Abstract
Previous studies suggest that tazarotene, a new member of the acetylenic class of RARβ/γ selective retinoids which is approved to treat a variety of skin diseases, exhibits an anti-proliferative effect in human basal cell carcinoma (BCC) by triggering caspase-dependent apoptosis. However, the detailed molecular mechanisms underlying the anti-tumor activity of tazarotene are poorly understood. This study aims at investigating the molecular mechanisms of tazarotene-induced apoptosis in human BCC cells. Our results are the first to demonstrate that tazarotene induces mitochondria-dependent cleavage of caspase-9 and -3 and PARP in BCC cells by producing reactive oxygen species (ROS) and activating caspase-8 through both ROS and death receptor signaling. These events are accompanied by a decrease in BCL-2 and BCL-xl anti-apoptotic proteins as well as by survivin and XIAP, two IAP family members. Furthermore, our results presented for the first time that tazarotene triggers a convergence of the intrinsic and extrinsic apoptotic pathways via the caspase-8-truncated Bid signaling pathway. Collectively, these data provide insights into the molecular mechanisms underlying tazarotene-induced apoptosis in human BCC cells, suggesting that this compound is a potential anti-skin cancer drug.
Introduction
Basal cell carcinoma (BCC) is the most common type of skin cancer worldwide, and its incidence is increasing (Diepgen and Mahler, 2002; Kasper et al., 2012). BCC is characterized by a non-aggressive behavior and is, therefore, a good candidate for treatment with traditional and experimental therapies (Wennberg, 2000). Surgical excision and cryosurgery are currently considered the standard treatments for BCC (Marghoob, 1997; Martinez and Otley, 2001). In addition, a number of treatment options are now available to patients for whom surgery is contraindicated. Topical immunotherapy, chemotherapy, and photodynamic therapy can be proposed for the treatment of select patients. The lesion location, lesion size, recurrence status, clinical type, patient's age, ease of treatment, and cost dictate which topical treatment is utilized (Bastiaens et al., 1998; Urosevic and Dummer, 2002; Urosevic et al., 2003).
Numerous retinoids have exerted striking effects on proliferation, differentiation, and apoptosis in various cancers, including leukemia and non-melanoma skin cancers (Robert et al., 2006; Lens and Medenica, 2008; Carr et al., 2011). Tazarotene and tazarotenic acid, a free acid metabolite of tazarotene, are new members of the acetylenic class of RARβ/γ–selective retinoids that are approved to treat a variety of skin diseases, such as psoriasis, acne, and photoaging (Roeder et al., 2004; Dando and Wellington, 2005; Tanghetti et al., 2011). Recently, clinical studies have also indicated that long-term topical treatment with Tazarotene gel can cure one-fourth to one-half of human BCC cases without significant toxicity (Bianchi et al., 2004; Orlandi et al., 2004). Additional in vitro and in vivo studies have indicated that tazarotene exerts its anti-proliferative effects by triggering caspase-dependent apoptosis in BCC (Orlandi et al., 2004). Although these findings suggest that tazarotene possesses anti-cancer activity in BCC cells, the details of the molecular mechanisms underlying the anti-tumor activity of tazarotene are poorly understood. Therefore, our present study aimed at elucidating the molecular mechanisms of tazarotene's anti-cancer activity in human BCC cells, focusing on the apoptotic death signaling pathway. We report that tazarotene induces apoptosis in BCC cells by producing reactive oxygen species (ROS) that activate caspase-8 at the death-inducing signaling complex. Activation of caspase-8 subsequently induces mitochondria-dependent activation of the caspase-9 and caspase-3 cascades. In addition, we found that tazarotene induces cell cycle arrest at the G0/G1 phase of the cell cycle. These events are concordant with decreased BCL-2 and BCL-xl anti-apoptotic proteins and downregulation of the IAP family proteins survivin and XIAP.
Materials and Methods
Cell culture and reagents
The human BCC cell line (BCC-1/KMC) was a kind gift from Dr. L.C. Chiang at Kaohsiung Medical University (Yen et al., 1996). The cells were grown in RPMI 1640 medium (GIBCO/Life Technologies) containing 10% fetal bovine serum (GIBCO/Life Technologies) and maintained at 37°C in a humidified incubator containing 5% CO2. Dantrolene dimethyl sulfoxide (DMSO; Sigma-Aldrich Co.) was used to dissolve and dilute Tazarotene (Allergan) to produce a 100 mM stock solution.
Cell viability assay
Cell proliferation was measured using the 3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazoliumbromide (MTT) assay (Sigma-Aldrich Co.). Aliquots between 3×104 and 4×104 cells/well were dispensed into 24-well tissue culture plates in 1 mL of medium and then incubated with 0, 6.125, 12.5, 25, 50, and 100 μM tazarotene (0%, 0.1% DMSO) for 12, 24, or 48 h. Then, 200 μL of MTT (5 mg/mL) was added to each well and incubated for an additional 4 h at 37°C. After removing the supernatant, color was developed by the addition of 600 μL of DMSO to each well. The absorbance was read at 570 nm using a microplate reader (Sunrise Microplate Reader; TECAN).
Cell cycle analysis by flow cytometry
Cell cycle analysis was performed using flow cytometry. BCC cells were seeded into 6-cm tissue culture dishes at a density of 3×105 cells/dish in 3 mL of medium and then incubated with 0, 25, 50, and 100 μM tazarotene for 12, 24, and 48 h. At the indicated time point, cells were trypsinised, washed with phosphate-buffered saline (PBS), and fixed in 70% ethanol at −20°C overnight. The fixed cells were washed with PBS, stained in a solution containing RNase A (Sigma-Aldrich Co.) and propidium iodide (PI; Sigma-Aldrich Co.) for 30 min at room temperature, and analyzed by an Accuri™ C5 cytometer CAT. NO. 657214. (BD Biosciences). The percentage of cells in the sub-G1 (indicative of apoptosis), G0/G1, S, and G2/M phases was determined using Accuri™ C6 software version 1.0264.21 software.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay
Apoptosis was analyzed using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining method. TUNEL staining was performed using a commercially available kit (Guava TUNEL Kit; Millipore). In this assay, BCC cells were seeded into 6-cm tissue culture dishes at a density of 3×105 cells/dish in 3 mL of medium and then incubated with 0, 25, 50, and 100 μM tazarotene for 24 h. After incubation, the cells were collected and washed with PBS. The cells were then fixed with 1% paraformaldehyde at 4°C for 60 min, washed twice with wash buffer, re-suspended in 70% ethanol, and incubated at −20°C for 2 h. Cells were collected by centrifugation and washed twice with rinsing buffer. Cells were stained with TUNEL reaction mixtures at 37°C for 60 min. After incubation, the cells were analyzed by flow cytometry (FACSCalibur; BD BioSciences).
Transient transfection and RNA interference
For Fas-associated death domain (FADD) siRNA delivery, 1×105 BCC cells were grown in six-well plates and were then transfected with 400 pmol of FADD siRNA (Santa Cruz) using Lipofectamine 2000 reagent. Cells were harvested at 24 h after transfection, and cell viability was assessed in the presence or absence of 100 μM tazarotene for an additional 24 h using the MTT assay.
