Abstract
Epothilones are relatively new tubulin-poison anticancer drugs. Iso-fludelone (KOS-1803) is a synthetic third generation epothilone drug discovered at Memorial Sloan Kettering Cancer Center, and currently in Phase I clinical trials. We report an LC-MS/MS assay for the sensitive, accurate and precise quantitation of Iso-fludelone in 0.2 mL of human plasma. Validation was performed according to FDA guidance. The assay comprised of KOS-1724 as the internal standard and an MTBE liquid-liquid extraction with a water wash step. Separation was achieved with an YMC-Pack ODS-AQ column and an isocratic mobile phase of 0.1% formic acid in acetonitrile and water (70:30, v/v) at 0.3 mL/min for 4 min. Chromatographic separation was followed by electrospray, positive-mode ionization tandem mass spectrometric detection in the multiple reaction monitoring (MRM) mode. The assay was linear from 0.1– 300 ng/mL and was accurate (−9.41–7.07%) and precise (1.03–13.7%) which fulfilled FDA criteria for validation. Recovery from plasma was 73.9–79.7% and ion suppression was negligible (−22.8 to −31.3%). Plasma freeze thaw stability (99.97–105.7%), stability for 11 months at −80 °C (94.93–107.9%), and stability for 6 h at room temperature (94.75–105.5%) were all acceptable. This assay is currently being applied to quantitate Iso-fludelone in clinical samples.
Keywords: KOS-1803 (Iso-fludelone), epothilone, LC-MS/MS, assay, validation
1. Introduction
While molecularly targeted anticancer agents may show remarkable response rates, progression is often inevitable, and subsequently cytotoxics, such as microtubule-interaction agents continue to play a major role in disease control in cancer treatment. While vinca alkaloids possess a microtubule destabilizing activity, the taxanes, notably paclitaxel and docetaxel, are considered microtubule stabilizers [1]. Like the taxanes, epothilones promote microtubule stability, disrupting cell division and promoting apoptosis. Reported advantages of epothilones over taxanes include effectiveness against multidrug-resistant (MDR) tumor cells in vitro and in vivo [2]. Ixabepilone is the only FDA approved epothilone derivative [3], but suffers from the need of cremophor-ethanol in the formulation. In further optimizing the pharmacological properties of the epothilones, several analogues have been characterized, including Iso-fludelone [4]. Iso-fludelone (KOS-1803) is a synthetic third generation epothilone drug discovered at Memorial Sloan Kettering Cancer Center [5]. It has shown remarkable in vitro metabolic stability and preclinical in vivo activity in several xenografts models. The incorporation of the isoxazole moiety conferred improved metabolic stability, and the trifluoromethyl moiety improved solubility, eliminating the need for cremophor-ethanol [5].
We aimed to develop and validate a quantitative Iso-fludelone assay in human plasma to support an ongoing first-in-human dose-finding trial (ClinicalTrials.gov Identifier: NCT01379287). Related compounds have low doses and long half-lives. Dehydelone (KOS-1584) has a maximum tolerated dose of 36 mg/m2, a volume of distribution of approximately 300 L/m2, and displays a terminal half-life of 30 h [4], while ixabepilone is dosed at 40 mg/m2 over 3 h, has a volume of distribution of 1500 L, displays a half-life of approximately 35 h [6], and has a 168 h post-dose plasma concentration of less than 10 ng/mL [7]. Given the clinical starting dose of Iso-fludelone at 1 mg/m2, we aimed to develop and validate an assay with a lower limit of quantitation of 0.1 ng/mL in human plasma.
2. Experimental
2.1. Chemicals and reagents
Iso-fludelone (KOS-1803), KOS-1724 (Figure 1), were provided by Memorial Sloan Kettering (NewYork, NY, USA). Acetonitrile and water were obtained from Fisher Scientifc (Fair Lawn, NJ, USA) and methyl t-butyl ether (MTBE) was obtained from Honeywell Burdick and Jackson (Morristown, NJ, USA). Formic acid was obtained from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade. Control human plasma was produced by centrifuging whole blood (Central Blood Bank, Pittsburgh, PA, USA) for 20 min at 2000× g at room temperature. Nitrogen for evaporation of samples was purchased from Valley National Gases, Inc. (Pittsburgh, PA, USA). Nitrogen for mass spectrometric applications was purified with a Parker Balston Nitrogen Generator (Parker Balston, Haverhill, MA, USA).
