, GHSR knockout mice show reduced voluntary ethanol intake and diminished ethanol-induced conditioned place preference (Jerlhag et al, 2009). Similarly, ghrelin knockout mice show attenuated alcohol-induced release of dopamine and alcohol-induced locomotor simulation (Jerlhag et al, 2011), as well as decreased alcohol intake and ethanol-induced conditioned place preference (Bahi et al, 2013). Thus, targeting the ghrelin system may have a significant potential for treatment of drug abuse and addition.
The current study compared two ghrelin receptor antagonists, [D-Lys3]-GHRP-6 (DLys) and JMV2959 on alcohol intake in C57BL/6J mice. These two antagonists have been shown to block or reduce alcohol intake in various models. DLys is a peptide-based antagonist of GHSR (Traebert et al, 2002). Kaur and Ryabinin (2010) found DLys reduces ethanol intake and preference, while also blocking ethanol-induced c-Fos expression in the Edinger-Westphal nucleus, a region affiliated with ethanol sensitivity and intake. DLys has also been shown to block ethanol reward seeking in rats (Hajnal et al, 2012). JMV2959 is a small molecule-type specific antagonist of ghrelin receptors (Moulin et al, 2007). Bahi et al. (2013) has shown that the reduction of ethanol intake/preference by JMV2959 is only seen in wild-type C57BL/6 mice and not their GHSR knockout littermates, indicating that the effects of JMV2959 on these behaviors are mediated by GHSR. Recent studies have shown that, in response to ethanol, JMV2959 administration changes dopamine release (Jerlhag et al, 2009), novelty seeking (Hansson et al, 2012), and ventral tegmental Ghsr mRNA expression (Suchankova et al, 2013). Behaviors associated with alcohol consumption, like conditioned place preference or operant self-administration, have also been suppressed by exogenous treatment with JMV2959 (Bahi et al, 2013; Landgren et al, 2011). Thus, the potential of ghrelin antagonism as a possible mechanism for alcohol abuse treatment exists.
A majority of the above studies have tested ghrelin or its antagonist on an acute basis. To more fully determine the potential of ghrelin antagonism as a treatment for alcohol abuse, studies need to test specific drugs on a chronic level. In order to assess whether a drug is pharmacologically relevant for treatment it must show effectiveness without tolerance. Thus in the present study, two different ghrelin antagonists (DLys and JMV2959) were tested over a period of 4-12 consecutive days. As per previously referenced effects of ghrelin antagonism on ethanol drinking behavior, we expected both drugs to reduce ethanol intake and preference. Additionally, to determine if these drugs were specific for ethanol, water and food intakes were measured daily.
Methods
Subjects
Adult male C57BL/6J mice (6-7 weeks old) were obtained from Jackson Laboratories West (Davis, CA). Upon arrival mice were individually housed with food and water available ad libitum on a 12-hour reverse light cycle (lights off at 07:00). Mice were split into three experiments and assigned to receive a daily intraperitoneal injection of either saline or a ghrelin antagonist. All procedures were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee.
Drugs
Two different ghrelin receptor antagonists were used; [D-Lys3]-GHRP-6 (DLys) (Tocris Bioscience, USA, Cat #1922) and JMV2959 (Aeterna Zentaris, Gemany). Sterile saline (0.9% sodium chloride) was used as vehicle and compound dissolvent for all experiments. Experiment 1: JMV2959 was injected at a dose of 12 mg/kg. This dose was chosen based on preliminary work done in our lab. Experiment 2: DLys was injected at a dose of 15 mg/kg, which was the dose used in a previous experiment showing a reduction in ethanol intake (Kaur and Ryabinin, 2010). Experiment 3: JMV2959 was injected at a dose of 9 mg/kg, an intermediate dose between 12 mg/kg and 6 mg/kg, which did not have effects in our preliminary studies. All solutions of drugs were prepared daily and mixed by vortexing for at least 30 seconds. Injections were given using 28G ½ ml syringes and side of intraperitoneal injection was alternated every day. Ethanol solutions and water were given in 25 ml graduated cylinders fitted with a sipper tube which allowed for readings to the nearest 0.1 ml.