Measurement of ROS by flow cytometry
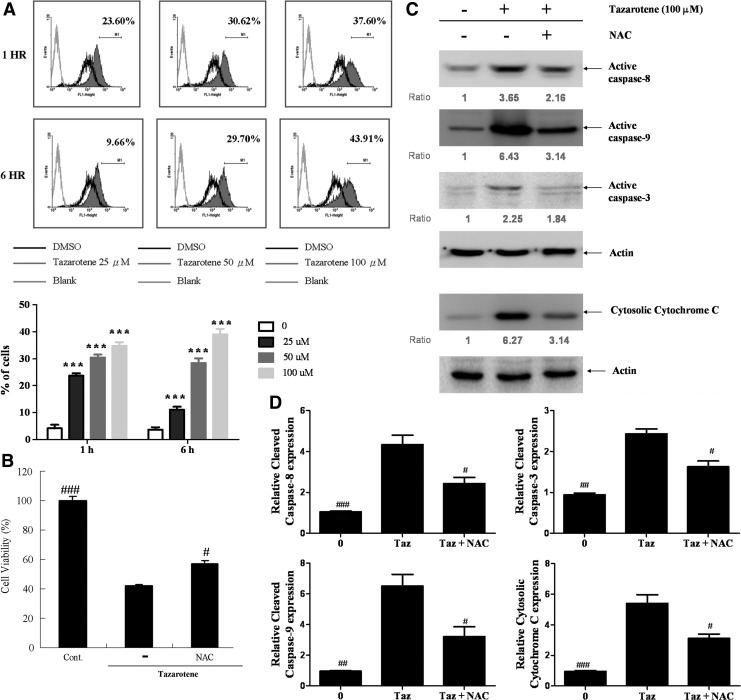
ROS generation was measured after staining BCC cells with 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Molecular Probes). BCC cells were seeded into 6-cm tissue culture dishes at a density of 3×105 cells/dish in 3 mL of medium. The medium was removed at 1 or 6 h after tazarotene treatment, and culture medium containing 5 μM DCFDA was added under low-light conditions. The cells were incubated for 30 min at 37°C, and the level of ROS was analyzed by flow cytometry. To examine whether ROS participated in the tazarotene-induced reduction in cell viability, BCCs were grown overnight and then pre-treated with 5 mM N-acetylcysteine (NAC; Sigma-Aldrich) for 1 h. Cells were then treated with the 100 μM tazarotene for 24 h. The treated cells were incubated with 5 mg/mL MTT for 4 h at 37°C. After removing the supernatant, color was developed by the addition of 600 μL of DMSO to each well. The absorbance was read at 570 nm using a microplate reader.
Measurement of mitochondrial membrane potential
To measure the mitochondrial membrane potential (MMP), BCC cells were incubated with JC-1 (Invitrogen) and then assessed for red (J-aggregate) fluorescence. For this assay, BCC cells were seeded into 6-cm tissue culture dishes at a density of 3×105 cells/dish in 3 mL of medium and then incubated with 0, 25, 50, and 100 μM tazarotene for 24 h. The treated cells were trypsinised, washed with PBS, and resuspended with 1 mL of culture medium. Each treatment was stained with 2 μL of JC-1 stock solution (final concentration 10 μg/mL) for 10 min at 37°C. The cells were collected by centrifugation and washed with PBS. Each treatment group was then re-suspended in 1 mL of PBS and analyzed by flow cytometry (FACSCalibur; BD BioSciences).
Analysis of death receptors and ligands by flow cytometry
The BCC cells were seeded into 6-cm tissue culture dishes at 3×105 cells/dish in 3 mL of medium and then incubated with 0, 25, 50, and 100 μM tazarotene for 12 h. At the indicated time point, cells were trypsinised and washed with PBS. The treated cells were then stained with anti-DR4-FITC, anti-DR5-FITC, anti-Fas-FITC, anti-TRAIL-FITC, or anti-FasL-FITC (eBioscience) at 4°C for 30–45 min. The expression level of each molecule was determined by flow cytometry (FACSCalibur; BD BioSciences) and analyzed using WinMdi software (Scripps Research Institute).
In vitro assay for cytochrome c release
BCC cells were seeded into 10-cm tissue culture dishes at a density between 8×106 and 10×106 cells/dish in 8–10 mL of medium and then incubated with 0, 25, 50, and 100 μM tazarotene for 24 h. Cells were collected by centrifugation. Cytosolic fractions were isolated using the Mitochondria/Cytosol Fraction Kit (BioVision). The quality of the cytosolic fraction was estimated by Western blotting using an anti-cytochrome c antibody (BD Pharmingen).
Western blot analysis
The BCC cells were incubated with 0, 25, 50, and 100 μM tazarotene for 24 h, lysed in 2% sodium dodecyl sulfate (SDS; 10 mM ethylenediaminetetraaceticacid, 50 mm Tris base, 10% SDS, pH 8.0), and boiled at 100°C for 10 min. Protein concentrations were determined using the BCA Protein Assay Reagent (PIERCE). Proteins were separated by electrophoresis on a 12% or 15% SDS-polyacrylamide gel electrophoresis (PAGE) gel and then transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% non-fat milk at room temperature and then incubated with primary antibodies against caspase-3, caspase-8, caspase-9, Bcl-2, Bcl-Xl, Bak, Bax, Bid, truncated Bid (tBid), COX IV, XIAP, cleaved PARP, and Survivin (Cell signaling) at 4°C overnight. After washing, the membranes were incubated with horseradish peroxidase-labeled secondary antibodies (The Jackson Laboratory) for 2 h. The membranes were then incubated with the enhanced chemiluminescence system and developed using the LAS3000 system (Fujifilm). Densitometric analysis was performed with ImageJ software (National Institute of Health).
Caspase inhibitor assay
The BCC cells were seeded into 24-well tissue culture plates at between 3×104 and 4×104 cells/well. The cells were grown overnight and then pre-treated with inhibitors of caspase-3 (Ac-DEVD-CMK; Calbiochem), caspase-8 (Z-IETD-FMK; Calbiochem), and caspase-9 (Z-LEHD-FMK; Calbiochem) for 1 h. Cells were then treated with 100 μM tazarotene for 24 h. The treated cells were incubated with 5 mg/mL MTT for 4 h at 37°C. After removing the supernatant, color was developed by the addition of 600 μL of DMSO to each well. The absorbance was read at 570 nm using a microplate reader.
Statistical analysis
All statistically analyses were performed with the GraphPad Prism software package version 4.0. In addition, cell viability was evaluated by analysis of variance test between groups followed by Tukey's test to determine the significance of differences between pairs of groups.
Results
Tazarotene exerts potent cytotoxicity in BCC cells
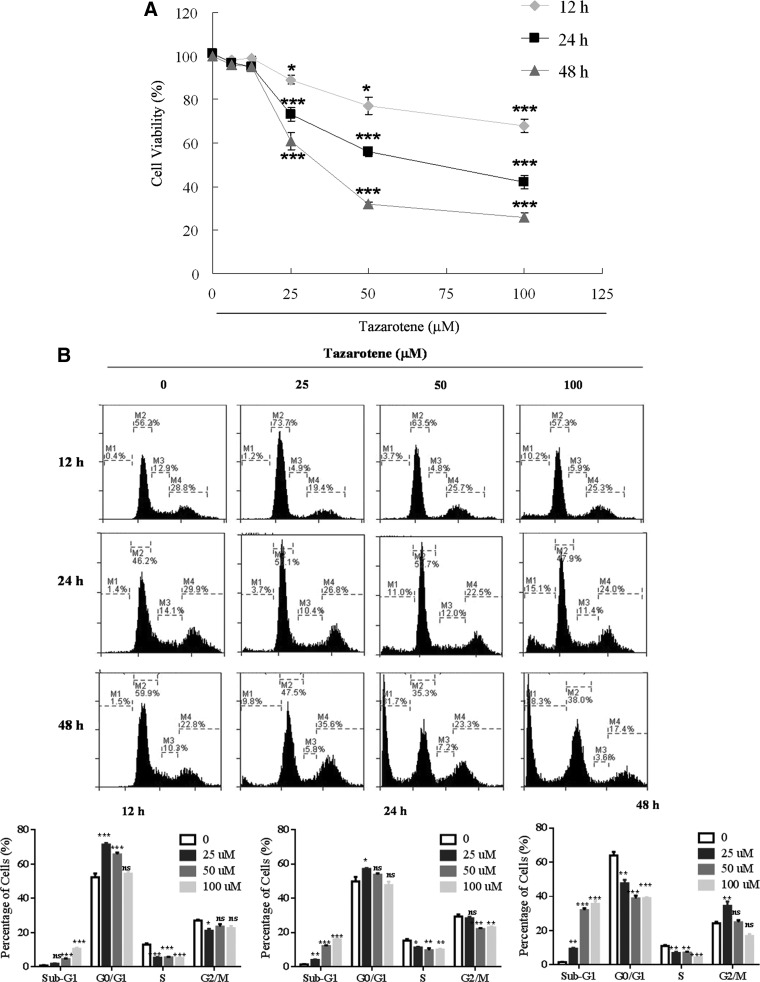
To determine the effect of tazarotene on cell growth, human BCC cells were treated with various doses of tazarotene for 12, 24, or 48 h, and cell viability was measured using the MTT assay. As shown in Figure 1A, tazarotene significantly reduces BCC cell viability in a dose- and time-dependent manner.