Figure 1.
Chemical structures of Iso-fludelone and internal standard (KOS-1724).
2.2. Chromatography
The LC system consisted of an Agilent (Palo Alto, CA, USA) 1200 SL autosampler and binary pump, a YMC-Pack ODS-AQ (3 μm, 100 mm × 2 mm) column, (YMC America, Inc., Allentown, PA, USA), and an isocratic mobile phase. Mobile phase solvent A consisted of 0.1% (v/v) formic acid in acetonitrile, and mobile phase solvent B consisted of 0.1% (v/v) formic acid in water. The mobile phase composition was 70% solvent A and 30% solvent B, pumped at a flow rate of 0.3 mL/min. The overall run time was 4 min.
2.3. Mass spectrometry
Mass spectrometric detection was carried out using an ABI SCIEX (San Jose, CA, USA) 4000Q hybrid linear ion trap tandem mass spectrometer with electrospray ionization operated in the positive, and multiple reaction monitoring (MRM) mode. The settings of the mass spectrometer were as follows: curtain gas 40, IS voltage 5500 V, probe temperature 500 °C, GS1 40, GS2 40, declustering potential 50 V, collision energy 22 V. Quadrupoles were set to unit resolution and dwell time of 100 msec. The MRM m/z transitions monitored for Iso-fludelone and KOS-1724 were 528.3 to 340.4, and 475.5 to 368.3, respectively. The HPLC system and mass spectrometer were controlled by Analyst software (version 1.4.2), and data was collected with the same software. The analyte-to-internal standard ratio was calculated for each standard by dividing the area of each analyte peak by the area of the respective internal standard peak for that sample. Standard curves of the analytes were constructed by plotting the analyte-to-internal standard ratio versus the known concentration of analyte in each sample. Standard curves were fit by linear regression with weighting by 1/y2, followed by back calculation of concentrations.
2.4. Preparation of calibration standards and quality control samples
Stock solutions of Iso-fludelone and KOS-1724 were prepared independently at 1 mg/mL in acetonitrile and stored at −80 °C. On the day of assay, these solutions were serially diluted (in steps of 10-fold) in acetonitrile to obtain the lower calibration working solutions of 100, 10, 1, 0.1, and 0.01 μg/mL. These calibration working solutions were diluted in human plasma to produce the following analyte concentrations: 0.1, 0.3, 1, 3, 10, 30, 100, 300 ng/ml. For each calibration series, zero and blank samples were also prepared from control plasma. Quality control (QC) stock solutions were prepared independently from separate weightings and diluted in human plasma to produce 200-μL aliquots of the following Iso-fludelone concentrations, stored at −80 °C: QC Lower Limit (QCLL) 0.1 ng/mL, QC Low (QCL) 0.25 ng/mL; QC Mid (QCM) 5.0 ng/mL, and QC High (QCH) 250 ng/mL.
2.5. Sample preparation
Ten μL of KOS-1724 internal standard (IS) solution (100 ng/mL in acetonitrile) were added to each 200 μL standard, QC, or sample plasma in an Eppendorf tube. One mL of MTBE were added to precipitate proteins, after which the sample was vortexed for 1 min on a vortex Genie-2 set at 10 (VWR Scientific Products, Bohemia, NY, USA) and centrifuged for 5 min at 16,000 x g, at room temperature. Samples were then flash frozen using dry ice and methanol, and the MTBE layer was decanted into a second Eppendorf. Two hundred μL of HPLC water was added to the MTBE layer and vortexed for 5 sec. Samples were then flash frozen again and the MTBE layer was decanted into 12 × 75 mm borosilicate glass tube and evaporated to dryness under nitrogen at 37°C. Dried residues were reconstituted in 100 μL of acetonitrile/water 60/40 (v/v). The supernatants were transferred to an autosampler vial (6 mm crimp top round bottom 0.3 mL glass vial, Agilent Technologies, Santa Clara, CA) and were capped and placed into the autosampler (kept at 4 °C) of the LC-MS/MS system. Five μL from each vial were injected onto the LC-MS/MS system with the first set of calibration samples being injected twice and discarding of the first injection data set.
