Abstract
We analyzed the association of age at antiretroviral therapy (ART) initiation with CD4+ T cell count recovery, death, and loss to follow-up (LTFU) among HIV-infected adults in Zambia. We compared baseline characteristics of patients by sex and age at ART initiation [categorized as 16–29 years, 30–39 years, 40–49 years, 50–59 years, and 60 years and older]. We used the medication possession ratio to assess adherence and analysis of covariance to measure the adjusted change in CD4+ T cell count during ART. Using Cox proportional hazard regression, we examined the association of age with death and LTFU. In a secondary analysis, we repeated models with age as a continuous variable. Among 92,130 HIV-infected adults who initiated ART, the median age was 34 years and 6,281 (6.8%) were aged ≥50 years. Compared with 16–29 year olds, 40–49 year olds (–46 cells/mm3), 50–59 year olds (–53 cells/mm3), and 60+ year olds (–60 cells/mm3) had reduced CD4+ T cell gains during ART. The adjusted hazard ratio (AHR) for death was increased for individuals aged ≥40 years (AHR 1.25 for 40–49 year olds, 1.56 for 50–59 year olds, and 2.97 for 60+ year olds). Adherence and retention in care were poorest among 16–29 year olds but similar in other groups. As a continuous variable, a 5-year increase in age predicted reduced CD4+ T cell count recovery and increased risk of death. Increased age at ART initiation was associated with poorer clinical outcomes, while age <30 years was associated with a higher likelihood of being lost to follow-up. HIV treatment guidelines should consider age-specific recommendations.
Introduction
Widespread use of antiretroviral therapy (ART) has substantially reduced acquired immunodeficiency syndrome (AIDS)-related mortality and increased life expectancy for persons living with human immunodeficiency virus (HIV) infection.1–3 Recently, HIV treatment programs in resource-limited settings (RLS) have begun to highlight the emergence of an older population of HIV-infected adults.4 Worldwide, the Joint United Nations Program on HIV/AIDS (UNAIDS) estimates that 2.8 million older adults (age ≥50 years) are living with HIV/AIDS.5 Within sub-Saharan Africa (SSA), nearly 1 in 10 HIV-infected adult is 50 years of age and older.6
Although aging of the HIV/AIDS epidemic is welcome evidence of HIV treatment program success, recent reports from SSA suggest that older patients may be more likely to experience adverse ART outcomes when compared to their younger counterparts,6–8 possibly as a result of late ART initiation and/or delayed CD4+ T cell count recovery. Late ART initiation could be due to lower HIV/AIDS knowledge9,10 or lower HIV testing rates among older compared to younger adults.11 Once older adults initiate ART, they may experience reduced immune recovery, which is a risk factor for both AIDS and non-AIDS-associated conditions.12
In many prior analyses, age has been dichotomized at 50 years,8,13,14 based in part on the World Health Organization's (WHO) definition of old age.15 Yet aging is a continuous physiologic process whose effects on ART outcomes may become clinically apparent before or after that arbitrary threshold. For example, studies from SSA have reported that 40–49 year olds on ART may have significantly reduced CD4+ T cell count gains16 and increased mortality6 compared with their younger counterparts. In contrast, another study observed increased in mortality only among 60+ year olds.7 As the population of older HIV-infected adults grows, the health of this group will increasingly impact HIV programs in RLS.17 We investigate here the association of age with ART outcomes.
Materials and Methods
To assess treatment outcomes among older HIV-infected patients on ART, we explored the association of age at ART initiation with subsequent immunologic recovery, death, and loss to follow-up. We analyzed data from a well-characterized cohort of HIV patients in Lusaka, the capital city of Zambia.18,19 According to Zambian Ministry of Health guidelines, from 2004 to 2010, HIV-infected adults (age≥16) years were eligible for ART with WHO stage 4 disease, a CD4+ T cell count <200 cells/mm3, or WHO stage 3 disease and a CD4+ T cell count <350 cells/mm3. In 2011 ART eligibility was expanded to all patients with either WHO stage 3 or 4 disease or CD4+ T cell count <350 cells/mm3.
For this analysis, we defined baseline as the date of ART initiation, and categorized baseline age as 16–29 years, 30–39 years, 40–49 years, 50–59 years, and 60 years and above. At baseline all patients undergo a history and physical examination, WHO clinical disease staging, and laboratory studies that include a CD4+ T cell count. Stratified by age category, we compared baseline characteristics using the Jonckheere–Terpstra test for categorical variables and the Wilcoxon signed-rank test for continuous variables.