Procedures
For all experiments, animals were allowed to acclimate to their environment for one week. All mice received at minimum four saline injections (0.1 cc) prior to drug administration to habituate them to the procedure. The control (CON) groups were injected with an equal volume of saline compared to experimental groups (0.01 ml/g). Ethanol, preference, water, and food intake was measured 4-hours following the injection (15:00) and again 24-hours after the injection (11:00 next day). Ethanol (10% v/v) and water intake was measured by reading graduated cylinders to the nearest 0.1 ml. Food was weighed (g) and the difference was taken as amount consumed. All measurements for consummatory behaviors were converted from raw data (ml or g) into g/kg. For ethanol, density was accounted for; hence, data was quantified using the formula: [intake (ml) × ethanol density (0.0789 g/cm3)] ÷ body weight (kg). Every other day the bottles were switched to control for possible side preference and body weights were measured.
Experiment 1
Mice were given 24-hour access to ethanol (10% v/v) using a two bottle choice paradigm (n=12/group) for four days prior to drug administration. JMV2959 (12 mg/kg) was injected 4-hours into the dark phase (11:00). Mice were euthanized immediately after the 4 hours of drinking on the final day of the experiment and trunk blood was collected for blood ethanol content analysis. Using an Analox GL5, 5μl of sera was analyzed in duplicates to assess possible differences in blood ethanol content between mice treated with ghrelin antagonist or vehicle.
Experiment 2
A different set of mice were used (n=12/group). The procedures were conducted exactly as in Experiment 1 except that testing was conducted for 4-days and D-Lys-3-GHRP-6 (15 mg/kg) was injected 4-hours after lights off (11:00).
Experiment 3
Since repeated injections of DLys in Experiment 2 had no effects on any measures after day 1, mice used for Experiment 2 were reused in Experiment 3; however, mice that were previously in the control group were now assigned to the drug group and vice versa. Mice were given 2-days without injections after the final DLys treatment, but had continuous access to ethanol, water and food. Following the 2-day washout, mice (n=12/group) were injected with JMV2959 (9 mg/kg) or vehicle for eight consecutive days. The following four days were used to test long-lasting effects of JMV2959 and its effects on reinstatement of drinking. On the ninth day, drug administration was discontinued, but all mice were still injected with saline and still had access to ethanol, food, and water. On days 10-11, mice were deprived of ethanol, but were still injected with saline. Side preference was still calculated to determine if mice drink more water out of the expected “ethanol” side. The last day (12) reintroduced drug administration and ethanol access to test for possible deprivation-induced increases in ethanol intake/preference. As In Experiment 1, mice were euthanized immediately after the 4 hours of drinking on the final day of the experiment for analysis of blood ethanol concentration.
Statistical Analyses
For all experiments, during times of consecutive drug and ethanol administration, repeated measures ANOVAs were run with days as the within group factor and drug as the between group factor (experiment 1: 12×2; experiment 2: 4×2; experiment 3: 8×2). Repeated measures tests were run to assess ethanol intake, preference, water intake, food intake, and body weights. Any significant within subjects interactions were further assessed with post-hoc independent samples t-tests on each day of treatment. For experiment 2, planned comparisons tested between group differences on day-1 using an independent samples one tailed t-test. For experiment 3, planned comparisons assessed each individual day during deprivation (days 9-12) between groups using independent samples one tailed t-tests. Finally, blood ethanol content for experiments 1 and 3 were analyzed between groups using an independent samples one tailed t-test.
