Abstract
There have been recent calls to examine the efficacy of drug-combination therapies in the treatment of substance use disorders. The purpose of the present study was to examine the ability of a novel stimulant-opioid combination to reduce cocaine self-administration, and to compare these effects to those of each drug administered alone. To this end, male Long-Evans rats were implanted with intravenous catheters and trained to self-administer cocaine under positive reinforcement contingencies. Once self-administration was acquired, rats were divided into four different groups and treated chronically for 20 days with (1) saline, (2) the psychomotor stimulant and monoamine releaser amphetamine, (3) the mu/kappa opioid agonist butorphanol, or (4) a combination of amphetamine and butorphanol. During chronic treatment, cocaine self-administration was examined on both fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement. On the FR schedule, butorphanol significantly decreased cocaine self-administration, but this effect was not enhanced by amphetamine. On the PR schedule, amphetamine and butorphanol non-significantly decreased cocaine self-administration when administered alone but significantly decreased cocaine self-administration when administered in combination. These data suggest that under some conditions (e.g., when the response requirement of cocaine is high), a dual stimulant-opioid pharmacotherapy may be more effective than a single-drug monotherapy.
Keywords: amphetamine, butorphanol, cocaine, fixed ratio, progressive ratio, self-administration
1.0 Introduction
In 2012, 639,000 Americans tried cocaine for the first time and 1.1 million Americans met diagnostic criteria for a cocaine use disorder (SAMHSA, 2013). Despite the continuing public health problems associated with cocaine abuse, there are currently no FDA-approved medications for the treatment of cocaine use disorders. Previous research has indicated that endogenous mu and kappa opioids play a critical role in cocaine reward and reinforcement (Gianoulakis, 2009; Wee and Koob, 2010), and the mixed mu/kappa agonists butorphanol and nalbuphine reduce cocaine self-administration in laboratory animals (Winger et al., 1992; Mello et al., 1993; Lynch et al., 1998; Kuzmin et al., 2000; Negus and Mello, 2002). Despite these positive findings, controlled studies with opioids in cocaine-abusing populations have failed to demonstrate consistent efficacy on measures of cocaine self-administration (e.g., Walsh et al., 2001). In recent years, stimulant-based therapies employing indirect dopamine agonists have shown positive responses in treatment-seeking populations (Dackis et al., 2005; Mooney et al., 2009). Although the use of stimulant drugs represents a significant advance in medication development, approximately half of the participants in those studies either dropped out or did not show a consistent treatment response. Clearly, the need for effective medications for the treatment of cocaine use disorders still exists.
The majority of previous research examining medications for the treatment of substance use disorders has focused on single-drug monotherapies. Several investigators have argued that substance use disorders may benefit from a combination of medications (e.g., Stoops and Rush, 2014), and the National Institute on Drug Abuse (NIDA) called for additional preclinical and clinical research on novel drug combinations for the treatment of substance use disorders (National Advisory Council on Drug Abuse, 2010). A few studies have examined the effects of drug combinations on measures of drug self-administration, and these studies have reported varying degrees of success (e.g., Mariani et al., 2012; Schmitz et al., 2012; Wee et al., 2012). One drug combination that was effective at reducing drug self-administration in both animals and humans was an opioid/stimulant combination consisting of a mu opioid agonist (buprenorphine or methadone) and the monoamine releaser d-amphetamine (Grabowski et al., 2004; Mello and Negus, 2007; Greenwald et al., 2010). This combination was well tolerated, produced minimal adverse effects, and significantly reduced drug self-administration under a variety of conditions. Such findings are significant because one goal of any combination therapy is to maximize the therapeutic efficacy of treatment while minimizing the potential for side effects that would otherwise limit its use.