Following ART initiation, patients were asked to attend the clinic for clinical visits and to collect antiretrovival drugs at 2, 6, and 12 weeks, and then every 3–6 months, based upon clinical status. CD4+ T cell count was monitored every 6 months and viral load was measured only when treatment failure was suspected.20 At each visit, adherence was assessed through self-report and pharmacy refill records. For this analysis, we examined ART adherence using the medication-possession ratio (MPR). MPR described the percentage of time a patient had possession of ART, based on pharmacy refill records. At each ART dispensation, the pharmacy provided the patient with a refill appointment that corresponded to the date when the drugs would run out. If the patient refilled beyond that date, the number of late days was counted. We calculated the MPR as the cumulative number of late days divided by the time on ART, and then we multiplied the quotient by 100. MPR was categorized as optimal (≥95%), suboptimal (80–94%), and poor (<80%) on the basis of previously established thresholds, and then compared across age strata and by sex using a Chi-square test.20,21
Death was ascertained by report of a family member, clinic staff member, or community health worker. At the time of administrative censoring (January 31, 2011), all patients who were ≥60 days late for a scheduled appointment or ART refill were considered lost to follow-up (LTFU) and censored at 60 days after their last visit.22
Using analysis of covariance, we calculated the mean CD4+ T cell count change from baseline to 12 and 24 months of therapy for each age category, adjusted for sex, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared), WHO disease stage, baseline CD4+ T cell count, year of treatment initiation, ART regimen, and adherence. Our primary analysis of CD4+ T cell count change was a complete case analysis, but under a sensitivity analysis, we also used inverse probability weights (IPW) to address missing CD4+ counts. To describe the probability of death and LTFU, we utilized Kaplan–Meier survival analysis. We used multivariable Cox proportional hazard models to examine the association between age at treatment initiation and death and LTFU. In our analysis of death on ART, we performed a sensitivity analyses with the exclusion of patients who were LTFU and used IPW to adjust for the probability of LTFU.
Under a secondary analysis, we considered age at ART initiation as a continuous variable. Using multivariable logistic regression, we determined the odds of having a CD4+ T cell count of 350 cells/mm3 or above at 12 and 24 months of ART for a 2-year, 5-year, and 10-year increase of age at therapy initiation. In a similar fashion, we also repeated multivariable Cox proportional hazard models for death and LTFU.
To explore the possible contribution of comorbid medical conditions on the risk of death, we analyzed the proportion of patients with baseline renal impairment (defined as creatinine clearance <90 ml/min by the Modification of Diet in Renal Disease equation23), abnormal liver function tests (defined as alanine aminotransferase greater than the upper limit of normal, 33 U/liter), and overweight or obesity (defined as BMI ≥25) at ART initiation. We compared the proportion of patients with each condition by age category using the Jonckheere–Terpstra test. We defined multimorbidity as having two or more of the above conditions at ART initiation. To determine the possible contribution of age-associated comorbid conditions on the risk of death, we included multimorbidity in Cox models as a secondary analysis.
We used SAS version 9.2 (SAS Institute, Cary, NC) and Stata version 12 (Statacorp, College Station, TX) for statistical analysis of this deidentified observational data. Ethical approval was obtained from the University of Zambia (Lusaka, Zambia) and the University of North Carolina at Chapel Hill (Chapel Hill, NC).
Results
Between April 1, 2004 and January 31, 2011, within 22 public-sector clinics in Lusaka, 92,130 HIV-infected adults initiated ART. The median duration of follow-up was 536 days [interquartile range (IQR), 172–1,172 days]. The median age at ART initiation was 34 years (range, 16–95 years). Of the total, 6,281 (6.8%) were 50 years of age or older, with 5,018 (5.4%) aged 50–59 and 1,263 (1.4%) aged 60–95 years. As age increased, the proportion of women decreased from 77% of 16–29 year olds to 45% of 60–95 year olds (p for trend <0.001).
At baseline, BMI, CD4+ T cell count, and first-line regimen were clinically similar with increasing age category. However, the proportion of women with WHO stage 3 or 4 disease increased with age from 50% of 16–29 year olds to 65% of 60–95 year olds (p for trend <0.001), in part due to increased prevalence of tuberculosis with increasing age. Across all age strata, relative to women, men had lower median CD4+ T cell counts (135 vs. 163 cells/mm3) and a larger proportion of men had WHO stage 3 or 4 disease (69% vs. 53%; both p<0.001). Contrary to the trend observed in women, the proportion of men with WHO stage 3 or 4 disease decreased slightly with increasing age, from 72% for 16–29 year olds to 65% for 60–95 year olds (p for trend <0.001), in part due to decreased prevalence of tuberculosis with increasing age. With increasing age, tenofovir use increased among women from 37% to 47% and decreased among men from 53% to 42% (both p for trend <0.001). In Table 1 we display the baseline characteristics of the cohort, by age and sex.
Table 1.