Results
Experiment 1: Effects of 12 mg/kg JMV2959 Injections (Figure 1)
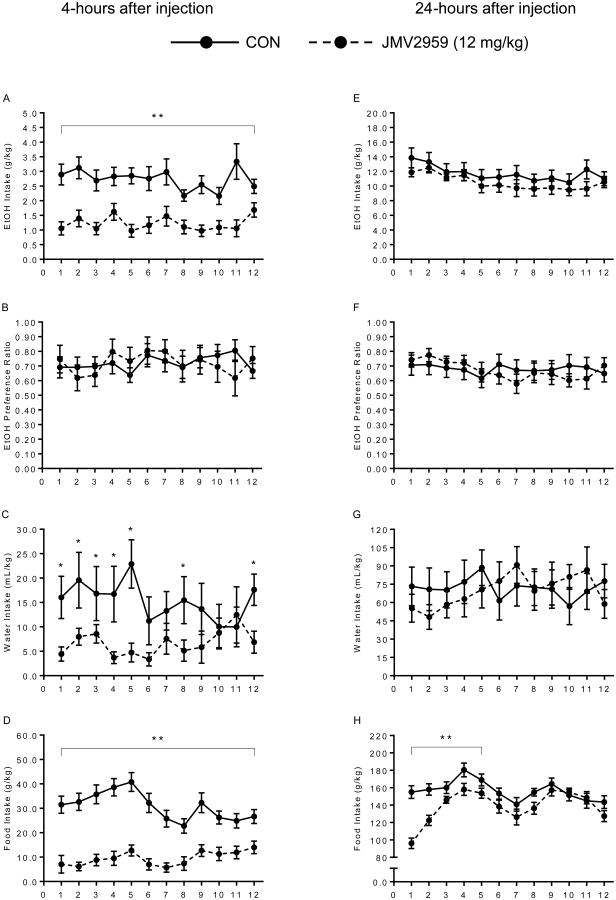
Figure 1.
Experiment 1 – Control group (solid line) versus 12 mg/kg JMV2959 (dotted line). JMV2959 effectively reduced ethanol (A: **p<0.0001), water (C: *p<0.05), and food (D: **p<0.0001) intake over the 12-days of treatment without changing ethanol preference. Food intake was also suppressed at 24-hours for the first 5-days of treatment (H: **p<0.05). Right column shows data taken 4-hours after injection (A-D). Left column shows data taken 24-hours after injection (E-H).
Treatment with 12 mg/kg JMV2959 or saline led to a between subjects main effect on voluntary ethanol intake [F(1,22)=35.79, p=0.0001], showing that the JMV2959 group drank significantly less than the CON group 4-hours after injection. The reduction in voluntary ethanol consumption was present all 12-days of treatment and no within group differences were found. However, despite a decrease in ethanol intake, preference for ethanol was not different between groups [F(1,16)=0.04, p=0.85]. There were within group treatment × day interactions for water and food intake at the 4-hour check point [F(11,242)=2.92, p=0.037; F(11,242)=1.92, p=0.001; respectively]. While the interactive effects were present for all days in food intake, the JMV2959 altered water intake mainly at the beginning of treatment days 1-5. Between groups, water and food intake was decreased by treatment with JMV2959 compared to saline [F(1,22)=4.55, p=0.04 and F(1,22)=68.56, p=0.0001; respectively]. These results suggest that 12 mg/kg of JMV2959 has a global suppressive effect of general consummatory behavior. In contrast to the 4-hour measurements, we saw no decreases in ethanol and water intake from CON levels 24-hours post-injection with JMV2959 [F(1,22)=1.04, p=0.32 and F(1,22)=0.02, p=0.90, respectively]. There was a within subjects interaction of treatment × day [F(11,242)=4.40, p=0.0001] and a between subjects effect showing that JMV2959 still had an effect on food intake 24-hours after the injection [F(1,22)=9.61, p=0.005], with mice in the drug group showing less consumption. Post-hoc analysis indicated that food intake at 24-hours was only different between groups for the first 5 days, with no difference seen for the remainder of testing (days 6-12). The difference in blood ethanol concentrations between groups did not reach statistical significance [t(22)=1.44, p=0.08, data not shown]. Despite the reduced intake in food during the 12-days of treatment, there was no difference in body weights between groups [M≈23g; F(1,22)=1.03, p=0.32, data not shown].
