Abstract
This case-controlled clinical study was undertaken to investigate to what extent pulmonary function in individuals with chronic Spinal Cord Injury (SCI) is affected by posture. Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1), Maximal Inspiratory Pressure (PImax) and Maximal Expiratory Pressure (PEmax) were obtained from 27 individuals with chronic motor-complete (n=13, complete group) and motor-incomplete (n=14, incomplete group) C2-T12 SCI in both seated and supine positions. Seated-to-supine changes in spirometrical (FVC and FEV1) and airway pressure (PImax and PEmax) outcome measures had different dynamics when compared in complete and incomplete groups. Patients with motor-complete SCI had tendency to increase spirometrical outcomes in supine position showing significant increase in FVC (p=.007), whereas patients in incomplete group exhibited decrease in these values with significant decreases in FEV1 (p=.002). At the same time, the airway pressure values were decreased in supine position in both groups with significant decrease in PEmax (p=.031) in complete group and significant decrease in PImax (p=.042) in incomplete group. In addition, seated-to-supine percent change of PImax was strongly correlated with neurological level of motor-complete SCI (ρ= −.77, p=.002). These results indicate that postural effects on respiratory performance in patients with SCI can depend on severity and neurological level of SCI, and that these effects differ depending on respiratory tasks. Further studies with adequate sample size are needed to investigate these effects in clinically specific groups and to study the mechanisms of such effects on specific respiratory outcome measures.
Keywords: Spinal Cord Injury, Respiratory Function, Posture
1. Introduction
Respiratory dysfunction is a major cause of morbidity and mortality in patients with SCI (Center, 2013; Garshick et al., 2005). Individuals with SCI are also at increased risk of sleep disordered breathing (Burns et al., 2000). Major causes of such dysfunction are respiratory motor control deficits associated with paresis, paralysis and spasticity of trunk and respiratory muscles (Gracies, 2005; Ovechkin et al., 2010; Schilero et al., 2009; Terson de Paleville et al., 2011). In general clinical practice and research the pulmonary function test (PFT) is used to evaluate respiratory motor function. In people with SCI, this test includes spirometrical and maximum airway pressure measures acquired with the test subject in the seated position (American Thoracic Society/European Respiratory Society, 2002; Brusasco et al., 2005; Jain et al., 2006; Miller et al., 2005; Stolzmann et al., 2008). While normative values are corrected for age, height, gender and race, they may also vary depending on body position (Manning et al., 1999; Segizbaeva et al., 2013) and functional capacity of the respiratory muscles (Rehder, 1998). It has been shown that healthy individuals exhibit significantly lower spirometrical and airway pressure outcomes in supine compared to seated position (Badr et al., 2002; Meysman and Vincken, 1998; Navajas et al., 1988; Vilke et al., 2000). It is not clear how postural changes affect respiratory function in individuals with SCI. In contrast to healthy controls, individuals with chronic SCI showed higher FVC and FEV1 outcomes in supine compared to seated position (Chen et al., 1990; Estenne and De Troyer, 1987; Maeda et al., 1990). However, it has been shown that paraplegic patients with thoracic SCI can reach higher spirometrical values in seated position (Baydur et al., 2001). The dynamics of the maximum airway pressure measures in response to the seated-to-supine postural change in SCI population are less known. Compared to supine position, individuals with acute tetraplegic injury exhibited higher PEmax and PImax outcomes in the semirecumbent position (Alvisi et al., 2012). At the same time, significant seated-to-supine decrease in PEmax has been found in the group of patients with motor-complete and incomplete cervical SCI (Sankari et al., 2014). Therefore, since postural factors may have differential impacts in healthy individuals and persons with SCI, it suggests that neurological level and severity of SCI may have complex effects on postural variations in spirometrical and maximum airway pressure measures. This study was undertaken to investigate the impact of postural changes on spirometrical and maximum airway pressure measures in patients with motor-complete and motor-incomplete chronic SCI.