Characteristics of HIV-Infected (N=92,130) Zambia Adults Who Initiated Antiretroviral Therapy During 2004–2011, by Age and Sex at Therapy Initiation
| Age, years | ||||||
|---|---|---|---|---|---|---|
| Sex, characteristic | 16–29 | 30–39 | 40–49 | 50–59 | 60–95 | |
| Women | n=20,902 | n=23,670 | n=8,870 | n=2,501 | n=573 | p* |
| BMIa | 20 (18–23) | 21 (18–23) | 21 (19–24) | 21 (19–24) | 20 (18–23) | <0.001 |
| WHO disease stage | ||||||
| 1 or 2 | 10,294 (50) | 10,431 (45) | 3,412 (39) | 913 (37) | 193 (35) | <0.001 |
| 3 or 4 | 10,181 (50) | 12,808 (55) | 5,285 (61) | 1535 (63) | 360 (65) | |
| Tuberculosis | 1,141 (6) | 1,425 (6) | 562 (6) | 176 (7) | 45 (8) | <0.001 |
| CD4+ T cell count, cells/mm3 | 169 (93–247) | 148 (80–220) | 144 (80–215) | 157 (85–229) | 164 (97–247) | <0.001 |
| Year of ART start | ||||||
| 2004–2006 | 5,331 (26) | 7,459 (32) | 3,245 (37) | 825 (33) | 151 (26) | <0.001 |
| 2007–2011 | 15,572 (75) | 16,211 (69) | 5,625 (63) | 1,676 (67) | 422 (74) | |
| First-line ART regimen | ||||||
| AZT+XTC+NVP/EFV | 6,001 (29) | 6,519 (28) | 2,272 (26) | 621 (25) | 121 (21) | <0.001 |
| D4T+XTC+NVP/EFV | 6,311 (30) | 7,189 (30) | 2,640 (30) | 650 (26) | 155 (27) | |
| TDF+XTC+NVP/EFV | 7,798 (37) | 9,254 (39) | 3,712 (42) | 1129 (45) | 271 (47) | |
| Other | 788 (4) | 708 (3) | 246 (3) | 101 (4) | 26 (5) | |
| Died during follow-up | 1,426 (7) | 1,872 (8) | 782 (9) | 274 (11) | 86 (15) | <0.001 |
| Lost to follow-up | 5,745 (27) | 5,214 (22) | 1,878 (21) | 510 (20) | 146 (25) | <0.001 |
| Men | n=6,242 | n=17,348 | n=8,817 | n=2,517 | n=690 | |
|---|---|---|---|---|---|---|
| BMI | 19 (17, 21) | 19 (18, 21) | 20 (18, 22) | 20 (18, 22) | 20 (18, 22) | <0.001 |
| WHO disease stage | ||||||
| 1 or 2 | 1,742 (28) | 4,928 (29) | 2,749 (32) | 806 (33) | 240 (35) | <0.001 |
| 3 or 4 | 4,386 (72) | 12,119 (71) | 5,904 (68) | 1,659 (67) | 438 (65) | |
| Tuberculosis | 728 (12) | 1900 (11) | 923 (11) | 206 (8) | 51 (7) | 0.028 |
| CD4+ T cell count cells/mm3 | 149 (77, 226) | 130 (64, 205) | 123 (63, 196) | 129 (66, 204) | 140 (78, 216) | <0.001 |
| Year of ART start | ||||||
| 2004–2006 | 1,625 (26) | 5,214 (30) | 3,047 (35) | 910 (36) | 227 (33) | <0.001 |
| 2007–2011 | 4,617 (74) | 12,134 (70) | 5,770 (65) | 1,607 (64) | 463 (67) | |
| First-line ART regimen | ||||||
| AZT+XTC+NVP/EFV | 1,455 (23) | 4,599 (27) | 2,540 (29) | 744 (30) | 201 (29) | <0.001 |
| D4T+XTC+NVP/EFV | 1,319 (21) | 3,717 (21) | 2,036 (23) | 615 (24) | 160 (23) | |
| TDF+XTC+NVP/EFV | 3,288 (53) | 8,595 (50) | 3,980 (45) | 1,046 (42) | 288 (42) | |
| Other | 180 (3) | 437 (3) | 261 (3) | 112 (4) | 41 (6) | |
| Died during follow-up | 692 (11) | 1,815 (10) | 1,039 (12) | 335 (13) | 132 (19) | <0.001 |
| Lost to follow-up | 2,074 (33) | 4,699 (27) | 2,141 (24) | 624 (25) | 156 (23) | <0.001 |
Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Wilcoxon trend test used for continuous variables and Jonckheere–Terpstra test used for categorical variables.
Data are the number (%) of patients or median value (interquartile range).
ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; XTC, lamivudine or emtricitabine; IQR, interquartile range; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization; 3TC, lamivudine.