2.6. Validation procedures
2.6.1. Calibration curve and lower limit of quantitation (LLQ)
Iso-fludelone was injected into our analytical system at decreasing concentrations to determine the minimal concentration with a signal-to-noise ratio of at least 5:1. Calibration standards and blanks were prepared (see section 2.4) and analyzed in triplicate to establish the calibration range with acceptable accuracy and precision.
2.6.2. Accuracy and precision
The accuracy and precision of the assay were determined by analyzing samples with Iso-fludelone at the LLQ concentration and QC concentrations in a minimum of 6 replicates in 3 analytical runs together with independently prepared, triplicate calibration curves.
Accuracy was calculated at each test concentration as: (mean measured concentration / nominal concentration) x 100%.
Precision was calculated by ANOVA as described [8]. Back-calculated concentrations of calibration and QC samples were entered with the run number as factor. The intra-assay and inter-assay precisions were calculated from the resulting mean squares of the within runs and mean squares of the between runs, respectively.
2.6.3. Selectivity and specificity
To investigate whether endogenous matrix constituents interfered with the assay, blank extracts generated from six individual lots of control human plasma were analysed in triplicate. Any endogenous interference at the retention time of Iso-fludelone should be <20% of the lower limit of quantitation. In addition, no significant interfering peaks should be observed at the retention times of the internal standard.
2.6.4. Extraction recovery and ion-suppression
The extraction recovery of Iso-fludelone from human plasma samples was determined by comparing QC concentrations, in replicates of four, extracted and injected onto the LC-MS/MS system (processed samples). Neat samples of analyte, diluted in reconstitution solvent, were analyzed. Solutions of Iso-fludelone spiked into extracted matrix blanks at concentrations representing 100% extraction recovery were also analyzed by LC-MS/MS in replicates of four (fortified samples). Extraction recovery was determined by comparing the individual peak areas of processed samples to the mean peak area of the fortified samples. Ion-suppression was determined by comparing the peak areas of fortified samples to the mean peak area of the neat samples. The same experiments were performed to determine the extraction recovery of the internal standard KOS-1724 at the working concentration used for the assay.
2.6.5. Stability
Long term stability experiments were performed for Iso-fludelone which compared fresh stock solution made from powder stock to stock solution made up 6 months and 14 months prior and stored at −80 °C. Samples were processed in triplicate and analysed by LC-MS/MS. In addition, a stock solution of 1 mg/ml of both Iso-fludelone and internal standard were aliquoted separately into replicates of 8. Four samples were then processed immediately while the other four sat untouched on the bench top at room temperature for 6 h. Samples were then diluted 10,000 fold and analyzed by LC-MS/MS. The stability of Iso-fludelone in plasma at −80 °C was determined by assaying 6 QCL, QCM, and QCH samples before and after 4, 7, and 11 months of storage. Six QCLL were also analyzed before and after 3, 6, and 10 months of storage at −80 °C. The effect of three freeze/thaw cycles on Iso-fludelone at all four QC concentrations in plasma were evaluated in replicates of 6 by assaying samples after freezing and thawing on 3 separate days and comparing them to freshly prepared samples. The stability of Iso-fludelone in plasma during sample preparation was evaluated by assaying samples before and after 6 h of sitting untouched on the benchtop at room temperature. Autosampler stability was performed to re-evaluate processed samples at 24 h, 48 h, and 72 hour time periods. Samples remained sitting in a refrigerated autosampler at 4 °C until reinjection. Deviations of the re-injected result from the initial injection result were calculated.