FIG. 1.
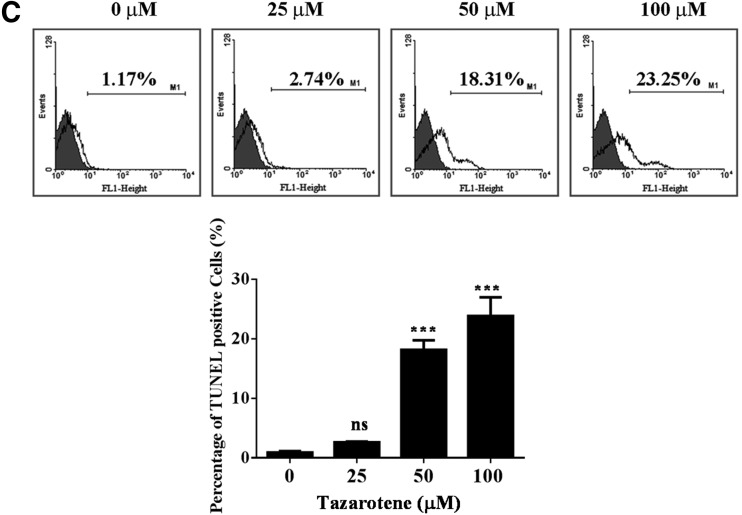
Tazarotene-induced cytotoxicity and apoptosis in basal cell carcinoma (BCC) cells. (A) BCC cells were treated with various concentrations of tazarotene for 12, 24, or 48 h, and cell viability was measured with 3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazoliumbromide assay as described in the “Materials and Methods” section. The data represent the mean±standard deviation (SD) from three wells. The data are representative of three independent experiments with similar results. (B) BCC cells were treated with various concentrations of tazarotene for 12, 24, or 48 h. Cells were trypsinized, propidium iodide stained, and analyzed by flow cytometry. The means±SD of the experimental triplicates are presented in the bar graph at the bottom. (C) BCC cells were treated with various concentrations of tazarotene for 24 h. The cells were then subjected to terminal deoxynucleotidyl transferase dUTP nick-end labeling staining and flow cytometric analysis. The means±SD of the experimental triplicates are presented in the bar graph at the bottom. nsp>0.05, *p<0.05, **p<0.01, and ***p<0.001, compared with the 0.1% dimethyl sulfoxide (DMSO)-treated group.
Tazarotene-induced apoptosis in BCC cells
To determine whether tazarotene-induced growth inhibition occurs via cell cycle blockade, BCC cells were subjected to flow cytometric analysis. BCC cells were exposed to a series of different tazarotene concentrations for 12, 24, or 48 h. Figure 1B shows that 25 and 50 μM tazarotene treatment for 12 h and 25 μM tazarotene treatment for 24 h caused transient G0/G1 phase cell cycle arrest. In addition, the sub-G1 population, which is typically considered an apoptosis-related hypo-diploid DNA content peak, significantly increased in a dose-dependent manner at 12, 24, and 48 h after tazarotene treatment. To further confirm the observed tazarotene-induced apoptosis, we utilized flow cytometry to analyze TUNEL staining in cells treated with various concentrations of tazarotene for 24 h. As shown in Figure 1C, the percentage of TUNEL-positive BCC cells increased in a concentration-dependent manner. Taken together, these observations suggest that the anti-proliferative effect of tazarotene in BCC cells results, at least in part, from its ability to induce apoptosis.
Tazarotene alters MMP in BCC cells
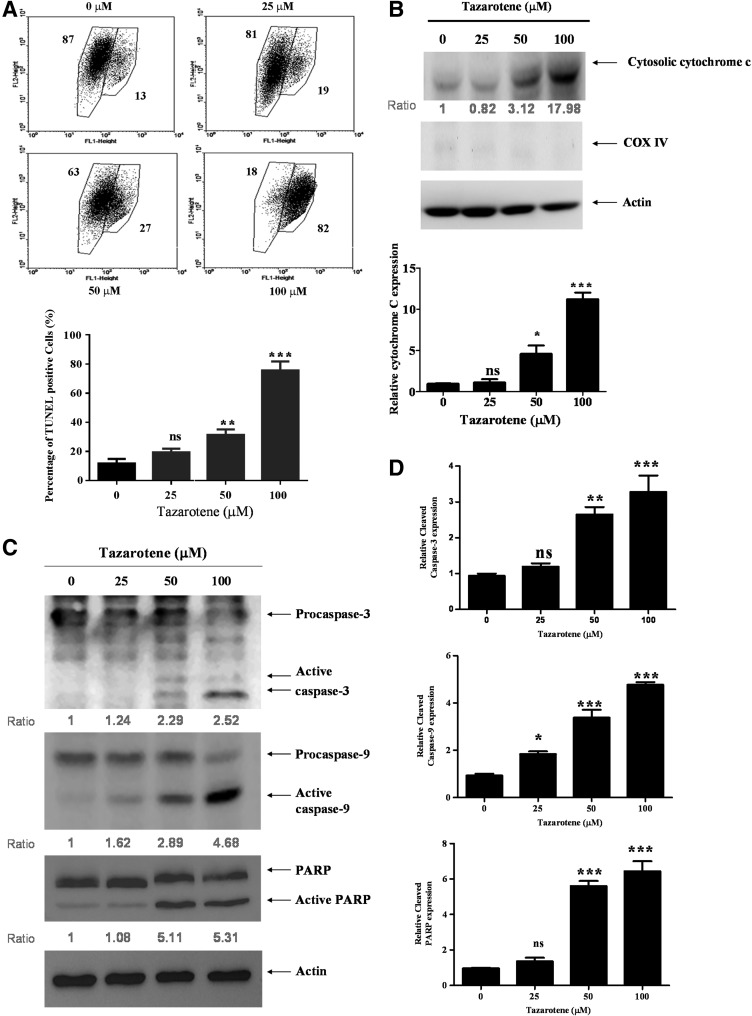
Mitochondria play an essential role in apoptotic signaling given that both the intrinsic and extrinsic apoptotic pathways converge at the mitochondria to induce mitochondrial membrane permeabilization (MMP) (Wang, 2001; Danial and Korsmeyer, 2004). To examine whether the mitochondria-mediated apoptotic pathway is involved in tazarotene-induced apoptosis, we determined the effect of tazarotene on MMP (Δψm) using flow cytometry to analyze staining with the fluorescent cationic dye JC-1. Loss of Δψm is an indicator of mitochondrial damage during apoptosis. After cells were treated with tazarotene, red fluorescence was detected in BCC cells in a concentration-dependent manner, suggesting that tazarotene treatment results in a reduction in Δψm (Fig. 2A) and that mitochondrial dysfunction is involved in tazarotene-induced apoptosis.
FIG. 2.