During the initial 12 months of ART, the median MPR was 98% (IQR, 90–100%). The proportion of patients with optimal adherence (i.e., ≥95%) by 12-month MPR was 61%, while 26% had suboptimal (i.e., 80–94%) and 13% had poor adherence (i.e., <80%). We did not observe substantial differences in adherence levels between women and men. For example, the proportion of men with poor 12-month adherence (14%) was similar to that of women (13%). When examined by age category, adherence was optimal for 58% of 16–29 year olds, 61% of 30–39 year olds, 63% of 40–49 year olds, 62% of 50–59 year olds, and 60% of 60–95 year olds. A slightly larger proportion of young patients (16–29 year olds) had poor adherence compared with 30+ year olds (15% vs. 12%).
At baseline, CD4+ T cell counts were documented for 88,858 (96%) patients. Of those with a baseline measurement, repeat CD4+ T cell counts were available for 50,456 (58%) of individuals at 12 months and 43,152 (49%) at 24 months. We observed a decrease in CD4+ T cell count gains with increasing age at ART initiation (p for trend <0.001). Over the initial 12 months of ART, 16–29 year olds experienced an adjusted mean increase of 239 cells/mm3 [95% confidence interval (CI), 231–246]; in contrast, the average increases were 205 cells/mm3 (95% CI, 201–209) for 30–39 year olds, 193 cells/mm3 (95% CI, 188–197) for 40–49 year olds, 186 cells/mm3 (95% CI, 179–193) for 50–59 year olds, and 179 cells/mm3 (95% CI, 164–193) for 60+ year olds. At 24 months of therapy, we observed similar trends (Fig. 1). In sensitivity analyses that adjusted for missing data, the trend observed in the primary analysis was similar.
FIG. 1.
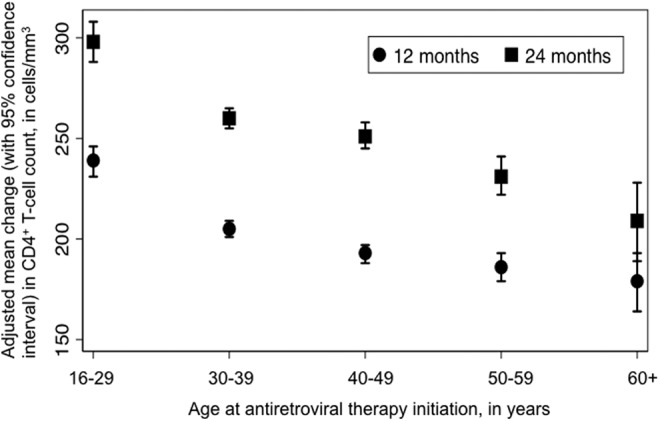
CD4+ T cell count gains at 12 and 24 months of antiretroviral therapy, by age at therapy initiation.
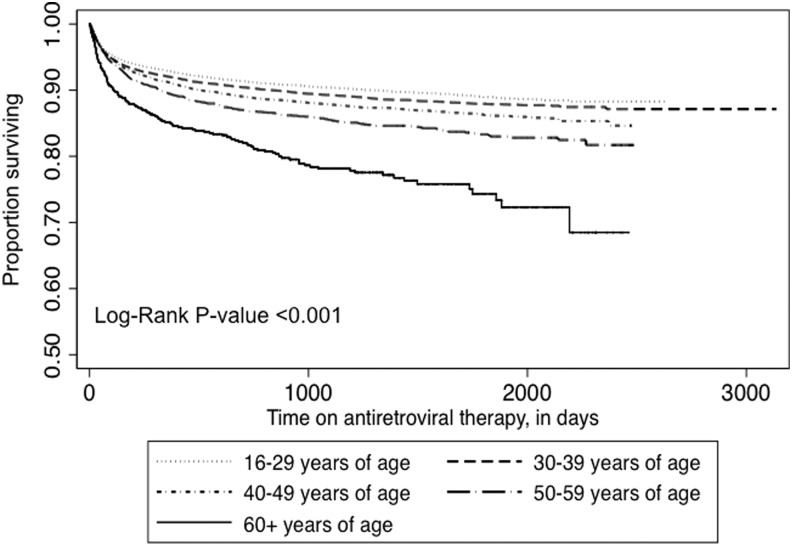
During follow-up, 8,453 (9.2%) patients died. In Kaplan–Meier analysis, the risk of death increased as age category increased (p<0.001, by the log-rank test; Fig. 2). During the initial 90 days of ART, 60+ year olds had a disproportionately high risk of death (unadjusted hazard ratio 2.13, 95% CI, 1.76–2.59). Male sex, BMI <18, lower CD4+ T cell count, and WHO stage 3 or 4 disease were also associated with early mortality (all p<0.001). In unadjusted models that considered the entire time on ART, relative to 16–29 years, increasing age at ART initiation was associated with the risk of death (Table 2). After adjustment for other factors associated with death during ART including sex, BMI, baseline WHO stage, and baseline CD4+ T cell count, individuals aged 40–49 years [AHR 1.25 (95% CI, 1.08–1.45)], 50–59 years [AHR 1.56 (95% CI, 1.28–1.92)], and 60+ years [AHR 2.97 (95% CI, 2.21–3.99)] experienced increased mortality. In sensitivity analyses that adjusted for LTFU, mortality hazard ratios were similar.