2.6.6. Dilutional integrity
To dilutional integrity, the ability to dilute samples from above the upper limit of quantitation to within the validated concentration range, plasma samples containing Iso-fludelone above the upper limit of quantitation were diluted to within the assay range. Plasma samples (N = 6) with analyte concentrations of 3000 ng/mL were diluted 30-fold (to 100 ng/mL) with control plasma, then extracted and analyzed.
2.6.1. Anti-coagulant cross-validation
Heparinized plasma or citrate plasma was used separately to construct calibration curves, and applied to analyze duplicate QC samples at each level prepared in citrate plasma. Back-calculated concentrations of the QCs were compared.
2.7. Application of the assay to patient samples
To demonstrate the applicability of our assay, we quantitated concentrations of Iso-fludelone in the plasma of a patient dosed 8 mg/m2 by 3 h intravenous infusion. Prior to participation in the study, the subject gave written, informed consent as approved by MSKCC Institutional Review Board. Blood samples were collected into heparinized tubes before and at 1, 2 h after start of infusion, 5 min before the end of the 3 h infusion, and 0.5, 1, 2, 3, 5, 7, 24, 48, and 72 h after dosing. Each sample was centrifuged at approximately 1000 × g at 4 °C for 5 min, and the resulting plasma was stored at −70 °C until analysed.
3. Results and Discussion
3.1. Method development
Because we have experience with the epothilone compound ixabepilone, our starting point was the ixabepilone assay which covers a concentration range of 2–500 ng/mL in 50 μL plasma, extracted with acetonitrile, separated on a an YMC-Pack ODS column and detected with an ABI4000 mass spectrometer in positive mode using an analogue as an internal standard [9]. Iso-Fludelone and the candidate internal standards were infused into the mass spectrometer in single quadropule operation mode. Voltages and gas flows were varied to optimize the [M+H]+ signal. Next, the detector was operated in triple-quadrupole mode and collision voltage was varied to optimize the signal of a product ion. The YMC-Pack ODS column yielded adequate retention and peak shape for Iso-fludelone. The mobile phase was simplified to an isocratic system of 0.1% formic acid in acetonitrile and water (70:30, v/v). We explored acetonitrile protein precipitation and MTBE liquid-liquid extraction. The main advantage of acetonitrile was the potential of direct injection of the supernatant. However, in striving for high sensitivity by increasing the injection volume, this compromised peak shape. Therefore, the cleaner MTBE extraction, with dry-down, reconstitution, and consequently concentration, was pursued.
We had several potential analogue internal standards at our disposal, which were co-injected with Iso-fludelone utilizing a preliminary gradient (elution 1.86 min): KOS-1724 (1.97 min), KOS-1591 (2.07 min), and KOS-1584 (2.21 min) [5]. After determining the performance of a triplicate calibration curve in plasma with all IS candidates, none stood out, and we chose KOS-1724 as internal standard based on the retention time closest to Iso-fludelone. In this manner, we aimed to increase the robustness of the assay with respect to unforeseen matrix effects. We were able to reduce the run-time to 4 min with adequate performance.
At this point, we focused on the performance of the assay close to the lower limit, which was inadequate at 0.1 ng/mL with too small a signal-to-noise ratio. Although the ixabepilone assay employed negative ionization, which usually results in a lower background signal, switching to positive ionization enhanced the Iso-fludelone signal more than 40-fold. Next, we varied the injection from 5–20 μL, and settled at 5 μL to prevent saturation of the response ratio at 300 ng/mL. Adequate performance of the calibration curve was compromised by accuracy deviations of calibrators of approximately 50%, especially at the lower end of the calibration curve. First, replicate control plasma samples were extracted and each extract was repeatedly injected to determine whether the background was specific to an extract or an individual injection/chromatography run. As it appeared that some extracts, but not plasma batches were inherently dirtier, we applied the water wash step, which resulted in an improvement of the assay performance by decreasing deviations in calibrator accuracy. A range from 0.1–300 ng/mL was optimal, as at a concentration of 500 ng/mL, the response ratio was showing saturation.