Effects of 15 mg/kg D-Lys3-GHRP-6 Injections (Figure 2)
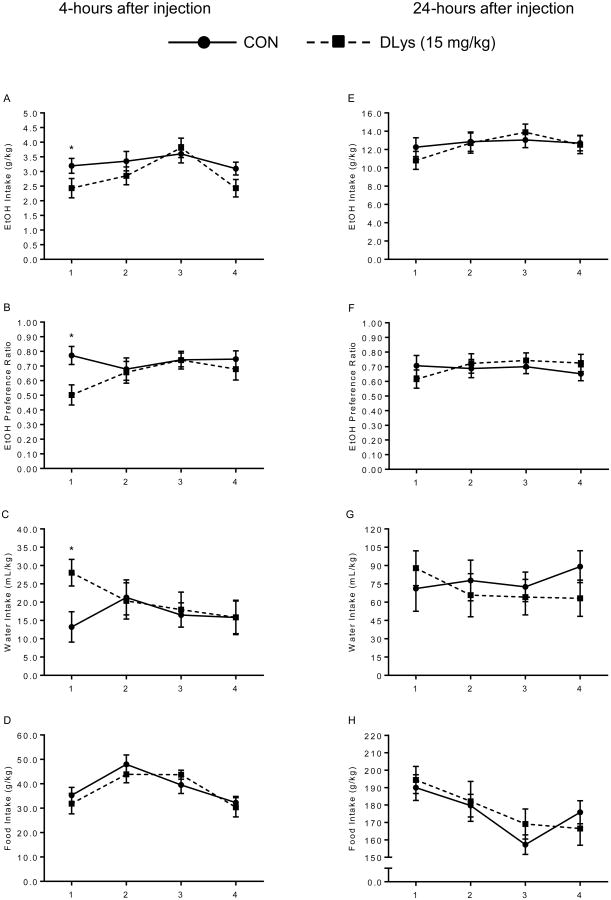
Figure 2.
Experiment 2 – Control group (solid line) versus 15 mg/kg DLys (dotted line). No long-term effects over days of treatment were found between groups at either time point. On day-1, DLys reduced ethanol intake (A: *p=0.04) and preference (B: *p=0.004), while also increasing water intake (C: *p=0.007) and leaving food intake unaffected. Right column shows data taken 4-hours after injection (A-D). Left column shows data taken 24-hours after injection (E-H).
Repeated-measures 2×4 ANOVAs found only one significant within group interaction of treatment × day on ethanol preference [F(3,66)=2.91, p=0.041]. There were no statistically significant group effects of treatment on ethanol intake, preference, water intake, or food intake over the 4-days of treatment at both 4- and 24-hours post-injection. However, based on previous work in our lab (Kaur and Ryabinin, 2010) and expected outcome, planned comparisons assessed the first day for all dependent variables 4-hours after treatment, and found that DLys decreased voluntary ethanol consumption compared to CON [t(22)=1.84, p=0.04]. Additionally, ethanol preference was reduced in mice treated with DLys [t(22)=2.93, p=0.004], which corresponded to an increase in water intake by the DLys group compared to CON [t(22)=2.69, p=0.007]. Although, ethanol, preference, and water intake were affected on the first day by DLys, food intake remained stable and non-different between groups. Body weight was also not different between groups [F(1,22)=0.01, p=0.92]. This suggests a specific, albeit transient, pharmacological effect of DLys treatment on ethanol related consummatory behaviors. Since the drug was not effective in regulating measured behaviors past day 1, we discontinued the experiments after four days of treatment and tested these mice for effectiveness of a lower dose of JMV2959.