2. Materials and Methods
2.1 Neurological assessment
The study was approved by the University of Louisville Institutional Review Board in compliance with all the institutional and federal regulations concerning the ethical use of human volunteers for research studies. Neurological level and clinical severity of the spinal cord lesion were determined using the American Spinal Cord Injury Association Impairment Scale (AIS) according to the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) (Kirshblum et al., 2011). The ISNCSCI categories were based on the clinical estimation of voluntary contraction strengths from upper-limb (C5 to T1) and lower-limb (L2 to S1) muscles. Those with AIS-A or AIS-B were classified as motor-complete SCI subjects (complete group) and participants with AIS-C or AIS-D were classified as motor-incomplete SCI subjects (incomplete group). A sensory level of injury was determined by light touch and pin prick for C2 through S5 dermatomes (Marino et al., 2003).
2.2 Demographic and Clinical Characteristics
Twenty seven individuals, 40±14 (mean ± SD) years of age, 21 males and 6 females, 92±98 months after SCI participated in this study. Thirteen participants were classified as motor-complete and fourteen were diagnosed as motor-incomplete with cervical (n=15; 7 with motor-complete and 8 with motor-incomplete SCI) or thoracic (n=12; 6 with motor-complete and 6 with motor-incomplete SCI) neurological levels of SCI ranging from C2 to T12. (Tab. 1).
2.3 Pulmonary function test (PFT)
The participants have been tested in the afternoon when their morning routines were restricted to exclude the consumption of caffeine and alcohol and withholding from smoking. Participants have been asked to empty their bladder before testing. Standard spirometrical and maximum airway pressure measurements were taken in the seated and supine positions during every recording session. First, spirometry and airway pressure assessments have been performed in the seated position and then, after 30-min period, had been repeated in the supine position. FVC and FEV1 were obtained from the best of three acceptable attempts and were expressed as the percent of a predicted value (American Thoracic Society/European Respiratory Society, 2002; Hankinson et al., 1999; Hart et al., 2005). PEmax and PImax were assessed using MP45-36-871-350 differential pressure transducer (Validyne Engineering, Northridge, CA) with graphical representation visible during recording. The PImax was measured during maximal inspiratory effort beginning at residual volume and PEmax was measured during maximal expiratory effort starting from total lung capacity (American Thoracic Society/European Respiratory Society, 2002) using a three-way valve system with rubber tube as mouthpiece (Airlife 001504, Allegiance Healthcare Corp., McGaw Park, IL). The pressure meter incorporated a 1.5 mm diameter leak to prevent glottic closure and to reduce buccal muscle contribution during measurements (Griffiths and McConnell, 2007; Smyth et al., 1984). The assessment required that a sharp, forceful effort be maintained for a minimum of 2 seconds. The maximum pressure during each attempt was taken as the highest value that is sustained for one second (Smyth et al., 1984). Means from three airway pressure measurements separately in both seated and supine positions, when outcomes are not more than 20% different from each other, were used for calculations. For each subject, 2.9±1.2 (Mean ± SD) PFT recording sessions performed at least two weeks apart, were used to calculate mean value for each outcome measure.
2.4 Statistical analysis
Data sets were grouped according to subjects’ completeness and neurological level of SCI. Two groups (complete and incomplete) and four sub-groups (cervical complete, cervical incomplete, thoracic complete, and thoracic incomplete) were created. Comparisons between data obtained in seated and supine positions were made in each group using the paired two-tailed t-test. Statistical power values (β) for every comparison have been calculated using “G Power” software (www.gpower.hhu.de; UCLA, Los Angeles, CA). Spearman's rank correlation coefficient (ρ) for whole population, groups and sub-groups were calculated to evaluate the strength of relationships between functional changes (seated-to-supine percent change of FVC, FEV1, PEmax, and PImax) and neurological levels of SCI by assigning the numerical value to the participant’s level of SCI from 1 (C1) to 20 (T12). Significance was reached at p<0.05 with the level of significance (α) being set at 0.05.