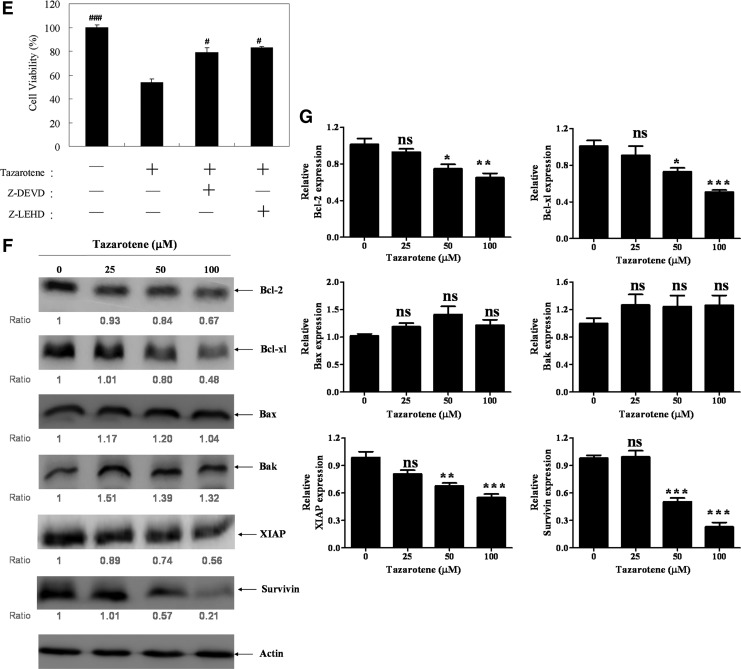
Tazarotene triggered apoptotic events in BCC cells. (A) BCC cells were stained with JC-1 fluorescent dye, and mitochondrial membrane potential (Δψm) was analyzed by flow cytometry. The means±SD of the experimental triplicates are presented in the bar graph at the bottom. (B) The cells were treated with indicated concentrations of tazarotene for 24 h. Cytosolic cell lysates were prepared, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with the indicated antibodies to detect cytochrome c. Cytochrome c oxidase subunit IV (COX IV) served as a mitochondrial marker. (C) Total cell lysates were prepared to detect the non-cleaved and cleaved forms of caspase-9, caspase-3, and PARP. (D) The bands were analyzed by ImageJ (National Institute of Health) and normalized to actin. The means±SD of the three independent experiments are presented in the bar graph. (E) The effect of caspase-3 and caspase-9 inhibitors on 100 μM tazarotene-induced cell viability. The data represent the mean±SD from three wells. The data are representative of three independent experiments with similar results. (F) Total cell lysates were prepared and subjected to SDS-PAGE followed by Western blotting with antibodies to detect the expression of Bcl-2, Bcl-xl, Bax, Bak, XIAP, and Survivin. (G) The bands were analyzed by ImageJ and normalized to actin. The means±SD of the three independent experiments are presented in the bar graph. nsp>0.05, *p<0.05, **p<0.01, and ***p<0.001, compared with the 0.1% DMSO-treated group. #p<0.05, ###p<0.001 compared with the 100 μM tazarotene-treated group.
Tazarotene-induced apoptosis involves the release of cytochrome c and caspase activation
Cytochrome c release from the mitochondria into the cytosol is an important event in the mitochondrial apoptotic pathway (Boehning et al., 2003). Mitochondrial dysfunction causes the activation of Apaf-1-associated caspase-9, which, subsequently, activates down-stream caspase-3 and induces the cleavage of PARP. In addition, Bcl-2 family proteins are frequently involved in mitochondrial dysfunction (Gross et al., 1999). To elucidate the relationships among the apoptotic proteins mentioned earlier, BCC cells were exposed to tazarotene for 24 h, lysed, and assessed by Western blot analysis. Cytochrome c expression was assessed in the cytosolic fractions, whereas the expression of caspase-3, caspase-9, and other apoptosis-related proteins was determined in whole cell lysates. As shown in Figure 2B, the data reveal a marked increase in cytosolic cytochrome c expression levels after 24 h of tazarotene treatment. As illustrated in Figure 2C and D, treatment with tazarotene induces the formation of the cleaved/activated forms of caspase-9, caspase-3, and PARP in BCC cells. Moreover, the treatment of BCC cells with a caspase-3 or caspase-9 inhibitor blocks tazarotene-induced decreases in cell viability, suggesting that the caspase-dependent pathway is involved (Fig. 2E). Exposure to tazarotene also results in the downregulation of anti-apoptotic proteins Bcl-2 and Bcl-xl; however, tazarotene does not significantly increase the expression of apoptotic proteins Bax and Bak (Fig. 2F, G). On receiving a death signal, mitochondria release Smac/DIABLO into the cytoplasm. Smac/DIABLO binds to IAP family members and relieves IAP-mediated inhibition of caspase-9 and caspase-3, thus promoting apoptosis (Deveraux and Reed, 1999). Therefore, we also examined the expression of two IAP family proteins, survivin and XIAP, using Western blot analysis. As shown in Figure 2F and G, XIAP and survivin protein expression is reduced on tazarotene treatment. Taken together, these findings suggest that treatment with tazarotene induces apoptosis primarily via the intrinsic mitochondrial pathway.
Activation of the caspase-8 pathway via tazarotene-induced apoptosis
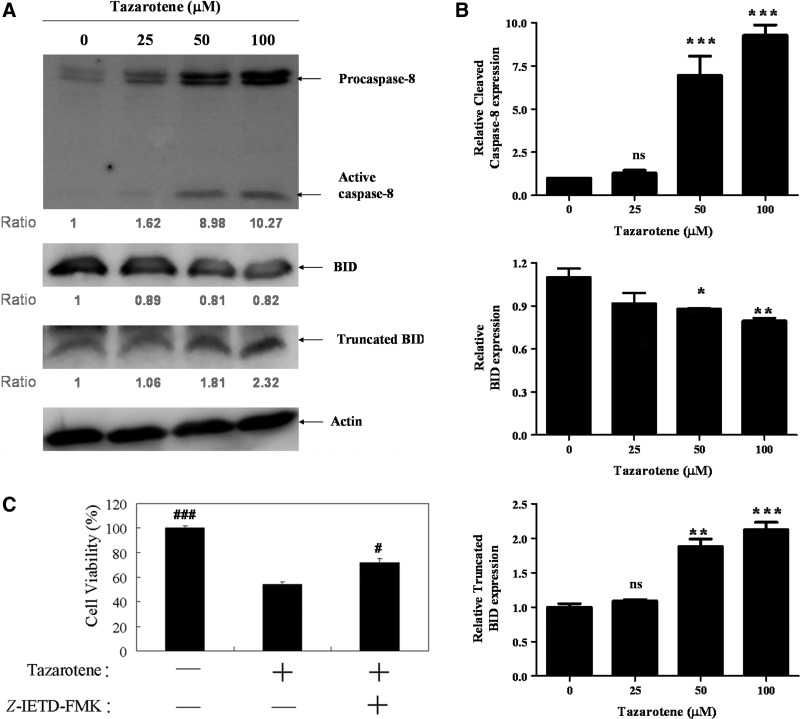
Caspase-8 can activate caspase-3 directly through cleavage or indirectly by inducing the cleavage of Bid (23 kDa) into tBid, which subsequently triggers mitochondria-dependent activation of the caspase-9 and caspase-3 cascades (Li et al., 1998). To determine whether tazarotene induces the activation of caspase-8, we treated BCC cells with various doses of tazarotene for 24 h. Our results indicate that tazarotene increases the expression of cleaved caspase-8 protein (Fig. 3A, B). In addition, Western blot analysis reveals that cleaved Bid expression increases after exposure to tazarotene (Fig. 3A, B).
FIG. 3.