FIG. 2.
Kaplan–Meier estimates of mortality during antiretroviral therapy, by age at therapy initiation.
Table 2.
Association of Age with the Risk of Death and Loss to Follow-Up Among HIV-Infected Zambian Adults Taking Antiretroviral Therapy
| Death | Loss to follow-up | |||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Age, years | ||||
| 16–29 | Reference | Reference | Reference | Reference |
| 30–39 | 1.11 (1.06–1.17) | 1.03 (0.90–1.17) | 0.77 (0.74–0.79) | 0.76 (0.72–0.79) |
| 40–49 | 1.25 (1.18–1.33) | 1.25 (1.08–1.45) | 0.69 (0.66–0.72) | 0.69 (0.65–0.73) |
| 50–59 | 1.50 (1.37–1.64) | 1.56 (1.28–1.92) | 0.70 (0.66–0.75) | 0.71 (0.64–0.78) |
| 60–95 | 2.31 (2.01–2.65) | 2.97 (2.21–3.99) | 0.87 (0.77–0.97) | 0.70 (0.58–0.85) |
| Sex | ||||
| Women | Reference | Reference | Reference | Reference |
| Men | 1.48 (1.42–1.55) | 1.38 (1.24–1.52) | 1.21 (1.18–1.24) | 1.33 (1.27–1.39) |
| BMIa | ||||
| <18 | Reference | Reference | Reference | Reference |
| 18–24.9 | 0.38 (0.36–0.39) | 0.70 (0.63–0.77) | 0.73 (0.71–0.75) | 0.89 (0.85–0.93) |
| ≥25 | 0.22 (0.20–0.24) | 0.62 (0.51–0.77) | 0.69 (0.66–0.72) | 0.87 (0.81–0.94) |
| WHO disease stage | ||||
| 1 or 2 | Reference | Reference | Reference | Reference |
| 3 | 2.31 (2.19–2.44) | 1.39 (1.23–1.56) | 1.00 (0.97–1.03) | 1.03 (0.99–1.08) |
| 4 | 3.49 (3.25–3.76) | 1.72 (1.44–2.04) | 1.24 (1.18–1.30) | 1.06 (0.98–1.15) |
| Baseline CD4+ T cell | ||||
| count, per 50 cells/mm3 increase | 0.81 (0.80–0.82) | 0.93 (0.91–0.95) | 1.04 (1.03–1.04) | 1.02 (1.01–1.03) |
| Year of ART start | ||||
| 2004–2006 | Reference | Reference | Reference | Reference |
| 2007–2011 | 0.64 (0.61–0.67) | 0.69 (0.60–0.79) | 1.71 (1.66–1.77) | 1.64 (1.55–1.73) |
| First-line ART regimen | ||||
| AZT+XTC+NVP/EFV | Reference | Reference | Reference | Reference |
| D4T+XTC+NVP/EFV | 1.87 (1.77–1.97) | 1.28 (1.15–1.43) | 1.18 (1.14–1.22) | 1.12 (1.07–1.17) |
| TDF+XTC+NVP/EFV | 1.18 (1.11–1.25) | 1.04 (0.87–1.24) | 1.48 (1.43–1.53) | 1.30 (1.21–1.38) |
| Other | 1.63 (1.44–1.86) | 0.97 (0.55–1.73) | 2.60 (2.42–2.78) | 1.86 (1.58–2.19) |
| Adherence, MPRb | ||||
| <80 | Reference | Reference | Reference | Reference |
| 80–94 | 0.75 (0.64–0.87) | 0.70 (0.60–0.82) | 0.56 (0.53–0.60) | 0.58 (0.55–0.62) |
| 95–100 | 0.66 (0.57–0.75) | 0.64 (0.56–0.74) | 0.40 (0.38–0.42) | 0.40 (0.38–0.42) |
Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Medication possession ratio (MPR) is calculated by dividing the number of days of ART possession (based on pharmacy data) by the number of days on ART and multiplying the quotient by 100.
ART, antiretroviral therapy; HR, hazard ratio; CI, confidence interval; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; XTC, either lamivudine or emtricitabine; WHO, World Health Organization.