3. Results
When analyzed for all participants (n=27), ranges of seated-to-supine change in PFT values for FVC and FEV1 varied from −7.5% to +29.8% and from −15.3% to +30.9%; whereas for PEmax and PImax those changes varied from −48.2% to +35.4% and from −38.8% to +36.4%, respectively. When analyzed in all subjects, only PEmax was decreased significantly in the supine as compared to seated position (p=.011). In the complete group (n=13), FVC were increased (p=.007) and PEmax were decreased (p=.031); whereas, in the incomplete group (n=14), both FEV1 and PImax were decreased (p=.002 and .042, respectively) in the supine position (Table 2). The analysis of the PFT outcomes depending on the completeness and neurological level of SCI revealed that there were significant increase (p=.033) in FVC in participants with cervical complete SCI (n=7) and decrease in FEV1 and PEmax (p=.30 and .40, respectively) in participants with cervical incomplete SCI (n=8). In participants with thoracic complete SCI (n=6), there was a decrease in PEmax (p=.041), whereas, in participants with thoracic incomplete SCI (n=6), FVC and FEV1 were decreased (p=.022 and .042, respectively) (Table 2). Change in the amounts of PImax correlated linearly with the level of neurological injury in the complete group (ρ=-.77, p=.002) (Fig.1). Changes in the other outcomes did not correlate with the levels of injury.
Table 2.
Pulmonary function outcomes in patients with chronic SCI in seated and supine positions
| Subjects | FVC (% predicted) |
FEV1 (% predicted) |
PEmax (cm H2O) |
PImax (cm H2O) |
|---|---|---|---|---|
| All subjects (n=27) | ||||
| Seated | 76.5 ± 19.6 | 66.6 ± 20.4 | 60.5 ± 26.6 | −74.3 ± 26.0 |
| Supine | 78.6 ± 16.9 | 65.5 ± 18.0 | 53.4 ± 25.5* | −69.3 ± 24.1 |
| Power (β) | 0.390 | 0.117 | 0.756 | 0.296 |
| p-value | 0.085 | 0.433 | 0.011 | 0.083 |
| Motor-complete (n = 13) | ||||
| Seated | 64.7 ± 13.8 | 56.5 ± 13.8 | 49.1 ± 22.7 | −72.0 ± 29.6 |
| Supine | 70.4 ± 11.4* | 59.2 ± 11.3 | 39.9 ± 14.4* | −69.1 ± 26.9 |
| Power (β) | 0.840 | 0.200 | 0.600 | 0.086 |
| p-value | 0.007 | 0.248 | 0.031 | 0.550 |
| Motor-incomplete (n = 14) | ||||
| Seated | 87.4 ± 18.0 | 76.1 ± 21.5 | 71.1 ± 26.2 | −76.5 ± 23.1 |
| Supine | 86.2 ± 18.0 | 71.4 ± 21.4* | 66.0 ± 27.4 | −69.6 ± 22.1* |
| Power (β) | 0.168 | 0.958 | 0.269 | 0.549 |
| p-value | 0.298 | 0.002 | 0.177 | 0.042 |
| Cervical Motor-complete (n = 7) | ||||
| Seated | 59.3 ± 8.1 | 49.4 ± 8.5 | 35.5 ± 18.7 | −53.9 ± 24.6 |
| Supine | 65.9 ± 7.3* | 54.8 ± 10.2 | 29.3 ± 7.4 | −62.1 ± 32.4 |
| Power (β) | 0.629 | 0.403 | 0.174 | 0.390 |
| p-value | 0.033 | 0.086 | 0.274 | 0.097 |
| Cervical Motor-incomplete (n = 8) | ||||
| Seated | 82.6 ± 18.1 | 68.7 ± 21.2 | 64.0 ± 28.6 | −65.7 ± 20.3 |
| Supine | 83.0 ± 18.3 | 64.5 ± 19.2* | 56.7 ± 22.2* | −60.5 ± 12.9 |
| Power (β) | 0.060 | 0.639 | 0.580 | 0.204 |
| p-value | 0.834 | 0.030 | 0.040 | 0.230 |
| Thoracic Motor-complete (n = 6) | ||||
| Seated | 71.0 ± 17.0 | 64.8 ± 14.7 | 65.1 ± 16.0 | −93.1 ± 19.6 |
| Supine | 75.6 ± 13.8 | 64.3 ± 11.0 | 52.2 ± 9.6 | −77.2 ± 18.0* |
| Power (β) | 0.275 | 0.050 | 0.440 | 0.599 |
| p-value | 0.154 | 0.892 | 0.076 | 0.041 |
| Thoracic Motor-incomplete (n = 6) | ||||
| Seated | 93.7 ± 17.4 | 85.8 ± 19.2 | 80.4 ± 21.4 | −90.9 ± 19.5 |
| Supine | 90.6 ± 18.2* | 80.6 ± 22.2* | 78.4 ± 30.8 | −81.8 ± 27.0 |
| Power (β) | 0.720 | 0.562 | 0.056 | 0.309 |
| p-value | 0.022 | 0.042 | 0.798 | 0.132 |
Values are means ± SD;
Significant difference compared with seated, p < 0.05 (paired t-test).