To ensure stabilization of the mass spectrometer response and reduce failure of calibrators in the first curve due to negative accuracy (curve splitting), we chose to inject the first standard curve twice while discounting the first injection from the quantitation process. This phenomenon has previously been described for ixabepilone [9], and is likely attributable to adsorption of analyte to surfaces. The closed ring structure of both Iso-Fludelone and ixabepilone are reminiscent of crowm-ethers, and these analytes likely are able to interact with metal ions on the surfaces in the analytical system. This physical process would eventually reach a steady-state once all possible active sites are occupied, and in line with this hypothesis, pre-injection improved assay performance.
3.2. Validation of the assay
3.2.1. Chromatography
Void time was determined to be 1.14 min. The retention time and corresponding capacity factors of the analyte and IS were: Iso-fludelone 2.22 min (k′ = 0.96), KOS-1724 2.34 min (k′ = 1.05). A representative chromatogram of Iso-fludelone at the LLQ concentration in plasma is displayed in Figure 2.
Figure 2.
A) Iso-fludelone chromatograms of control plasma (bottom line) and an LLQ plasma sample (upper line, 100 counts off-set). B) Internal standard chromatograms of control plasma (bottom line) and an LLQ plasma sample (upper line, 500 counts off-set).
3.2.2. Calibration curve and LLQ
According to the FDA guidance for bioanalytical method validation, the calibration curve describes the concentration-response relationship adequately if the observed deviation and precision are ≤ 20% for the LLQ and ≤ 15% for all other calibration concentrations. At least four of six calibration points should meet the above criteria [10].
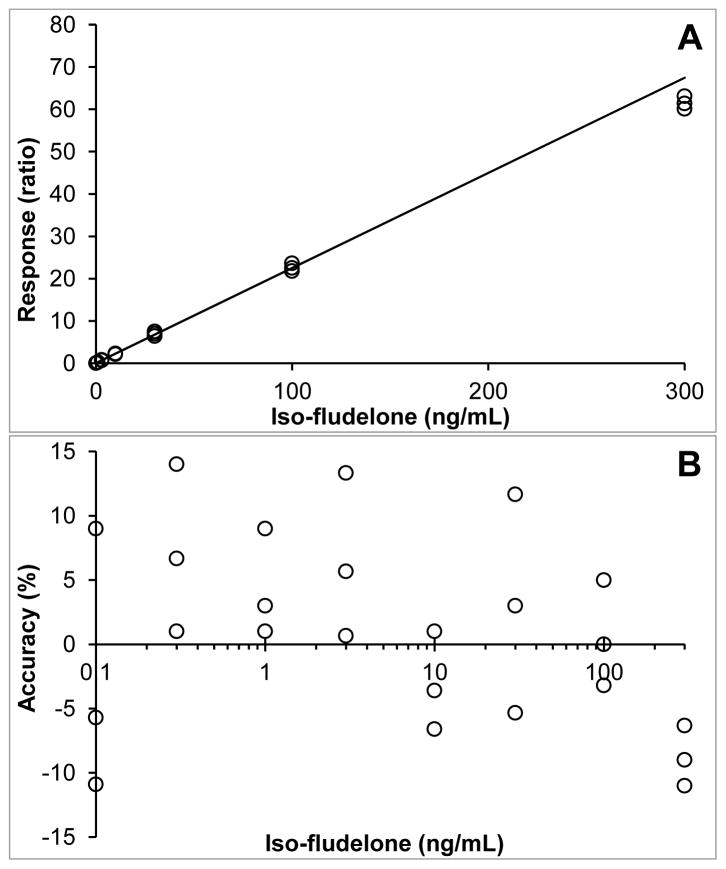
The selected assay range of 0.1–300 ng/mL fulfilled the FDA criteria for the LLQ concentration and the calibration curve. A representative calibration curve and corresponding coefficient of determination are shown in Figure 3. The mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variation due to the performance of the assay in different runs.