Effects of 9 mg/kg JMV2959 Injections (Figure 3)
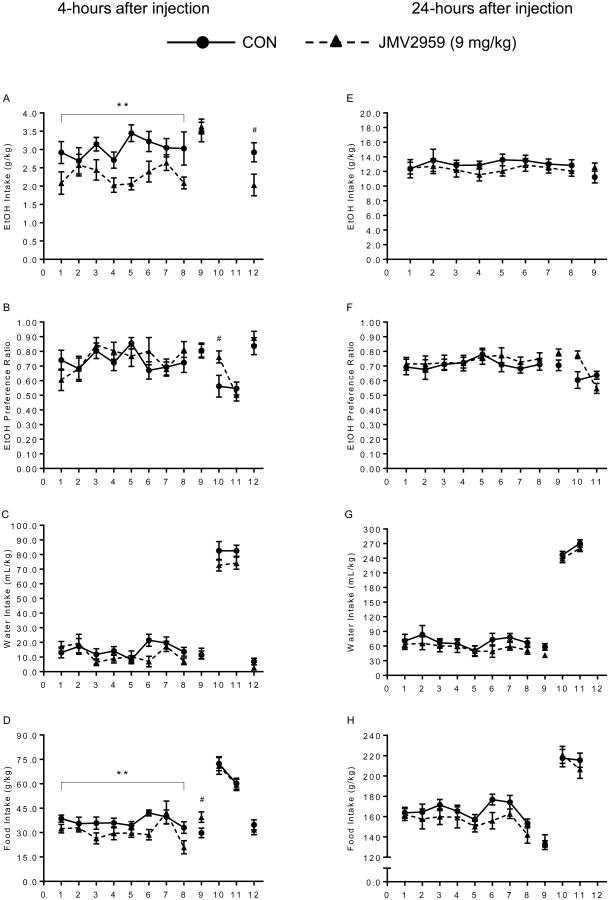
Figure 3.
Experiment 3 – Control group (solid line) versus 9 mg/kg JMV2959 (dotted line). During the first 8-days of treatment, JMV2959 reduced ethanol intake (A: **p=0.006) and food intake (D: **p=0.02) without affecting preference or water intake. On day-9, without drugs, there was no difference between groups, except for an increase in food intake (D: # p=0.02) by the JMV2959 group. On day-10, there was a side preference for the expected “ethanol” side seen in the JMV2959 group (B: # p=0.014). Finally, when ethanol and drugs were reintroduced (day-12), JMV2959 only reduced ethanol intake (A: # p=0.017). Right column shows data taken 4-hours after injection (A-D). Left column shows data taken 24-hours after injection (E-H).
Mice were treated consecutively for 8-days with JMV2959 and allowed access to ethanol, water, and food ad libitum. Repeated-measures 2×8 ANOVAs found no significant within group effects, but found significant between group differences in ethanol and food intake 4-hours after injection [F(1,22)=9.42, p=0.006; F(1,22)=6.38, p=0.019, respectively], with the JMV2959 group showing decreased intake compared to CON. However, the decrease in ethanol and food consumption attributed to ghrelin antagonism was not significant at 24-hours after the injection. At neither time point did JMV2959 or saline injections alter ethanol preference or water consumption [4-hour: F(1,22)=0.06, p=0.81 and F(1,22)=1.19, p=0.29; 24-hours: F(1,22)=0.19, p=0.66 and F(1,22)=0.77, p=0.39, respectively].
During the deprivation periods, planned independent samples t-tests were conducted on each individual day to test differences between established groups. Without any drug treatment, mice in the JMV2959 group showed equal ethanol intake, preference, and water intake compared to CON at both 4- and 24-hour time points (p>0.05). However, there was an increase in food intake the day after (4-hour) the last drug injection in the JMV2959 group [t(22)=2.23, p=0.02]. When both ethanol and drug was discontinued, there was no difference in water or food consumption between groups. Interestingly, the JMV2959 group displayed a side preference for the bottle where the ethanol would have been on the first day of deprivation [t(22)=2.37, p=0.014]. On the final day, when ethanol and drug was reintroduced, JMV2959 treated mice showed a decrease only in ethanol intake [t(22)=2.27, p=0.017], while preference, water, and food intake remained equal between groups. Blood ethanol content was not significantly different between groups [t(22)=0.07, p=0.94, data not shown]. Although food intake was suppressed only at 4-hours, overall body weights were not different between groups [F(1,22)=0.07, p=0.79].