Fig. 1.
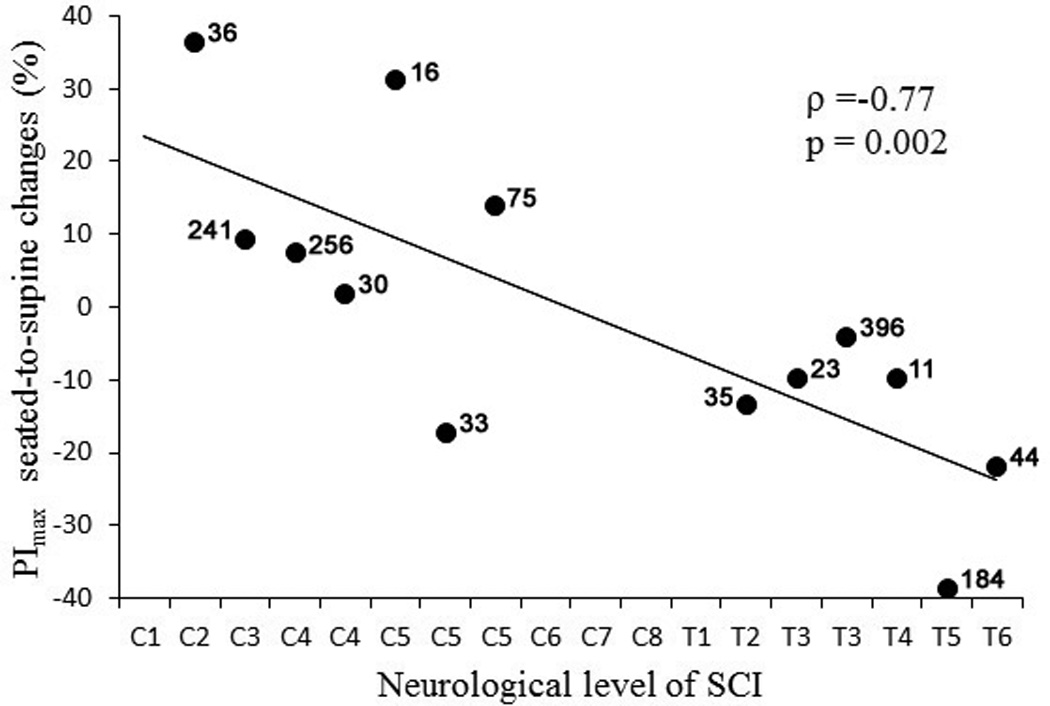
Scatter plot of seated-to-supine percent change in PImax values vs. neurological level of injury in participants with both cervical and thoracic motor-complete SCI. The time after injury shown above each individual data point. Note that those with cervical lesions largely had an increase in PImax while subjects with thoracic lesion showed a decrease. Also note that there are no correlation between data points and time after injury.
4. Discussion
The major conclusion of the current study is that PFT measures assessed in seated and supine positions in patients with chronic SCI depend on severity and neurological level of SCI, and that these postural effects vary in specific respiratory tasks. Our findings indicate that spirometrical (FVC and FEV1) and airway pressure (PEmax and PImax) measures exhibit differential dynamics when posture is changed from seated to supine position. In contrast to subjects with motor-complete SCI, those with incomplete injury exhibited decrease in spirometrical values. At the same time, compared to seated position, the airway pressure outcomes were decreased in supine position in both complete and incomplete groups.