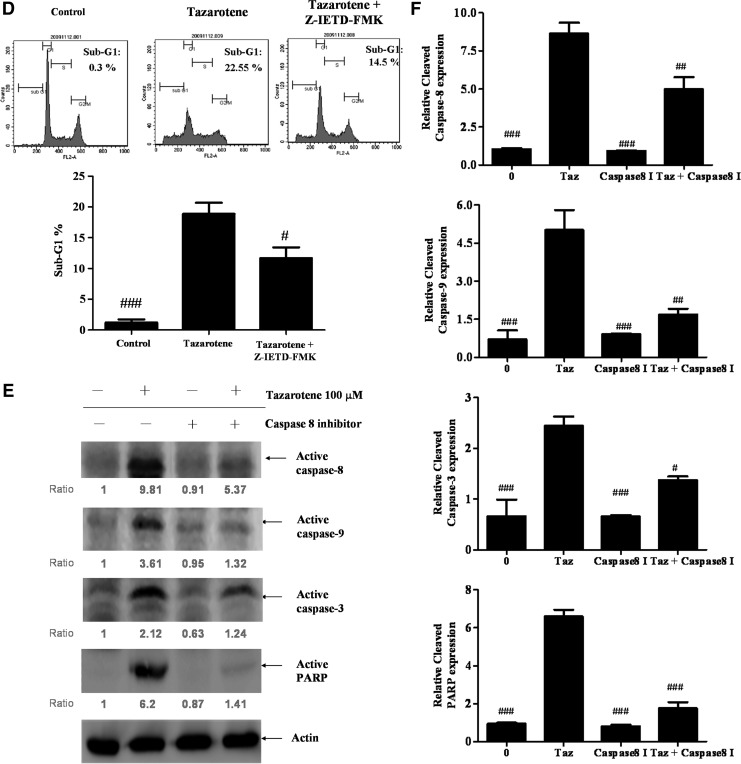
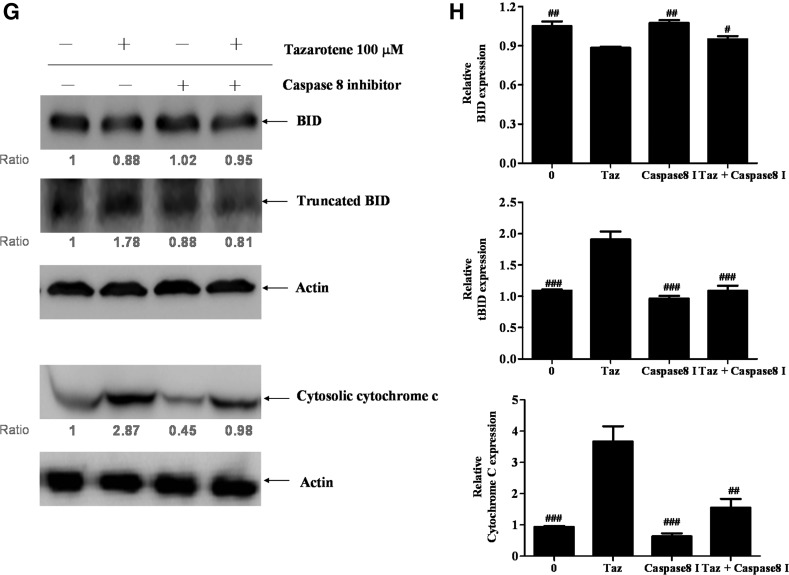
Tazarotene-induced activation of the caspase-8-truncated Bid (tBid) cascade and the effects of the caspase-8 inhibitor Z-IETD-FMK in BCC cells. (A) The cells were treated with indicated concentrations of tazarotene for 24 h. Total cell lysates were prepared, resolved by SDS-PAGE, and immunoblotted with the indicated antibodies to detect the non-cleaved and cleaved forms of caspase-8, Bid, and truncated Bid. (B) The bands were analyzed by ImageJ and normalized to actin. The means±SD of the three independent experiments are presented in the bar graph. nsp>0.05, *p<0.05, **p<0.01, and ***p<0.001, compared with the 0.1% DMSO-treated group. (C) The reverse effect of the caspase-8 inhibitor on 100 μM tazarotene-induced reduction in cell viability. The data represent the means±SD from three wells. The data are representative of three independent experiments with similar results. (D) The sub-G1 population, (E) caspase and (G) Bid cleavage, and cytosolic cytochrome c expression are presented. (F, H) The bands were analyzed by ImageJ and normalized to actin. The means±SD of the three independent experiments are presented in the bar graph. #p<0.05, ##p<0.01, and ###p<0.001, compared with the 100 μM tazarotene-treated group.
Caspase-8 inhibition by Z-IETD-FMK blocks tazarotene-induced cleavage of Bid, caspase-3, and caspase-9 as well as cytochrome c release
To determine whether caspase-8 induces caspase-9 and caspase-3 activation in tazarotene-treated BCC cells, caspase-8 was inhibited with z-IETD-fmk, a selective caspase-8 inhibitor. Our results indicate that pre-treatment with z-IETD-fmk significantly rescues cell viability (Fig. 3C), inhibits cell apoptosis (Fig. 3D), and prevents the cleavage of caspase-9, caspase-3, and PARP (Fig. 3E, F). tBid formation and cytochrome c release are also reduced when cells are pre-treated with z-IETD-fmk (Fig. 3G, H). Overall, these data indicate that caspase-8 plays an important role in tazarotene-induced apoptosis via the direct activation of effector caspase-8 and the indirect activation of the mitochondrial pathway via tBid.
Tazarotene induces the caspase-8 activation-dependent death receptor pathway
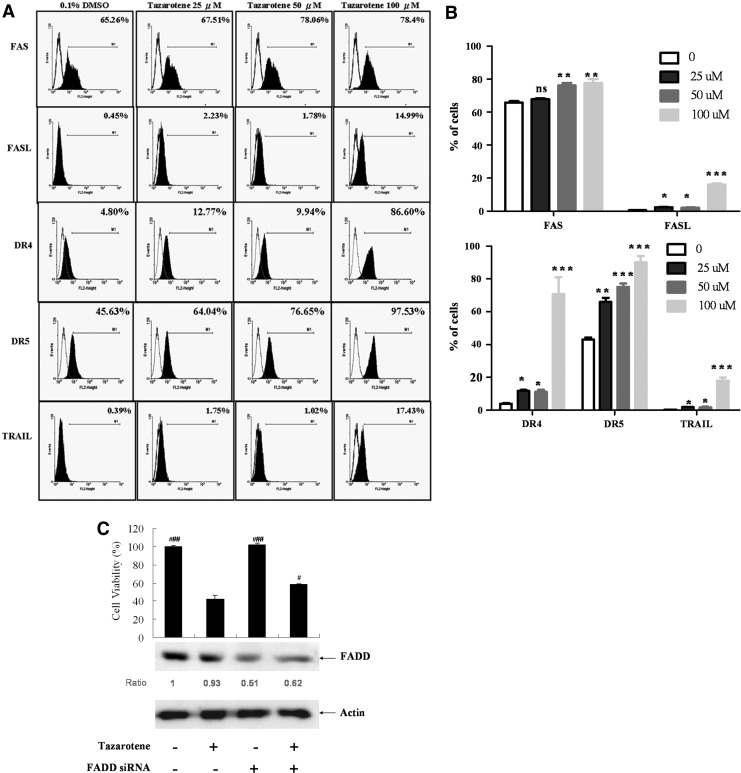
Caspase-8 cleavage can be induced through activation of death receptors, including TNF receptor-1 (TNF-R1), Fas (APO-1/CD95), TRAMP or death receptor-3 (DR3), and TRAIL receptor-1 and receptor-2 (TRAIL-R1/DR4, TRAIL-R2/DR5), via their respective ligands (Thorburn, 2004). Therefore, to explore whether a death receptor/ligand interaction was involved in tazarotene-induced caspase-8 activation and apoptosis, the expression levels of these factors on the surface were examined by flow cytometry after tazarotene treatment. As shown in Figure 4A and B, we observed that ≥25 μM tazarotene significantly increases Fas ligand (FASL), DR4, DR5, and TRAIL expression and ≥50 μM tazarotene significantly increases FAS expression after 12 h. Given that trimerization of the Fas and TRAIL receptors leads to the recruitment of the adaptor protein FADD and results in the activation of caspase-8 (Valmiki and Ramos, 2009), we further used FADD-targeted siRNA to confirm the relationship between the death receptor pathway and tazarotene-induced apoptosis. Our results indicate that FADD silencing rescues cell viability (Fig. 4C), thereby suggesting that tazarotene-induced caspase-8 activation is partially dependent on death receptor signaling.
FIG. 4.
Involvement of the death receptor pathway in tazarotene-triggered apoptosis. (A) Flow cytometric analysis of death receptors and death receptor ligand expression after 12 h of exposure to various tazarotene concentrations. (B) The means±SD of the experimental triplicates are presented in the bar graph. nsp>0.05, *p<0.05, **p<0.01, and ***p<0.001 compared with the 0.1% DMSO-treated group. (C) The reverse effect of transient transfection of Fas-associated death domain siRNA on 100 μM tazarotene-induced reduction in cell viability. The data represent the means±SD from three wells. The data are representative of three independent experiments with similar results.#p<0.05, ###p<0.001, compared with the 100 μM tazarotene-treated group.