The purpose of the present study was to examine the ability of a novel stimulant-opioid combination to reduce cocaine self-administration in an animal model of substance use, and to compare these effects to those of each drug administered alone. To this end, male rats were implanted with intravenous catheters and trained to self-administer cocaine under positive reinforcement contingencies. Once self-administration was acquired, rats were divided into different groups and treated chronically for 20 days with (1) the psychomotor stimulant and monoamine releaser d-amphetamine (amphetamine), (2) the mu/kappa opioid agonist butorphanol, (3) a combination of amphetamine and butorphanol, or (4) vehicle controls. Beginning 10 days after the initiation of chronic treatment, cocaine dose-effect curves were determined under both fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement. Each of these schedules measure different aspects of drug self-administration, with FR schedules more sensitive to satiety factors and PR schedules more sensitive to motivational factors controlling drug intake (Arnold and Roberts, 1997, Solinas et al., 2004, Oleson et al., 2011). Butorphanol and amphetamine were selected because both medications are commercially available for use in clinical populations, and because both drugs decrease cocaine self-administration when administered alone (Peltier et al., 1996; Mello et al., 1993; Lynch et al., 1998; Kuzmin et al., 2000; Negus and Mello, 2002, Negus and Mello, 2003; Chiodo and Roberts, 2009; Rush et al., 2010; Czoty et al., 2011; but see Walsh et al., 2001). Consequently, we predicted that each drug would decrease cocaine self-administration alone, but that greater decreases in cocaine self-administration would be observed when the drugs were administered in combination.
2.0 Methods
2.1 Subjects
Young, male, adult Long-Evans rats (250–280 g upon arrival) were obtained from Charles River Laboratories (Raleigh, NC, USA). All rats were housed individually in polycarbonate cages in a large colony room maintained on a 12-hr light-dark schedule (lights on: 0700). Excluding the brief period of lever-press training (see below), food and water were freely available in the home cage. All animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011), and the Davidson College Animal Care and Use Committee approved all procedures.
2.2 Apparatus
All experimental sessions were conducted in commercially available operant conditioning chambers from Med Associates, Inc. (St Albans, VT, USA). Each chamber was equipped with two response levers located on the forward wall, one white stimulus light located above each lever, one food receptacle located between the two levers, and one houselight located at the rear of the chamber. Food pellets were delivered via a pellet dispenser located behind the forward wall. Infusion pumps mounted outside the chamber delivered drug infusions via Tygon tubing protected by a stainless steel spring and attached to a counter-balanced swivel suspended above the chamber. All experimental events were programmed and data were collected through software and interfacing supplied by Med Associates, Inc.
2.3 Lever-Press Training
Approximately one week after arrival, all rats were food restricted to no less than 90% of their free-feeding body weight and trained to lever press using food reinforcement. In these sessions, each lever press produced a 45 mg grain pellet on a fixed ratio (FR1) schedule of reinforcement. Each training session lasted 2 hr or until 40 reinforcers were delivered. If any rat failed to acquire the lever press response by the third day of training, the response was shaped by the experimenter using manually delivered food pellets. Training continued in this manner until rats acquired the maximum number of 40 reinforcers in any three training sessions. All rats met this criterion within 7 days and returned to unrestricted feed once they met the acquisition criterion.
2.4 Surgery
Following the completion of lever-press training, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8.0 mg/kg, ip) and surgically implanted with intravenous catheters (CamCaths, Cambridge, UK). Each catheter was inserted into the right jugular vein, was routed subcutaneously over the shoulder, and exited the body via a port mounted between the scapulae. Ketoprofen (5.0 mg/kg, sc) was given immediately after surgery as an analgesic, and a solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily in order to maintain patency and prevent infection. After 7 days, ticarcillin was discontinued and only heparinized saline was used to maintain catheter patency. All rats were given three days to recover before beginning self-administration testing.
2.5 Self-Administration Training
All self-administration training and testing sessions began with illumination of the house light, illumination of the white stimulus light above the response lever, and a noncontingent priming infusion of the specific dose of cocaine available during that session. During training, each lever press was reinforced on an FR1 schedule of reinforcement. On this schedule, each response activated an infusion pump that delivered 0.5 mg/kg cocaine (Research Triangle Institute, Research Triangle Park, NC, USA) over a 2.5–4.0 s duration (based on body weight). Simultaneous with each infusion, the stimulus light above the lever turned off to signal a 20 s time out in which cocaine was not available. After 20 s, the light turned on and cocaine was available on the FR1 schedule of reinforcement. No limit was placed on the maximum number of infusions that could be earned, other than those set by the session length and postinfusion timeout. All sessions terminated automatically after 120 min. Training continued in this manner for four consecutive days, at which time daily training sessions were discontinued.