In addition to deaths, 23,187 (25.1%) patients were LTFU during ART. While the oldest patients experienced the highest risk of death during ART, the youngest patients had the highest risk of LTFU relative to other groups (p<0.001, by the log-rank test; Fig. 3). Compared to 30+ year olds, 16–29 year olds experienced approximately 30% increased hazard of LTFU [AHR 1.32 (95% CI, 1.26–1.39)]. At 30 years of age and above, no substantial differences were found in the relative hazard of LTFU (Table 2).
FIG. 3.
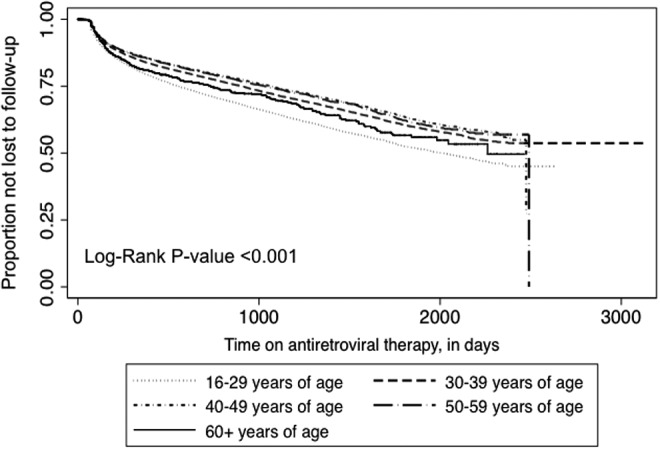
Kaplan–Meier estimates of losses to follow-up during antiretroviral therapy, by age at therapy initiation.
In a secondary analysis of age as a continuous variable, after adjustment for sex, BMI, WHO disease stage, baseline CD4+ T cell count, ART regimen, year of initiation, and adherence, each 5-year increase in age was associated with an 11% decreased odds of achieving a CD4+ T cell count ≥350 cells/mm3 at 12 months of therapy [adjusted odds ratio (AOR), 0.89 (95% CI, 0.88–0.90)]. A 5-year increase in age was also associated with a 10% increased risk of death [AHR 1.10 (95 CI, 1.07–1.13)] and a 14% decreased risk of LTFU [AHR, 0.86 (95% CI, 0.84–0.88)]. Findings were comparable when we used 2- and 10-year increments (data not shown).
With increasing age, the proportion of patients with multimorbidity increased. The proportion of patients with renal impairment increased with age category from 7.8% for 16–29 year olds to 46.6% for 60+ year olds and the proportion with overweight/obesity increased from 9.5% for 16–29 year olds to 14.6% for 50–59 year olds (both p for trend <0.001; Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). The proportion of patients with abnormal liver function tests peaked at 20.3% for 30–39 year olds and decreased with increasing age to 14.7% for 60+ year olds (p for trend <0.001). The frequency of multimorbidity at ART initiation increased with age from 3.0% for 16–29 year olds to 12% for 60+ year olds (p for trend <0.001). Although having multiple comorbidities was more common with increasing age, including multimorbidity in models did not change the hazard ratios for the risk of death (data not shown).
Discussion
In Lusaka's large public sector HIV treatment program, older age was associated with reduced CD4+ T cell gains and increased risk of death. Adherence and retention in care were poorest among 16–29 year olds, but similar among patients 30 years and older. As expected, those at the higher age ranges also had a higher prevalence of comorbidity at the time of ART initiation; however, the association between age and mortality remained after adjusting for these conditions.
A major strength of this analysis was our use of narrow age categories, stratifications made possible by the large number of patients 50+ years old (>6,000) in our cohort. Novel to our analysis was also the assessment of ART adherence by the MPR in older patients, an important aspect not discussed in recent analyses of age and ART outcomes.6,16 Finally, because aging is a continuous physiologic process we examined age at ART initiation in both narrow categories and as a continuous variable rather than dichotomizing it at an arbitrary cutoff. The results suggest that dichotomizing age at 50 years, as done in prior analyses,8,13,14 may not fully describe the impact of age on subsequent treatment outcomes.
Our analysis also had limitations that require discussion. First, because routine measurement of HIV viral load is too expensive for routine use in Zambia, we did not have virologic data for this analysis. Because uncontrolled viremia has been linked to immune recovery and death from both AIDS- and non-AIDS-associated causes,24 an analysis of viral suppression across age strata could have strengthened our conclusions. Second, we recognize that our ascertainment of death was incomplete, with some deceased patients being misclassified as LTFU. Because we think that the rate of misclassification was similar across age strata, we believe this misclassification would not have altered our mortality hazard ratios. We observed increased mortality at older age categories; however, we were unable to compare this with an HIV-uninfected population, limiting our ability to assess the interaction between HIV and age. Finally, during follow-up, missing CD4+ T cell counts were common. We conducted sensitivity analyses to account for missing data and observed similar results.