Tazarotene induces intracellular ROS
ROS are important players in apoptosis via the mitochondria-dependent pathway and caspase-8 activation (Boonstra and Post, 2004; Kim and Chung, 2007). However, no report has indicated that tazarotene promotes the generation of ROS. Hence, we investigated the ability of tazarotene to induce ROS production using the fluorescent probe DCFDA. As shown in Figure 5A, DCFDA fluorescence intensity significantly increases in a dose-dependent manner in cells treated with tazarotene compared with untreated cells. In addition, 2 h of pre-treatment with the oxidant scavenger NAC suppresses tazarotene-mediated reduction in cell viability compared with cells treated with tazarotene alone (Fig. 5B). This result further suggests that tazarotene-induced cytotoxicity is associated with the generation of ROS in human BCC cells.
FIG. 5.
The role of reactive oxygen species (ROS) in tazarotene-induced apoptosis and the reverse effect of oxidant scavengers. (A) BCC cells were exposed to various doses of tazarotene for 1 or 6 h, and ROS levels were analyzed by flow cytometry. The means±SD of the experimental triplicates are presented in the bar graph at the bottom. ***p<0.001, compared with the 0.1% DMSO-treated group. (B) Reversal of 100 μM tazarotene-induced reduction in cell viability. The data represent the means±SD from three wells. The data are representative of three independent experiments with similar results. (C) Caspase activation and cytosolic cytochrome c expression induced by the oxidant scavenger N-acetylcysteine in BCC cells. (D) The bands were analyzed by ImageJ and normalized to actin. The means±SD of three independent experiments are presented in the bar graph. #p<0.05, ##p<0.01, and ###p<0.001, compared with the 100 μM tazarotene-treated group.
ROS are responsible for tazarotene-induced apoptotic cell death
To investigate the contribution of ROS to tazarotene-induced apoptosis and caspase activation, BCC cells were pre-treated with NAC. We observed that NAC partially suppresses tazarotene-induced cleavage of caspase-8, -3, and -9 as well as cytochrome c release (Fig. 5C, D). These results suggest that ROS may be involved in tazarotene-induced BCC cell death and apoptosis.
Discussion
A clinical study suggests that topical treatment with tazarotene induces remission in BCC patients (Bianchi et al., 2004), but the mechanism of tazarotene action was not addressed. In the present study, we described the cellular and molecular events underlying the growth inhibitory effect of tazarotene in human BCC cells.
The disruption of MMP and cytochrome c release into the cytosol are early events in apoptotic signaling. The intrinsic and extrinsic apoptotic pathways converge at the mitochondria (Gross et al., 1999; Boehning et al., 2003; Wang, 2001; Danial and Korsmeyer, 2004; Sharma et al., 2012), and anti-apoptotic members of the Bcl-2 family also regulate the mitochondrial apoptotic pathway (Ran et al., 2005a; Wong and Puthalakath, 2008). With regard to the induction of the mitochondria-mediated apoptosis pathway, several mechanisms of action have been proposed for tazarotene, including increased Bax expression as reported in tazarotene-treated immortalized basal keratinocyte (C5N) cells (Orlandi et al., 2004). In addition, tazarotene increases the number of A375 human melanoma cells expressing Bax and decreases the number of cells expressing Bcl-2 (Ran et al., 2005b). In this study, our results indicate that the expression of anti-apoptotic Bcl-2 and Bcl-xl decreases in cell treated with tazarotene compared with the control group (Fig. 2F, G). However, the expression of the apoptotic proteins Bax and Bak is unaffected by tazarotene. The different effects of tazarotene on Bax expression is potentially explained by cell type differences.
Caspase-8 propagates the apoptotic signal by directly cleaving and activating downstream caspase-3 (Walczak and Krammer, 2000). However, no report has demonstrated that tazarotene induces caspase-8 activation. Our present study demonstrated, for the first time, that tazarotene could induce caspase-8 activation (Fig. 3A, B). In addition, caspase-8 inhibition significantly prevents apoptosis and caspase-3 cleavage (Fig. 3C, D). In addition, caspase-8 triggers Bid cleavage into tBid, which, subsequently, induces the release of cytochrome c from the mitochondria and caspase-9 activation (Li et al., 1998). Our results indicate that tazarotene triggers the caspase-8–Bid cleavage pathway (Fig. 3A, B). In addition, tazarotene-induced cytochrome c release is reduced in the presence of a caspase-8 inhibitor (Fig. 3G, H), suggesting that caspase-8 acts as an upstream executor of the mitochondrial apoptotic pathway in tazarotene-treated cells.
In this study, our results indicate that the expression of the death receptor and its ligands, in particular FASL, DR4, DR5, and TRAIL, is upregulated in BCC cells on treatment with 25–100 μM tazarotene; however, no statistically significant differences are observed between the 25 and 50 μM tazarotene treatment groups (Fig. 4A, B), which do not demonstrate extensive caspase-8 activation. Thus, in addition to the death receptor signaling pathway, we hypothesize that additional apoptotic factors, such as endoplasmic reticulum stress (Rao et al., 2004), Ca2+ influx (Chen et al., 2005), p38, JNK, and mitogen-activated protein kinase (MAPK) (Iwaoka et al., 2006; Cho et al., 2013), are potentially involved in tazarotene-induced caspase-8 activation in BCCs.
In this study, we observed that tazarotene-induced ROS generation was evident as early as 1 h after treatment. Moreover, NAC-mediated quenching of ROS generation confers partial protection against tazarotene-induced cell death (Fig. 5B) and apoptosis (Fig. 5C, D), suggesting that tazarotene-induced ROS may act as molecules to trigger BCC cell death. However, the role of ROS in apoptosis remains controversial (Jacobson, 1996). In particular, Mochizuki et al. (2006) recently reported that ROS generated by Nox4 transmit cell survival signals through the AKT–ASK1 pathway in pancreatic cancer cells. Thus, further investigation is required to determine the mechanism(s) linking the possible source of ROS generation, such as the NADPH oxidase (Nox) family (Mochizuki et al., 2006; Stasi et al., 2010; Rousset et al., 2013), in tazarotene-treated BCC cells.
In addition, the mechanism of ROS-induced caspase-8 activation remains unknown. ROS are implicated in the activation of MAPKs, and ASK1 is specifically required for the ROS-induced activation of JNK, p38, and apoptosis (Tobiume et al., 2001). A previous study demonstrated that p38 MAPK-mediated cell death triggered by ROS is associated with caspase-8 activation (Iwaoka et al., 2006). Thus, it is possible that ROS-induced caspase-8 activation after tazarotene treatment is mediated by MAPK activation. However, further investigation is required to elucidate the relationship between MAPK and caspase-8.
Cell cycle arrest is a common cause of cell growth inhibition and apoptosis (Pietenpol and Stewart, 2002). In this study, the proliferation of tazarotene-treated BCC cells was inhibited, and the cells were arrested in the G0/G1 phase of the cell cycle (Fig. 1B). Our results are consistent with previous studies in HL-60 human myeloblastic leukemia cells (Yen et al., 2004) and Tca8113 human tongue squamous cell carcinoma cells (Ran et al., 2005a, b) which demonstrated that tazarotene treatment inhibits proliferation by inducing G0/G1 phase arrest. Although the mechanism of tazarotene-induced G0/G1 arrest in BCC cells remains unclear and requires further study, a previous study demonstrated that tazarotene induces p73 expression (Papoutsaki et al., 2004). Additional previous studies demonstrate that p73 induces G1 arrest (Zhu et al., 1998; Ohtsuka et al., 2003), prompting the hypothesis that p73 plays a role in tazarotene-induced BCC G0/G1 phase arrest.