4.1 Spirometrical outcomes (FVC and FEV1)
It is known that healthy individuals (Vilke et al., 2000) as well as those with obstructive lung function, exhibit better spirometric outcomes in the upright position versus supine, which has been attributed to the higher lung compliance in supine position (Allen et al., 1985). In contrast, SCI patients show improved spirometrical values in the supine position (Baydur et al., 2001). Chen et al. reporting no difference in FVC values that were obtained in both seated and supine positions in paraplegics, but they found that in tetraplegics, these measurements were significantly higher in the supine position (Chen et al., 1990). Our results are consistent with their findings revealing significant increase in FVC in individuals with motor-complete SCI and significant decrease in FEV1 in individuals with motor-incomplete SCI (Table 2). In addition, in participants with thoracic motor-incomplete SCI, both FVC and FEV1 were significantly lower in supine position. These dynamics were not detected when compared cervical vs. thoracic SCI groups, indicating that the severity of SCI is a predominant factor over the level of SCI in the effects of postural changes onto the spirometric characteristics. Decrease in respiratory performance in patients with motor-incomplete SCI in supine position may play a role in predisposing them for sleep disordered breathing by having a more collapsible airway and hypoventilation under smaller lung volumes (Burns et al., 2000). At the same time, the results of the PFT may also depend on factors other than the amount of respiratory motor control loss features observed in SCI patients. These other factors may include a combination of restrictive and obstructive respiratory patterns associated with smoking, obesity, chronic obstructive pulmonary disease (COPD), skeletal deformations and/or airway stenosis.
4.2 Airway pressure outcomes (PEmax and PImax)
To our knowledge, no studies regarding seated-to-supine postural changes in PEmax and PImax in patients with chronic SCI considering severity of injury have been reported. In the acute phase of SCI, Alvisi and colleagues (2012) showed that, compared to the supine position, individuals with tetraplegic injury exhibit higher PEmax and PImax values when tested in the semirecumbent position. In contrast, our findings indicate that PEmax and PImax values show a tendency to decrease in supine position in patients with chronic SCI. The mechanisms of these differences might be attributed to the pathophysiological conditions associated with acute and chronic injury. Lasting up to 4–6 weeks after injury, the acute phase is associated with marked reduction or abolition of the reflex activity below the level of injury and can cause dramatic motor and autonomic control deficits (Krassioukov, 2009). As a consequence, acute SCI patients can develop tidal expiratory flow limitations associated with increased airway resistance and a reduced capacity of the respiratory muscles to respond to the increased load (Alvisi et al., 2012). With no discrimination to the completeness of injury, Sankari and coworkers (2014) detected significant decrease in PEmax in supine position in patients with cervical chronic SCI (Sankari et al., 2014). Our analysis indicates that PEmax and PImax tended to be lower in supine position as compared to seated in both complete and incomplete groups. However, in motor-complete SCI subjects, the seated-to-supine changes in PImax were strongly correlated with the level of injury showing increase in those with cervical injury whereas subjects with thoracic injury showed a decrease (Figure 1). Seated-to-supine range of change in airway pressure measures was higher (−48.2% to +36.4%) than the one in spirometrical outcomes (−7.5% to +30.9%). This finding indicates that posture has bigger effect on maximum airway pressure than on spirometrical measures.
4.3 Limitations of the study
This study was primarily limited by its relatively small sample size. It’s always a challenge to form the homogeneous groups of highly diverse but limited SCI population. However, as a result of this investigation, a statistical power analysis suggests that further studies could warrant stronger conclusions.
The window for the postural changes investigated in this study might vary in patients with different time since injury. Despite the wide range of time after injury in our population (Table 1), our analysis did not reveal functional correlations associated with this factor (Figure 1). This can partially be attributed to multi-factorial heterogeneity of the population related to the levels, completeness and severity of injury and limited numbers of subjects to form specific groups.
When analyzed in all subjects, our result are representative of chronic SCI population showing that 80.7% of SCI have occurred among males (Center, 2014). However, the hypothesis that postural effects on pulmonary function may be different when assessed separately in males and females, should be investigated in future studies in groups with an appropriate sample size.
Although the dynamics of functional seated-to-supine changes in our population have often corresponded with subjective feelings during task performance, we did not grade these sensations. This analysis could be an important addition to these future investigations.
By using a limited cohort of clinically diverse SCI subjects, it was not feasible to evaluate the impact of combinatory effects of the restrictive and obstructive respiratory patterns associated with smoking, obesity, COPD, skeletal deformations, airway stenosis and other conditions.
Table 1.