MPR-based adherence and program retention were lower for 16–29 year olds when compared to the rest of the cohort, highlighting the need for interventions to strengthen health behaviors in HIV-infected adolescents and young adults. However, adherence did not vary across age strata among those aged 30 years and above (the majority of the cohort). This contrasts with European and U.S. HIV-infected adults aged 50 years and older who consistently report better adherence than their younger counterparts. In upper-income settings, better adherence among older patients may partially compensate for the decrease in immune recovery experienced by 50+-year-old HIV-infected adults.25 Previous studies of age and ART outcomes in SSA have not reported adherence levels; as such, our analysis should be replicated in other settings.
Our finding that 40–49 year olds taking ART experienced reduced CD4+ T cell count recovery and increased mortality is similar to reports from both upper-income settings25 and other HIV treatment programs in SSA. In West Africa, 40–49 year olds experienced smaller average CD4+ T cell count increases compared with younger adults.16 In urban South Africa, relative to 18–29 year olds, 40–49 year olds had an adjusted mortality hazard of 1.24 (95% CI, 0.98–1.57) at 12 months post-ART initiation,26 which is similar to the effect size observed in this analysis. Among HIV-infected adults from multiple small and medium-sized African programs in nine countries, 40–49 year olds experienced 21% increased mortality hazard during the first 90 days of ART and 59% increased mortality hazard from 90 days to 2 years of ART.6 Our analysis suggests that age 60+ years may be an important risk factor for early mortality. Among 40–49 year olds and 50–59 year olds increases in mortality appeared to manifest only after a longer duration on ART.
Although we did not have the data regarding cause of death as part of this analysis, we suspect that non-AIDS-defining events (NADEs), which increase in frequency with age, contributed to the higher mortality observed in older HIV-infected Zambians. In upper-income settings, NADEs—more common among HIV-infected versus HIV-uninfected individuals—have replaced opportunistic infections as the leading causes of death for HIV-infected individuals.27,28 In RLS, data are limited on NADEs, but in one study in Botswana, HIV-infected adults experienced similar or even increased rates of NADEs, compared with a U.S.-based HIV-infected cohort.29 Using the limited data available, we observed increases in multimorbidity with greater age. However, inclusion of these conditions into our multivariable models did not diminish the strength of the association between age and death. This area requires further research, because our data on comorbid conditions were very limited.
While men consistently initiate ART at a more advanced WHO stage than women,30,31 we observed that older women might also initiate ART at an advanced WHO stage. Most analyses of age and ART outcomes in SSA have not stratified by sex. The one report that is available did not observe an association between advanced clinical disease and increasing age among women.13 Although opt-out HIV testing at antenatal care settings reaches women of child-bearing age, older women may have decreased exposure to HIV testing, which may have contributed to our finding. In addition, African adults aged 50 years and older, particularly women, have lower HIV/AIDS knowledge compared with younger adults,11 which could have resulted in delays in HIV testing, linkage to care, and ART initiation.
This report and others from SSA have now demonstrated that age at ART initiation is an important predictor of outcomes; therefore, the WHO HIV treatment guidelines should consider inclusion of a section on the health needs of older HIV-infected adults. As they experience increased early mortality and reduced immune recovery during ART, older adults require support with adherence and retention on ART, particularly in the initial 90 days of treatment. In addition, the integration of interventions such as smoking cessation and monitoring of blood pressure and cholesterol into routine HIV care should be emphasized among older patients who are at risk for NADEs.32
In conclusion, in a large HIV treatment program in Lusaka, Zambia, older patients initiating ART experienced decreased immune recovery and increased mortality compared to younger patients. Adherence was similar among patients 30+ years old. Approaches to improve the outcomes of older HIV patients in SSA could include adherence interventions to optimize CD4+ gains on ART and screening for comorbid conditions. Additional investigation in resource-constrained settings is needed to optimize the care of this emerging group.