Discussion
The effectiveness of two ghrelin antagonists in suppressing ethanol consumption was tested following daily treatments and differing results were found. DLys was found to suppress ethanol intake/preference only on the first day of treatment 4-hours after injection. No difference in consummatory behaviors between DLys and vehicle treated groups were detected at 24-hours post-injection. Treatment with the higher dose of JMV2959 (12mg/kg) resulted in a consistent suppression of ethanol intake at 4-hours but no effects after 24-hours. However, unlike DLys, JMV2959 had no effect on preference and also consistently decreased water and food intake at the 4-hour mark, which stabilized to control levels at 24-hours post-injection. The lower dose of JMV2959 (9mg/kg) was effective at decreasing ethanol intake without significantly altering water intake. However, much like the higher dose, food intake was decreased and preference was unaltered with no effects seen 24-hours post injection. Thus, DLys appears to have a specific effect on reducing ethanol related consummatory behaviors, but is only effective on the first day. On the other hand, JMV2959 has a robust effect consistently decreasing ethanol intake across days, but lacks effects on preference and decreases water and food intake. The effect of JMV2959 seems to be due to a general gross reduction in all consummatory behaviors.
Until recently, ghrelin and its antagonists have been used to study and treat eating related disorders. A wealth of new studies have shown that ghrelin (both agonists and antagonists) have a powerful effect on drug responses and subsequent use. Specifically, ghrelin antagonists (JMV2959) when injected alone do not appear to change general behavioral activity which includes locomotion, catalepsy, immobility, and stereotyped activity (Sustkova-Fiserova et al, 2014). Ghrelin itself shows reinforcement properties akin to drugs of abuse by causing increases in locomotion, release of dopamine in the accumbens, and induction of conditioned place preference (Jerlhag, 2008). In the current study, although in agreement with previous works showing DLys reduces ethanol intake (Kaur and Ryabinin, 2010; Hajnal et al, 2012), the effect was only seen 4-hours post-injection on the first day. In a previous study from our lab, it was shown that following a 4-hour 2-bottle ethanol access, mice treated with DLys show decreased drinking and associated lower blood ethanol contents (BEC). Although BECs were not assessed in the DLys group, behaviorally, DLys had the specificity of only blocking ethanol related consumption following its first administration, the data suggest a quick tolerance to the drug which my hinder its ability to provide adequate treatment for alcohol abuse disorders. BECs were measured in Experiments 1 and 3. There was no difference in BECs between groups of treatment. However, our animals had 24-hour access to ethanol and the time of blood sampling was substantially past typical circadian peak of consummatory behaviors in mice (Freund, 1970; Goldstein and Kakihana, 1977). In relation to food consumption, infusion of ghrelin to rats via mini-pump over a period of 12-days (250 pmol/day) has been shown to increase both body weight and food intake (Nagazato et al, 2001), which is suppressed following an injection of DLys. Future research is needed to test whether several administrations of DLys throughout the day or increasing the dose may prolong the ability of DLys to suppress ethanol intake.
The ghrelin antagonist tested, JMV2959, is a potent GHSR-1a antagonist and has been shown to block endogenous ghrelin binding activity and alter the effects of drugs of abuse (review, Moulin et al, 2012). For our study, the effects of JMV2959 were more robust with respects to consistently reducing ethanol intake, which is in support of others that have seen this effect acutely (Jerlhag et al, 2009; Landgren et al, 2011; Bahi et al, 2013). Here we show that while JMV2959 was effective at suppressing ethanol consumption, at high doses it also suppresses water and food intake. Thus, without suppressing preference, JMV2959 appears to act on a global level to shut down all consumption while pharmacologically active. One potential confound was that the same mice from experiment 2 were used for experiment 3 and this may have influenced the results. However, the authors deem it unlikely since the effects of