IAPs suppress apoptosis by inhibiting activated effector caspases, such as caspase-3 and caspase-7. IAPs also inhibit cytochrome c-induced activation of caspase-9 (Deveraux and Reed, 1999). Furthermore, IAPs, such as survivin and XIAP, participate in the development of chemo-resistance in several cancer cells (Dubrez-Daloz et al., 2008; Fulda, 2008). To our knowledge, no study describing the relationship between the IAP protein family and tazarotene action has been reported. Thus, we are the first to report that tazarotene can significantly decrease survivin and XIAP protein expression (Fig. 2F, G).
Given that our results indicate that neither caspase inhibitors FADD siRNA nor NAC completely reverse tazarotene-induced growth inhibition (Figs. 2E, 3C, 4C and 5B), other factors/molecules may also regulate the induction of cell death. Tazarotene, a new member of the acetylenic class of RARβ/γ-selective retinoids (Roeder et al., 2004; Dando and Wellington, 2005; Tanghetti et al., 2011), has been shown to induce a concentration-dependent increase in RAR-beta expression in the immortalized basal keratinocyte (C5N) tumor cell line (Orlandi et al., 2004). Our current results also demonstrate that tazarotene induces RAR-beta and RAR-alpha but not RAR gamma in BCC cells (data not shown). Further studies are required to examine whether a RARβ/α-dependent pathway participates in tazarotene-induced cell cycle arrest and apoptosis.
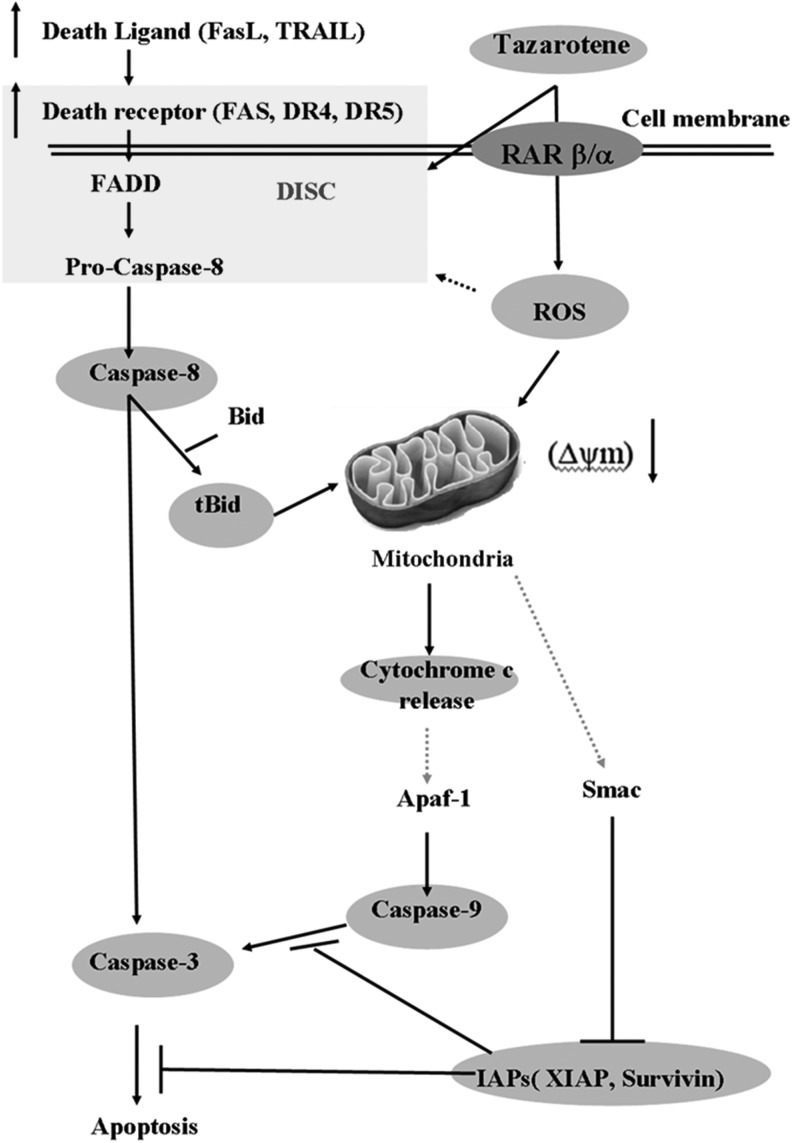
Tazarotene is now being tested in a clinical trial for skin diseases (Roeder et al., 2004; Dando and Wellington, 2005; Tanghetti et al., 2011). Many studies suggest that tazarotene can be used to treat skin cancers, including BCC (Bianchi et al., 2004). Our present study is the first to demonstrate that tazarotene induces apoptotic cell death in BCC via caspase-8 activation and mitochondrial damage, which is accompanied by decreased levels of anti-apoptotic members of the Bcl-2 and IAP protein families. Our data also reveal that activated caspase-8 triggers mitochondrial apoptotic events by inducing Bid cleavage. Furthermore, our data suggest that ROS play a role in the activation of caspase-8 and the induction of cytochrome c release (Fig. 6). Taken together, these results provide insights into the molecular mechanisms underlying tazarotene-induced apoptosis in human BCC cells and suggest that this compound may be a promising candidate for cancer treatment.
FIG. 6.
A hypothetical model of the signaling pathways involved in tazarotene-mediated apoptosis in BCC cells.
Acknowledgments
This work was supported by Grants TCVGH-NCHU1037606 for CCLIN and VGHKS 100-012 for C.-S.W. and in part by the Ministry of Education, Taiwan, R.O.C under the ATU plan.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- Bastiaens M.T., Hoefnagel J.J., Bruijn J.A., Westendorp R.G., Vermeer B.J., and Bouwes Bavinck J.N. (1998). Differences in age, site distributions, and sex between nodular and superficial basal cell carcinoma indicate different types of tumors. J Invest Dermatol 110,880–884 [DOI] [PubMed] [Google Scholar]
- Bianchi L., Orlandi A., Campione E., Angeloni C., Costanzo A., Spagnoli L.G., and Chimenti S. (2004). Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. Br J Dermatol 151,148–156 [DOI] [PubMed] [Google Scholar]
- Boehning D., Patterson R.L., Sedaghat L., Glebova N.O., Kurosaki T., and Snyder S.H. (2003). Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 5,1051–1061 [DOI] [PubMed] [Google Scholar]
- Boonstra J., and Post J.A. (2004). Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337,1–13 [DOI] [PubMed] [Google Scholar]
- Carr D.R., Trevino J.J., and Donnelly H.B. (2011). Retinoids for chemoprophylaxis of nonmelanoma skin cancer. Dermatol Surg 37,129–145 [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang X., Kubo H., Harris D.M., Mills G.D., Moyer J., Berretta R., Potts S.T., Marsh J.D., and Houser S.R. (2005). Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes 97,1009–1017 [DOI] [PubMed]
- Cho H.W., Park S.K., Heo K.W., and Hur D.Y. (2013). Methotrexate induces apoptosis in nasal polyps via caspase cascades and both mitochondria-mediated and p38 mitogen-activated protein kinases/Jun N-terminal kinase pathways. Am J Rhinol Allergy 27,e26–e31 [DOI] [PubMed] [Google Scholar]
- Dando T.M., and Wellington K. (2005). Topical tazarotene: a review of its use in the treatment of plaque psoriasis. Am J Clin Dermatol 6,255–272 [DOI] [PubMed] [Google Scholar]
- Danial N.N., and Korsmeyer S.J. (2004). Cell death: critical control points. Cell 116,205–219 [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., and Reed J.C. (1999). IAP family proteins—suppressors of apoptosis. Genes Dev 13,239–252(Review). [DOI] [PubMed] [Google Scholar]
- Diepgen T.L., and Mahler V. (2002). The epidemiology of skin cancer. Br J Dermatol 146,1–6 [DOI] [PubMed] [Google Scholar]
- Dubrez-Daloz L., Dupoux A., and Cartier J. (2008). IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle 7,1036–1046(Review). [DOI] [PubMed] [Google Scholar]
- Fulda S. (2008). Targeting inhibitor of apoptosis proteins (IAPs) for cancer therapy. Anticancer Agents Med Chem 8,533–539 [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J.M., and Korsmeyer S.J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13,1899–1911(Review). [DOI] [PubMed] [Google Scholar]
- Higuchi E., Chandraratna R.A., Hong W.K., and Lotan R. (2003). Induction of TIG3, a putative class II tumor suppressor gene, by retinoic acid in head and neck and lung carcinoma cells and its association with suppression of the transformed phenotype. Oncogene 22,4627–4635 [DOI] [PubMed] [Google Scholar]
- Iwaoka S., Nakamura T., Takano S., Tsuchiya S., and Aramaki Y. (2006). Cationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cells. J Leukoc Biol 79,184–191 [DOI] [PubMed] [Google Scholar]
- Jacobson M.D. (1996) Reactive oxygen species and programmed cell death. Biochem Sci 21,83–86 [PubMed] [Google Scholar]
- Kasper M., Jaks V., Hohl D., et al. (2012). Basal cell carcinoma—molecular biology and potential new therapies. J Clin Invest 122,455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.M., and Chung H.W. (2007). Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Commun 363,745–750 [DOI] [PubMed] [Google Scholar]