Figure 3.
A) Representative calibration curve used to quantitate Iso-fludelone in human plasma (response = 0.2248*conc + 0.0089; R2=0.9940); B) Accuracy of individual calibration points over the concentration range (log transformed).
3.2.3. Accuracy and precision
FDA guidance specifies that the accuracies for all tested concentrations should be within ±15%, and the precisions should not exceed 15% except for the LLQ, in which case these parameters should not exceed 20%[10].
The accuracies and intra- and inter-assay precisions for the tested concentrations (QCLL, QCL, QCM, QCH) were all within the defined acceptance criteria (Table 2).
Table 2.
Iso-fludelone assay performance data for the quantitation of calibration curves and quality control samples in human plasma.
| Concentration (ng/mL) | Accuracy (%) | Intra-assay precision (%) | Inter-assay precision (%) |
|---|---|---|---|
| 0.1 | −4.7 | 13.7 | NA |
| 0.3 | 5.7 | 6.8 | NA |
| 1 | 5.6 | 6.1 | 1.0 |
| 3 | 7.1 | 4.8 | 4.1 |
| 10 | −0.4 | 5.8 | NA |
| 30 | 5.7 | 7.6 | NA |
| 100 | 2.2 | 7.2 | 3.7 |
| 300 | 9.4 | 4.6 | 1.8 |
|
| |||
| 0.1 (QCLL) | −2.1 | 9.6 | NA |
| 0.25 (QCL) | 2.7 | 7.4 | 10.0 |
| 5.0 (QCM) | 8.3 | 4.34 | NA |
| 250 (QCH) | −7.0 | 4.9 | 7.1 |
Calibration curves: N=9; 3-fold results, each in 3 separate runs, for each concentration.
QC: N=18; 6-fold results, each in 3 separate runs, for each concentration.
NA, *The mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variation due to the performance of the assay in different runs.
3.2.4. Selectivity and specificity
According to FDA guidance, the signal at the LLQ must be at least 5 times the signal of any co-eluting peaks[10].
Chromatograms of six individual control plasma samples contained no co-eluting peaks >20% of the analyte areas at the LLQ concentration of Iso-fludelone. A representative chromatogram is displayed in Figure 2.
Concentrations of either Iso-fludelone (3000 ng/mL) or ISTD (1000 ng/mL) were analyzed, and relative signals were compared. Calculations revealed no significant cross talk of Iso-fludelone and IS, < 0.05% and <0.01%, respectively.
3.2.5. Extraction recovery and ion-suppression
FDA-guidance require that recovery is consistent and precise. A recovery of ≥70% with a variation of 15% is generally accepted[8, 10]. There is no specific guidance for the percentage of ion-suppression that is acceptable. Ultimately, the assay performance, as expressed in the precision and accuracy, is most relevant. However, a large and/or variable ion-suppression may explain poor assay performance.
The recoveries of Iso-fludelone at the three QC concentrations ranged from 73.9 to 79.7 %, with a coefficient of variation of 7.8 to 33.2 %. The ion-suppression ranged from −33.8 to −31.3 %, with a coefficient of variation of 5.7 to 27.4 % (Table 1)
Table 1.
Recoveries of Iso-fludelone and KOS-1724 from human plasma and their respective ion suppressions in human plasma extract, with coefficients of variation (CV).