DLys were non-effective after day 1, mice were allowed a 2-day washout, and the groups were switched so DLys mice now received vehicle and vehicle mice now received JMV2959. Furthermore, should DLys have retained any long-lasting effects, it is speculated that consumption of ethanol in the control mice of experiment 3 (former DLys group) would have been lower than controls in the other experiments (1 and 2) which was not seen. Additionally, it would have been expected that no difference between the former DLys group and new JMV2959 group would have been found. A recent study found, following 8-months of voluntary drinking, rats injected with JMV2959 daily for 5-day had reduced ethanol intake each day at both 1-hour and 24-hours post-injection (Suchankova et al, 2013). The current data only found a suppressing effect at 4-hours not 24. However, the difference in results may be due to our study using C57BL/6J mice that drink more (g/kg) than Wistar rats, the shorter duration of drinking prior to drug treatment, and the doses used. Suchankova et al found that JMV2959 suppressed increased drinking following ethanol deprivation in rats. In experiment 3, we found the JMV2959 group developed a side preference for the expected ethanol side on day 10 when no ethanol was present. This effect has implications for a possible learning mechanism affected by treatment with JMV2959. It is difficult to speculate on these findings, but it may be that JMV2959 is altering ethanol salience thus increasing motivation to seek out ethanol once treatment is discontinued. There may also be a possibility that JMV2959 facilitates memory in a way that allows the mice to learn the bottles will be switched every day. However, these are only speculations and further research is needed to determine what, if any, effects JMV2959 has on learning and memory. We did not see an increase in ethanol intake after its removal, suggesting the amount of drinking prior to deprivation was not enough to lead to this effect associated with dependence. However, JMV2959 was still effective in suppression of alcohol intake following ethanol deprivation, suggesting its potential use for preventing alcohol relapse.
Studies on mechanisms associated with basic survival, such as feeding, have proven essential towards understanding the mechanisms by which drugs of abuse act in the brain. In agreement with previous findings, we have shown that antagonism of the orexigenic hormone ghrelin has an ability to suppress high ethanol intake in mice. Extending previous findings we demonstrate that while each of the two tested drugs can raise its own concerns regarding their potential therapeutic use (quick development of tolerance for DLys and non-specific effects on consummatory responses for JMV2959), these concerns are not inherent in the ghrelin system as a target for drug development. Thus, lack of suppression of water and food consumption following administration of DLys, indicate that ghrelin antagonists can have selective effects on consumption of alcohol. On the other hand, consistent effects of JMV2959 on consumption of ethanol indicate that targeting the ghrelin receptor does not necessarily lead to tolerance to its effects. Therefore, this study along with others, suggest that ghrelin antagonists may have translational potential to treat human individuals suffering from alcohol abuse disorders. With this respect, endogenous ghrelin levels are positively correlated with alcohol craving (Addolorato et al, 2006; Leggio et al, 2012) andit has been recently shown that intravenous administration of ghrelin induces craving in active drinking alcoholics (Leggio et al, 2014). Thus, there is strong evidence suggesting that the ghrelin system plays a pivotal role in alcohol drinking among healthy and dependent individuals. The current study looked at the effects of ghrelin antagonisms shortly after ethanol was introduced. Future studies are aimed to determine whether these antagonists are effective once ethanol dependence is well established, producing a more translational model of the human population seeking treatment for alcohol use disorders. To conclude, ghrelin antagonism affects ethanol related behaviors, and it is pertinent to determine the effectiveness of their potential use to treat alcohol use disorders.
Acknowledgments
Support: NIH Grants – T32AA007468 and AA016647. JMV2959 provided by Aeterna Zentaris, Gemany.