Characteristics of participants
| Groups | Subjects (n=27) |
Sex | Age (yrs) |
Height (in.) |
Weight (lbs) |
Smoking | BMI | Level of SCI |
AIS category |
Time after SCI (mos) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Cervical | A33 | M | 51 | 74 | 232 | – | 29.8 | C2 | AIS-A | 36 |
| A38 | F | 37 | 69 | 113 | – | 16.7 | C4 | AIS-A | 256 | ||
| A41 | M | 19 | 72 | 150 | – | 20.3 | C4 | AIS-A | 30 | ||
| B18 | M | 46 | 72 | 155 | – | 21.0 | C3 | AIS-B | 241 | ||
| B17 | M | 42 | 75 | 217 | – | 27.1 | C5 | AIS-B | 16 | ||
| B12 | M | 23 | 75 | 180 | – | 22.5 | C5 | AIS-B | 33 | ||
| B11 | M | 23 | 68 | 185 | – | 28.1 | C5 | AIS-B | 75 | ||
| Thoracic | A37 | M | 25 | 74 | 115 | – | 14.8 | T3 | AIS-A | 23 | |
| A35 | M | 34 | 73 | 200 | – | 26.4 | T3 | AIS-A | 396 | ||
| A43 | M | 30 | 74 | 210 | † | 27.0 | T4 | AIS-A | 11 | ||
| A39 | M | 35 | 70 | 175 | – | 25.1 | T5 | AIS-A | 184 | ||
| A46 | F | 44 | 62 | 192 | – | 35.1 | T6 | AIS-A | 44 | ||
| B07 | M | 22 | 72 | 181 | – | 24.6 | T2 | AIS-B | 35 | ||
| Incomplete | Cervical | C19 | F | 59 | 62 | 130 | – | 23.8 | C4 | AIS-C | 38 |
| C27 | M | 57 | 70 | 190 | – | 27.3 | C4 | AIS-C | 36 | ||
| C26 | M | 33 | 72 | 165 | † | 22.4 | C6 | AIS-C | 7 | ||
| D33 | F | 60 | 62 | 200 | – | 36.6 | C2 | AIS-D | 26 | ||
| D16 | M | 45 | 61 | 200 | – | 37.8 | C3 | AIS-D | 109 | ||
| C23 | M | 64 | 71 | 182 | – | 25.4 | C3 | AIS-D | 32 | ||
| D21 | F | 22 | 62 | 125 | – | 22.9 | C5 | AIS-D | 72 | ||
| D34 | M | 67 | 74 | 212 | – | 27.2 | C6 | AIS-D | 130 | ||
| Thoracic | C16 | M | 35 | 72 | 185 | – | 25.1 | T1 | AIS-C | 75 | |
| C15 | M | 53 | 72 | 175 | – | 23.7 | T5 | AIS-C | 262 | ||
| C25 | M | 36 | 70 | 195 | – | 28.0 | T11 | AIS-C | 32 | ||
| C24 | F | 40 | 68 | 128 | – | 19.5 | T12 | AIS-C | 113 | ||
| C11 | M | 30 | 67 | 170 | – | 26.6 | T12 | AIS-C | 15 | ||
| D22 | M | 44 | 68 | 130 | – | 19.8 | T4 | AIS-D | 145 | ||
| Mean ± SD | N/A | 40 ± 14 | 70 ± 4 | 174 ± 33 | N/A | 25.3 ± 5.4 | N/A | N/A | 92 ± 98 | ||
Respiratory and postural trunk muscles have dual functions. In patients with SCI the seated posture is often unstable, requiring the use of the same muscles to perform both respiratory tasks and to maintain seated position. Identification of their specific role in respiratory performance depending on postural changes is extremely difficult task. The mechanisms of postural effects on respiratory function are more likely to have a combinatory nature. These potential mechanisms may include: specific patterns of respiratory muscle recruitment, effect of gravity, presence of uncontrolled muscular contraction (spasticity), changes in respiratory mechanics, and others (Ovechkin et al., 2010; Terson de Paleville et al., 2011). EMG activity of diaphragm and accessory respiratory muscles shows differential recruitment patterns and amplitude during different respiratory and non-respiratory maneuvers in neurologically intact individuals (Gandevia et al., 1990; Yokoba et al., 2003). It has been established that body position can affect the tone of trunk muscles (Stejskal, 1979) and that spasticity of these muscles can negatively affect pulmonary function in individuals with cervical SCI (Laffont et al., 2003). In non-injured individuals, in the supine position, lung compliance is lower than in the seated position which can be attributed to an increased blood volume of the lungs (Behrakis et al., 1983). In this position, there is reduced elastic recoil of the lungs (Behrakis et al., 1983) resulting in a lower functional residual capacity that is not evident in the sitting position (Rehder, 1998). Baydur and coworkers (2001) reported that there is an increase in inspiratory capacity in individuals with SCI that should be attributed to the effect of gravity (Baydur et al., 2001). In other words, a more functional positioning of the diaphragm pushing against a resistive abdominal content may enhance respiratory function. It has been suggested that, in the seated position, abdominal support using a custom girdle can reduce the sensation of respiratory efforts in patients with SCI by optimizing the operating lung volumes and decreasing abdominal compliance, leading to enhanced diaphragm performance (Hart et al., 2005). Our findings partially support these mechanisms suggesting that the changes in posture may impact PFT measures and that they can be different in each subgroup when level and severity of the injury are taken into consideration.