- Lens M., and Medenica L. (2008). Systemic retinoids in chemoprevention of non-melanoma skin cancer. Expert Opin Pharmacother 9,1363–1374(Review). [DOI] [PubMed] [Google Scholar]
- Li H., Zhu H., Xu C., and Yuan J. (1998). Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94,491–501 [DOI] [PubMed] [Google Scholar]
- Marghoob A.A. (1997). Basal and squamous cell carcinomas: what every primary care physician should know. Postgrad Med 102,139–159 [DOI] [PubMed] [Google Scholar]
- Martinez J.C., and Otley C.C. (2001). The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clin Proc 76,1253–1263 [DOI] [PubMed] [Google Scholar]
- Mochizuki T., Furuta S., Mitsushita J., Shang W.H., Ito M., Yokoo Y., Yamaura M., Ishizone S., Nakayama J., Konagai A., Hirose K., Kiyosawa K., and Kamata T. (2006). Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25,3699–3707 [DOI] [PubMed] [Google Scholar]
- Ohtsuka T., Ryu H., Minamishima Y.A., Ryo A., and Lee S.W. (2003). Modulation of p53 and p73 levels by cyclin G: implication of a negative feedback regulation. Oncogene 22,1678–1687 [DOI] [PubMed] [Google Scholar]
- Orlandi A., Bianchi L., Costanzo A., Campione E., Giusto Spagnoli L., and Chimenti S. (2004). Evidence of increased apoptosis and reduced proliferation in basal cell carcinomas treated with tazarotene. J Invest Dermatol 122,1037–1041 [DOI] [PubMed] [Google Scholar]
- Papoutsaki M., Lanza M., Marinari B., Nisticò S., Moretti F., Levrero M., Chimenti S., and Costanzo A. (2004). The p73 gene is an anti-tumoral target of the RARbeta/gamma-selective retinoid tazarotene. J Invest Dermatol 123,1162–1168 [DOI] [PubMed] [Google Scholar]
- Pietenpol J.A., and Stewart Z.A. (2002). Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 181–182,475–481(Review). [DOI] [PubMed] [Google Scholar]
- Ran L., Tan W., Tan S., Zhang R., Wang W., and Zeng W. (2005a). Effects of ATRA, acitretin and tazarotene on growth and apoptosis of Tca8113 cells. J Huazhong Univ Sci Technolog Med Sci 25,393–396 [DOI] [PubMed] [Google Scholar]
- Ran L.W., Tan S.S., Wang W.J., Qiu S., Lei X.B., Zeng WH. (2005b). Effects of all-trans-retinoic acid, acitretin and tazarotene on apoptosis and Bax/Bcl-2 expressions of human melanoma cells A375 and the significance. Di Yi Jun Yi Da Xue Xue Bao 25,972–974, 978 [Article in Chinese]. [PubMed] [Google Scholar]
- Rao R.V., Ellerby H.M., and Bredesen D.E. (2004). Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11,372–380 [DOI] [PubMed] [Google Scholar]
- Robert C., Delva L., Balitrand N., Nahajevszky S., Masszi T., Chomienne C., and Papp B. (2006). Apoptosis induction by retinoids in eosinophilic leukemia cells: implication of retinoic acid receptor-alpha signaling in all-trans-retinoic acid hypersensitivity. Cancer Res 66,6336–6344 [DOI] [PubMed] [Google Scholar]
- Roeder A., Schaller M., Schäfer-Korting M., and Korting H.C. (2004). Tazarotene: therapeutic strategies in the treatment of psoriasis, acne and photoaging. Skin Pharmacol Physiol 17,111–118 [DOI] [PubMed] [Google Scholar]
- Rousset F., Nguyen M.V., Grange L., Morel F., and Lardy B. (2013). Heme oxygenase-1 regulates matrix metalloproteinase MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase activity in chondrocytes. PLoS One 8,e66478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi M.A., Scioli M.G., Arcuri G., Mattera G.G., Lombardo K., Marcellini M., Riccioni T., De Falco S., Pisano C., Spagnoli L.G., Borsini F., and Orlandi A. (2010). Propionyl-l-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol 30,426–435 [DOI] [PubMed] [Google Scholar]
- Sharma V., Anderson D., and Dhawan A. (2012). Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17,852–870 [DOI] [PubMed] [Google Scholar]
- Tanghetti E., Dhawan S., Green L., Ling M., Downie J., Germain M.A., Kasteler J.S., Kircik L., Oefelein M.G., and Draelos Z. (2011). Clinical evidence for the role of a topical anti-inflammatory agent in comedonal acne: findings from a randomized study of dapsone gel 5% in combination with tazarotene cream 0.1% in patients with acne vulgaris. J Drugs Dermatol 10,783–792 [PubMed] [Google Scholar]
- Thorburn A. (2004). Death receptor-induced cell killing. Cell Signal 16,139–144(Review). [DOI] [PubMed] [Google Scholar]
- Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., and Ichijo H. (2001). ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2,222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic M., and Dummer R. (2002). Immunotherapy for nonmelanoma skin cancer: does it have a future? Cancer 94,477–485 [DOI] [PubMed] [Google Scholar]
- Urosevic M., Maier T., Benninghoff B., Slade H., Burg G., and Dummer R. (2003). Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Arch Dermatol 139,1325–1332 [DOI] [PubMed] [Google Scholar]
- Valmiki M.G., and Ramos J.W. (2009). Death effector domain-containing proteins. Cell Mol Life Sci 66,814–830(Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H., and Krammer P.H. (2000). The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 256,58–66 [DOI] [PubMed] [Google Scholar]
- Wang X. (2001). The expanding role of mitochondria in apoptosis. Gene Dev 15,2922–2933 [PubMed] [Google Scholar]
- Wennberg A.M. (2000). Basal cell carcinoma—new aspects of diagnosis and treatment. Acta Derm Venereol Suppl (Stockh) 209,5–25 [PubMed] [Google Scholar]
- Wong W.W., and Puthalakath H. (2008). Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life 60,390–397(Review). [DOI] [PubMed] [Google Scholar]
- Yen A., Fenning R., Chandraratna R., Walker P., and Varvayanis S. (2004). A retinoic acid receptor beta/gamma-selective prodrug (tazarotene) plus a retinoid X receptor ligand induces extracellular signal-regulated kinase activation, retinoblastoma hypophosphorylation, G0 arrest, and cell differentiation. Mol Pharmacol 66,1727–1737 [DOI] [PubMed] [Google Scholar]
- Yen H.T., Chiang L.C., Wen K.H., Tsai C.C., Yu C.L., and Yu H.S. (1996). The expression of cytokines by an established basal cell carcinoma cell line (BCC-1/KMC) compared with cultured normal keratinocytes. Arch Dermatol Res 288,157–161 [DOI] [PubMed] [Google Scholar]
- Zhu J., Jiang J., Zhou W., and Chen X. (1998). The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res 58,5061–5065 [PubMed] [Google Scholar]