References
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, Farnetti S, Domenicali M, D'Angelo C, Vonghia L, Mirijello A, Cardone S, Gasbarrini G. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Bahi A, Tolle V, Fehrentz JA, Brunel L, Martinez J, Tomasetto CL, Karam SM. Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides. 2013;43:48–55. doi: 10.1016/j.peptides.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152:743–747. doi: 10.1530/eje.1.01905. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Gustafsson T, Holst JJ, Brismar K, Rojdmark S. Alcohol intake and its effect on some appetite-regulating hormones in man: influence of gastroprotection with sucralfate. Endocr Res. 2012;37:154–162. doi: 10.3109/07435800.2012.662662. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Rodriguez J, Schul D, Hughes S, Kniffin T, Hart N, Eitan S, Brunel L, Fehrentz JA, Martinez J, et al. Attenuation of cocaine-induced locomotor sensitization in rats sustaining genetic or pharmacologic antagonism of ghrelin receptors. Addict Biol. 2012;17:956–963. doi: 10.1111/j.1369-1600.2011.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G. Alcohol consumption and its circadian distribution in mice. J Nutr. 1970;100:30–36. doi: 10.1093/jn/100.1.30. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Kakihana R. Circadian rhythms of ethanol consumption by mice: a simple computer analysis for chronopharmacology. Psychopharmacology (Berl) 1977;52:41–45. doi: 10.1007/BF00426598. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND, Thanos PK. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One. 2012;7:e49121. doi: 10.1371/journal.pone.0049121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson C, Shirazi RH, Naslund J, Vogel H, Neuber C, Holm G, Anckarsater H, Dickson SL, Eriksson E, Skibicka KP. Ghrelin influences novelty seeking behavior in rodents and men. PLoS One. 2012;7:e50409. doi: 10.1371/journal.pone.0050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211:415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Engel JA. Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend. 2011;117:126–131. doi: 10.1016/j.drugalcdep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Landgren S, Egecioglu E, Dickson SL, Engel JA. The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol. 2011;45:341–347. doi: 10.1016/j.alcohol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012;22:211–228. doi: 10.1007/s11065-012-9209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Yoon SJ, Choi B, Kim TS, Woo YS, Kim W, Myrick H, Peterson BS, Choi YB, Kim YK, Jeong J. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40:76–79. doi: 10.1093/alcalc/agh108. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 Suppl 1:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U, Kornhuber J, Bleich S. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29:2154–2157. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol. 2011;17:86–94. doi: 10.1111/j.1369-1600.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17:452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Schwandt ML, Oot EN, Dias AA, Ramchandani VA. Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo-controlled study. Psychoneuroendocrinology. 2013;38:3085–3091. doi: 10.1016/j.psyneuen.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.019. Advance online publication. DOI: http://dx.doi.org/10.1016/j.biopsych.2014.03.019. [DOI] [PMC free article] [PubMed]
- Moulin A, Brunel L, Boeglin D, Demange L, Ryan J, M'Kadmi C, Denoyelle S, Martinez J, Fehrentz JA. The 1,2,4-triazole as a scaffold for the design of ghrelin receptor ligands: development of JMV2959, a potent antagonist. Amino Acids. 2012;44:301–314. doi: 10.1007/s00726-012-1355-2. [DOI] [PubMed] [Google Scholar]
- Moulin A, Demange L, Berge G, Gagne D, Ryan J, Mousseaux D, Heitz A, Perrissoud D, Locatelli V, Torsello A, et al. Toward potent ghrelin receptor ligands based on trisubstituted 1,2,4-triazole structure. 2. Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem. 2007;50:5790–5806. doi: 10.1021/jm0704550. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- NCBI. Gene 2693 (database online) Bethesda MD: National Center for Biotechnology Information; 2013. [Updated November 17, 2013]. [Google Scholar]
- Spoth M, Willenbring ML. The past and future of research on treatment of alcohol dependence. Alcohol Res Health. 2010;33:55–63. [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS One. 2013;8:e71284. doi: 10.1371/journal.pone.0071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustkova-Fiserova M, Jerabek P, Havlickova T, Kacer P, Krsiak M. Ghrelin receptor antagonism of morphine-induced accumbens dopamine release and behavioral stimulation in rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3466-9. ahead of print. [DOI] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol. 2002;14:580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Clifford PS, Rodriguez J, Hughes S, Eitan S, Brunel L, Fehrentz JA, Martinez J. Pharmacologic antagonism of ghrelin receptors attenuates development of nicotine induced locomotor sensitization in rats. Regul Pept. 2011;172:77–80. doi: 10.1016/j.regpep.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) [Accessed November 15, 2013];Alcohol Fact Sheet [WHO Web site] 2011 Feb; Available at: http://www.who.int/mediacentre/factsheets/fs349/en/#.
- Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12:17–21. doi: 10.1111/j.1369-1600.2006.00026.x. [DOI] [PubMed] [Google Scholar]