| Concentration (ng/mL) | Recovery (%) | CV (%) | Ion suppression (%) | CV (%) |
|---|---|---|---|---|
| Iso-fludelone | ||||
| 0.25 | 73.85 | 33.20 | −27.64 | 27.35 |
| 5.0 | 79.53 | 11.62 | −31.33 | 10.17 |
| 250 | 79.73 | 7.83 | −22.83 | 5.68 |
| KOS-1724 | ||||
| 10.0 | 61.50 | 25.12 | 9.64 | 11.58 |
N=4
3.2.6. Stability
Stability in biological samples is acceptable when ≥ 85% of the analyte is recovered. The stability of Iso-fludelone and KOS-1724 under varying conditions is reported in Table 3. The stability of Iso-fludelone stock solution at −80 °C, for 6 and 14 months with intermittent thawing for use, was 105.7% and 94.2%, respectively. The stability of Iso-fludelone and KOS-1724 stock solution at room temperature for 6 h was 107.2% and 97.7%, respectively. The stability of Iso-fludelone QCs after 3 freeze thaw cycles (−80 °C to RT) was between 100.0 and 105.7%. Long-term stabilities in plasma at −80 °C were adequate with recoveries between 94.9 and 107.9%. The absolute responses of plasma extracts of Iso-fludelone at the QC concentrations, when reconstituted and kept in the autosampler at 4 °C for 24 h, 48 h and 72 h were 100.8 to 101.4%, 104.5 to 113.9, and 53.6 to 90.4, respectively of the initial responses. Reinjection runs at 24 h and 48 h passed the requirements of any run set by the FDA[10], but the 72 h reinjection run did not.
Table 3.
Stability of Iso-fludelone and KOS-1724 under varying conditions.
| Storage condition | Concentration (ng/mL) | Stability (%) | CV (%) | Replicates | |
|---|---|---|---|---|---|
| Stock solution 6 h | |||||
| Ambient temp. Iso-fludelone | 1 mg/ml | 107.2 | 9.10 | 4 | |
| Ambient temp. KOS-1724 | 1 mg/ml | 97.7 | 6.75 | 4 | |
| Stock solution 14 months | |||||
| −80°C | 1 mg/ml | 94.2 | 10.6 | 3 | |
| Plasma 3 freeze/thaw cycles | |||||
| −80 °C | QCL | 0.25 | 100.4 | 11.5 | 6 |
| QCM | 5.0 | 100.0 | 5.7 | 6 | |
| QCH | 250 | 105.7 | 8.2 | 6 | |
| Plasma 6 h | |||||
| Ambient temp. | QCL | 0.25 | 94.8 | 10.7 | 6 |
| QCM | 5.0 | 100.3 | 5.8 | 6 | |
| QCH | 250 | 105.5 | 6.3 | 6 | |
| Plasma 11 months | |||||
| −80 °C | * QCLL | 0.1 | 107.9 | 36.5 | 6 |
| QCL | 0.25 | 105.8 | 5.9 | 6 | |
| QCM | 5.0 | 94.9 | 11.0 | 6 | |
| QCH | 250 | 106.9 | 10.4 | 6 | |
QCLL long term stability at −80 °C was performed at 10 months
3.2.7. Dilutional integrity
Plasma samples (N = 6) with analyte concentrations of 3000 ng/mL were diluted 30-fold with control plasma had an accuracy of 104.0%, with a coefficient of variation of 6.4%.
3.2.8. Anti-coagulant cross validation
Differences in of back-calculated concentrations of all QC samples were within 6%.
3.3. Patient samples
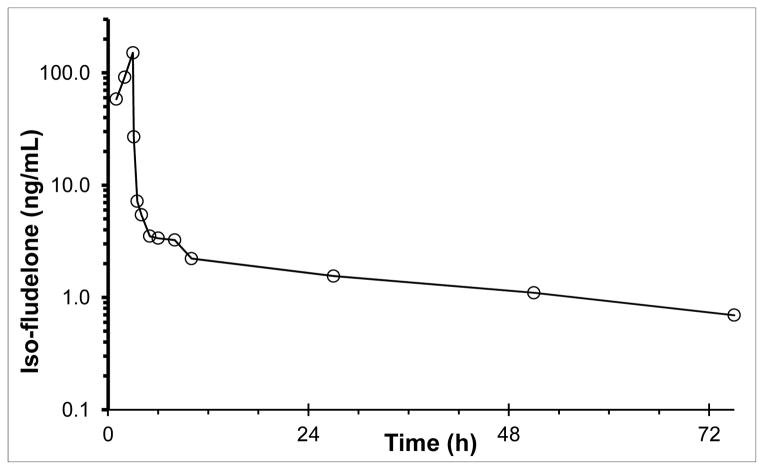
We applied the assay to samples obtained from a patient dosed at 8 mg/m2. The assay was capable of quantitating Iso-fludelone concentrations in all post-treatment samples out to 72 h (Figure 4)
Figure 4.