5. Conclusion
The effects of posture on respiratory function after SCI can depend on specificity of the respiratory tasks and are greater in those with higher neurological injury level and bigger loss of motor function. Both biomechanical and neuromuscular issues are candidate mechanisms for this observation. Therefore, additional studies are warranted to develop methods for evaluation and improving respiratory and trunk motor control and thereby reduce disability due to SCI-induced respiratory insufficiency. Future work should include adequately powered studies to further investigate our findings using invasive and non-invasive methods to accurately measure pulmonary function, airway pressure, air flow and respiratory sensation.
Acknowledgements
This work was supported by UofL/IRIG; KSCHIRT 9-10A; CDRF OA2-0802; Neilsen 1000056824; and NIH 1R01HL103750 grants.
References
- Allen SM, Hunt B, Green M. Fall in vital capacity with posture. Br J Dis Chest. 1985;79:267–271. [PubMed] [Google Scholar]
- Alvisi V, Marangoni E, Zannoli S, Uneddu M, Uggento R, Farabegoli L, Ragazzi R, Milic-Emili J, Belloni GP, Alvisi R, Volta CA. Pulmonary function and expiratory flow limitation in acute cervical spinal cord injury. Arch Phys Med Rehabil. 2012;93:1950–1956. doi: 10.1016/j.apmr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am.J.Respir.Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Badr C, Elkins MR, Ellis ER. The effect of body position on maximal expiratory pressure and flow. The Australian journal of physiotherapy. 2002;48:95–102. doi: 10.1016/s0004-9514(14)60203-8. [DOI] [PubMed] [Google Scholar]
- Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90:405–411. doi: 10.1152/jappl.2001.90.2.405. [DOI] [PubMed] [Google Scholar]
- Behrakis PK, Baydur A, Jaeger MJ, Milic-Emili J. Lung mechanics in sitting and horizontal body positions. Chest. 1983;83:643–646. doi: 10.1378/chest.83.4.643. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Crapo R, Viegi G, American Thoracic S, European Respiratory S. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- Burns SP, Little JW, Hussey JD, Lyman P, Lakshminarayanan S. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil. 2000;81:1334–1339. doi: 10.1053/apmr.2000.9398. [DOI] [PubMed] [Google Scholar]
- Center NSCIS. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2013;36:1–2. doi: 10.1179/1079026813Z.000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center NSCIS. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2014;37:117–118. doi: 10.1179/1079026813Z.000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Lien IN, Wu MC. Respiratory function in patients with spinal cord injuries: effects of posture. Paraplegia. 1990;28:81–86. doi: 10.1038/sc.1990.10. [DOI] [PubMed] [Google Scholar]
- Estenne M, De Troyer A. Mechanism of the postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis. 1987;135:367–371. doi: 10.1164/arrd.1987.135.2.367. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK, Plassman BL. Activation of human respiratory muscles during different voluntary manoeuvres. J Physiol. 1990;428:387–403. doi: 10.1113/jphysiol.1990.sp018218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM. Pathophysiology of spastic paresis, I: Paresis and soft tissue changes. Muscle and Nerve. 2005;31:535–551. doi: 10.1002/mus.20284. [DOI] [PubMed] [Google Scholar]