Supplementary Material
Acknowledgments
Trainee and investigator support were provided by the National Institutes of Health through the Fogarty Global Health Fellowship (R25TW009340) and the Vanderbilt-CIDRZ AIDS International Training and Research Program (D43TW001035). The findings and conclusions included herein are solely the responsibility of the authors and do not necessarily represent the official position of the National Institutes of Health, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, the Office of U.S. Global AIDS Coordinator, or the U.S. Agency for International Development.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Egger M, May M, Chene G, et al. : Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet 2002;360:119–129 [DOI] [PubMed] [Google Scholar]
- 2.Ivers LC, Kendrick D, and Doucette K: Efficacy of antiretroviral therapy programs in resource-poor settings: A meta-analysis of the published literature. Clin Infect Dis 2005;41:217–224 [DOI] [PubMed] [Google Scholar]
- 3.Laurent C, Ngom Gueye NF, Ndour CT, et al. : Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr 2005;38:14–17 [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Rammohan A, and Awofeso N: Ageing faster with AIDS in Africa. Lancet 2011;377:1131–1133 [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS: Report on the Global AIDS epidemic. UNAIDS, Geneva, Switzerland, 2006 [Google Scholar]
- 6.Grieg J, Casas EC, O'Brien DP, Mills EJ, and Ford N: Association between older age and adverse outcomes on antiretroviral therapy: A cohort analysis of programme data from nine countries. AIDS 2012;26(Suppl 1):S31–S37 [DOI] [PubMed] [Google Scholar]
- 7.Negin J, van Lettow M, Semba M, Martiniuk A, Chan A, and Cumming RG: Anti-retroviral treatment outcomes among older adults in Zomba district, Malawi. PLoS One 2011;6(10):e26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakanda C, Birungi J, Mwesigwa R, et al. : Association of aging and survival in a large HIV–infected cohort on antiretroviral therapy. AIDS 2011;25:701–705 [DOI] [PubMed] [Google Scholar]
- 9.Nicolosi A, Laumann EO, Glasser DB, Moreira ED., Jr.Palik A, and Gingell C: Sexual behavior and sexual dysfunctions after age 40: The global study of sexual attitudes and behaviors. Urology 2004;64:991–997 [DOI] [PubMed] [Google Scholar]
- 10.Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, and Waite LJ: A study of sexuality and health among older adults in the United States. N Engl J Med 2007;357:762–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negin J, Nemser B, Cumming R, Lelerai E, Amor YB, and Pronyk P: HIV attitudes, awareness and testing among older adults in Africa. AIDS Behav 2012;16(1):63–68 [DOI] [PubMed] [Google Scholar]
- 12.Baker JV, Peng G, Rapkin J, et al. : CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 2008;22:841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman J, Irlondo-Perez J, Hemingway-Foday J, et al. : Older adults accessing HIV care and treatment and adherence in the IeDEA central Africa cohort. AIDS Res Treat 2012;2012:725713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabar S, Kousignian I, Sobel A, et al. : Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French hospital database on HIV. AIDS 2004;18:2029–2038 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization: Definition of an older or elderly person. Health statistics and health information systems. Geneva, Switzerland, 2010 [Google Scholar]
- 16.Balestre E, Eholie SP, Lokossue A, et al. : Effect of age on immunological response in the first year of antiretroviral therapy in HIV-1-infected adults in West Africa. AIDS 2012;26:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negin J, Barnighausen T, Lundgren JD, and Mills EJ: Aging with HIV in Africa: The challenges of living longer. AIDS 2012;26(Suppl 1):S1–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stringer JS, Zulu I, Levy J, et al. : Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA 2006;296(7):782–793 [DOI] [PubMed] [Google Scholar]
- 19.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. : Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007;298(16):1888–1899 [DOI] [PubMed] [Google Scholar]
- 20.Goldman JD, Cantrell RA, Mulenga LB, et al. : Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses 2008;24(8):1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi BH, Cantrell RA, Zulu I, et al. : Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol 2009;38(3):746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi BH, Cantrell RA, Mwango A, et al. : An empirical approach to defining loss to follow-up among patients enrolled in antiretroviral treatment programs. Am J Epidemiol 2010;171(8):924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, and Roth D: A more accurate method to estimate glomerular filtration from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130(6):461–470 [DOI] [PubMed] [Google Scholar]
- 24.Althoff KN, Justice AC, Gange SJ, et al. : Virologic and immunologic response to HAART, by age and regimen class. AIDS 2010;24(16):2469–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, and Quesenberry CP: Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med 2007;167:684–691 [DOI] [PubMed] [Google Scholar]
- 26.Maskew M, Brennan AT, Macphail AP, Sanne IM, and Fox MP: Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care (Chicago) 2012;11(1):57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulet J, Fultz S, Rimland D, et al. : Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007;45:1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palella FJ, Baker PK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in HIV outpatient study. J AIDS 2006;43:27–34 [DOI] [PubMed] [Google Scholar]
- 29.Wester CW, Koethe JR, Shepherd BE, et al. : Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in a resource-replete versus resource-limited urban setting. AIDS 2011;25:1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornell M, Myer L, Kaplan R, Bekker L-G, and Wood R: The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health 2009;14(7):722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornell M, Schomaker M, Garone DB, et al. : Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: A multicentre cohort study. PLoS Med 2012;9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO, Geneva, Switzerland, 2013 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.