Plasma concentrations of Iso-fludelone in a patient after a 3-h infusion of 8 mg/m2 isofludelone.
4. Conclusion
Our objective was to develop and validate an analytical method for the sensitive quantitation of Iso-fludelone in human plasma. We accomplished this using reversed phase chromatography for separation with triple quadrupole mass spectrometric MRM detection. To meet the demanding analytical needs to support the phase I clinical trial of Iso-fludelone at low doses, and low expected concentrations, we chose the high-response positive ionization with an extra sample wash-step to reduce noise in the lower concentration range. The analytical method presented here allows the sensitive quantitation of Iso-fludelone in human plasma while meeting the FDA guidance [10], and is currently applied to the first-in-human clinical trial of Iso-fludelone (ClinicalTrials.gov Identifier: NCT01379287).
Highlights.
Iso-fludelone (KOS-1803) is a synthetic 3rd generation epothilone in Phase I trials
An LC-MS/MS assay from 0.1–300 ng/mL in 0.2 mL plasma was validated and applied
This assay is currently utilized to quantitate Iso-fludelone in clinical samples
Acknowledgments
This project used the UPCI Clinical Pharmacology Analytical Facility (CPAF) and was supported in part by award P30CA047904. We would like to thank Bristol-Myers Squibb for providing reference materials and collaborating on the development of Iso-Fludelone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabbatini P, Spriggs DR. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3079–3081. doi: 10.1200/JCO.2008.21.6481. [DOI] [PubMed] [Google Scholar]
- 2.Altmann KH, Memmert K. Progress in drug research Fortschritte der Arzneimittelforschung Progres des recherches pharmaceutiques. 2008;66:273, 275–334. doi: 10.1007/978-3-7643-8595-8_6. [DOI] [PubMed] [Google Scholar]
- 3.Rivera E, Lee J, Davies A. The oncologist. 2008;13:1207–1223. doi: 10.1634/theoncologist.2008-0143. [DOI] [PubMed] [Google Scholar]
- 4.Lam ET, Goel S, Schaaf LJ, Cropp GF, Hannah AL, Zhou Y, McCracken B, Haley BI, Johnson RG, Mani S, Villalona-Calero MA. Cancer Chemother Pharmacol. 2012;69:523–531. doi: 10.1007/s00280-011-1724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou TC, Zhang X, Zhong ZY, Li Y, Feng L, Eng S, Myles DR, Johnson R, Jr, Wu N, Yin YI, Wilson RM, Danishefsky SJ. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13157–13162. doi: 10.1073/pnas.0804773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghajanian C, Burris HA, 3rd, Jones S, Spriggs DR, Cohen MB, Peck R, Sabbatini P, Hensley ML, Greco FA, Dupont J, O’Connor OA. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1082–1088. doi: 10.1200/JCO.2006.08.7304. [DOI] [PubMed] [Google Scholar]
- 7.Beumer JH, Garner RC, Cohen MB, Galbraith S, Duncan GF, Griffin T, Beijnen JH, Schellens JH. Invest New Drugs. 2007;25:327–334. doi: 10.1007/s10637-007-9041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosing H, Man WY, Doyle E, Bult A, Beijnen JH. Journal of Liquid Chromatography & Related Technologies. 2000;23:329–354. [Google Scholar]
- 9.Xu XS, Zeng J, Mylott W, Arnold M, Waltrip J, Iacono L, Mariannino T, Stouffer B. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2010;878:525–537. doi: 10.1016/j.jchromb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Guidance for Industry-Bioanalytical Method Validation. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation; and Research Center for Veterinary Medicine; 2001. [Google Scholar]