- Griffiths LA, McConnell AK. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur.J.Appl.Physiol. 2007;99:457–466. doi: 10.1007/s00421-006-0367-6. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hart N, Laffont I, de la Sota AP, Lejaille M, Macadou G, Polkey MI, Denys P, Lofaso F. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Arch.Phys.Med.Rehabil. 2005;86:1447–1451. doi: 10.1016/j.apmr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Jain NB, Brown R, Tun CG, Gagnon D, Garshick E. Determinants of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC in chronic spinal cord injury. Arch.Phys.Med.Rehabil. 2006;87:1327–1333. doi: 10.1016/j.apmr.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34:547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol. 2009;169:157–164. doi: 10.1016/j.resp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Laffont I, Durand MC, Rech C, De La Sotta AP, Hart N, Dizien O, Lofaso F. Breathlessness associated with abdominal spastic contraction in a patient with C4 tetraplegia: a case report. Arch.Phys.Med.Rehabil. 2003;84:906–908. doi: 10.1016/s0003-9993(02)04898-0. [DOI] [PubMed] [Google Scholar]
- Maeda CJ, Baydur A, Waters RL, Adkins RH. The effect of the halovest and body position on pulmonary function in quadriplegia. J Spinal Disord. 1990;3:47–51. [PubMed] [Google Scholar]
- Manning F, Dean E, Ross J, Abboud RT. Effects of side lying on lung function in older individuals. Phys Ther. 1999;79:456–466. [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Meysman M, Vincken W. Effect of body posture on spirometric values and upper airway obstruction indices derived from the flow-volume loop in young nonobese subjects. Chest. 1998;114:1042–1047. doi: 10.1378/chest.114.4.1042. [DOI] [PubMed] [Google Scholar]
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- Navajas D, Farre R, Rotger MM, Milic-Emili J, Sanchis J. Effect of body posture on respiratory impedance. J Appl Physiol. 1988;64:194–199. doi: 10.1152/jappl.1988.64.1.194. [DOI] [PubMed] [Google Scholar]
- Ovechkin A, Vitaz T, Terson de Paleville D, Aslan S, McKay W. Evaluation of respiratory muscle activation in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2010;173:171–178. doi: 10.1016/j.resp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Rehder K. Postural changes in respiratory function. Acta anaesthesiologica Scandinavica. Supplementum. 1998;113:13–16. doi: 10.1111/j.1399-6576.1998.tb04980.x. [DOI] [PubMed] [Google Scholar]
- Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10:65–72. doi: 10.5664/jcsm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir.Physiol Neurobiol. 2009;166:129–141. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Segizbaeva MO, Pogodin MA, Aleksandrova NP. Effects of body positions on respiratory muscle activation during maximal inspiratory maneuvers. Adv Exp Med Biol. 2013;756:355–363. doi: 10.1007/978-94-007-4549-0_43. [DOI] [PubMed] [Google Scholar]
- Smyth RJ, Chapman KR, Rebuck AS. Maximal inspiratory and expiratory pressures in adolescents. Normal values. Chest. 1984;86:568–572. doi: 10.1378/chest.86.4.568. [DOI] [PubMed] [Google Scholar]
- Stejskal L. Postural reflexes in man. Am J Phys Med. 1979;58:1–25. [PubMed] [Google Scholar]
- Stolzmann KL, Gagnon DR, Brown R, Tun CG, Garshick E. Longitudinal change in FEV1 and FVC in chronic spinal cord injury. Am.J.Respir.Crit Care Med. 2008;177:781–786. doi: 10.1164/rccm.200709-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terson de Paleville DG, McKay WB, Folz RJ, Ovechkin AV. Respiratory Motor Control Disrupted by Spinal Cord Injury: Mechanisms, Evaluation, and Restoration. Transl Stroke Res. 2011;2:463–473. doi: 10.1007/s12975-011-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilke GM, Chan TC, Neuman T, Clausen JL. Spirometry in normal subjects in sitting, prone, and supine positions. Respir Care. 2000;45:407–410. [PubMed] [Google Scholar]
- Yokoba M, Abe T, Katagiri M, Tomita T, Easton PA. Respiratory muscle electromyogram and mouth pressure during isometric contraction. Respir.Physiol Neurobiol. 2003;137:51–60. doi: 10.1016/s1569-9048(03)00092-2. [DOI] [PubMed] [Google Scholar]