2.6 Drug Treatment
Immediately following the final training session, rats were divided randomly into four groups: water-saline (WAT-SAL), water-butorphanol (WAT-BUT), amphetamine-saline (AMP-SAL), and amphetamine-butorphanol (AMP-BUT). All rats assigned to butorphanol groups (WAT-BUT, AMP-BUT) received one daily injection of butorphanol (5.0 mg/kg, ip), whereas rats assigned to the saline groups (WAT-SAL; AMP-SAL) received one daily injection of saline (1.0 ml/kg, ip). All rats assigned to amphetamine groups (AMP-SAL; AMP-BUT) received an amphetamine solution (0.05 mg/ml) as their sole source of drinking water, whereas rats assigned to the water groups (WAT-SAL; WAT-BUT) received only tap water in their drinking bottle. Amphetamine (dextroamphetamine sulfate) was dissolved in ordinary tap water and obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA). Butorphanol tartrate was dissolved in physiological saline and purchased from Sigma Chemical Company (St. Louis, MO, USA). All rats remained in their respective treatment conditions for 20 consecutive days until the completion of the study.
The doses of butorphanol and amphetamine were selected on the basis of previous studies reporting moderate effects of these doses on diverse measures of elicited and emitted behavior (Monder, 1981; Kanarek and Marks-Kaufman, 1988; Jänicke et al., 1990; Lynch et al., 1998; Smith et al., 1999). The pretreatment interval for butorphanol (15 minutes before each daily session) was selected on the basis of its time course to produce opioid-mediated antinociceptive effects (Smith et al., 1999; Cook et al., 2000) and reduce cocaine self-administration (Lynch et al., 1998). The routes of administration were selected on the basis of pharmacokinetic considerations of how these drugs are used in clinical populations. For instance, amphetamine is typically administered orally to maintain stable levels of drug concentrations over time for narcolepsy, obesity, and attention-deficit hyperactive disorder (ADHD). Butorphanol is typically administered acutely, either via parenteral injection or nasal spray, for the management of acute pain states. Pilot tests conducted in our lab revealed that these dosing parameters did not produce any adverse health effects.
Throughout the 20-day treatment period, body weight and fluid consumption was recorded daily, and catheters were flushed with heparinized saline at 24-hr intervals.
2.7 Self-Administration Testing
After 10 consecutive days of treatment, all rats received one training session in which responding was reinforced with 0.5 mg/kg cocaine on a PR schedule of reinforcement. On this schedule, the ratio value (i.e., response requirement) for each infusion of cocaine increased progressively over the course of the session according to the following ratio values: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, and 573 (for complete algorithm, see Suto et al., 2002). Each session continued until a breakpoint was reached, with breakpoint defined as the number of reinforcers obtained before 1 hr elapsed with no infusions. All other experimental events were identical to those described in the initial training sessions. Over the next four consecutive days, breakpoints were determined for 0.0 (saline), 0.1, 0.3, and 1.0 mg/kg cocaine in separate test sessions. Doses of cocaine and saline were tested in a pseudorandom order with the stipulation that no more than two ascending or descending doses could be tested in a row.
Following testing on the PR schedule, contingencies changed again and responding was reinforced on an FR1 schedule of reinforcement. All experimental events on this schedule were identical to those described in the initial training sessions, except the dose of cocaine differed during each test session. Tests were conducted with 0.0 (saline), 0.03, 0.1, 0.3, and 1.0 mg/kg cocaine. Doses of cocaine and saline were tested in a pseudorandom order with the stipulation that no more than two ascending or descending doses could be tested in a row.
During the period of behavioral testing, butorphanol or saline was always injected 15 min before each daily session. Water or amphetamine was continuously available in the home cage, and fluid consumption was measured prior to each test session. Rats that lost catheter patency were not included in the statistical analysis of the drug self-administration data; however, they were not removed from the study and continued to receive daily drug treatment so that their data could be included in the statistical analysis of body weight and fluid consumption.
2.8 Data Analysis
A total of 58 rats received the full 20 days of drug treatment and were included in the statistical analysis of body weight and fluid consumption: WAT-SAL (n = 14), WAT-BUT (n = 13), AMP-SAL (n = 15), and AMP-BUT (n=16). A total of 51 rats maintained patent catheters for the duration of the study and were included in the statistical analysis of the drug self-administration data: WAT-SAL (n = 10), WAT-BUT (n = 11), AMP-SAL (n = 15), and AMP-BUT (n=15). Body weight and fluid consumption data were examined by 2-way, mixed-factor ANOVA, with group serving as a between-subjects factor and day serving as the repeated measure. Drug self-administration data were examined by 2-way, mixed factor ANOVA, with group serving as a between-subjects factor and dose serving as the repeated measure. Post-hoc tests were conducted on all possible between-group comparisons using the Bonferroni-Holm correction for multiple comparisons.
3.0 Results
3.1 Fluid Consumption and Body Weight
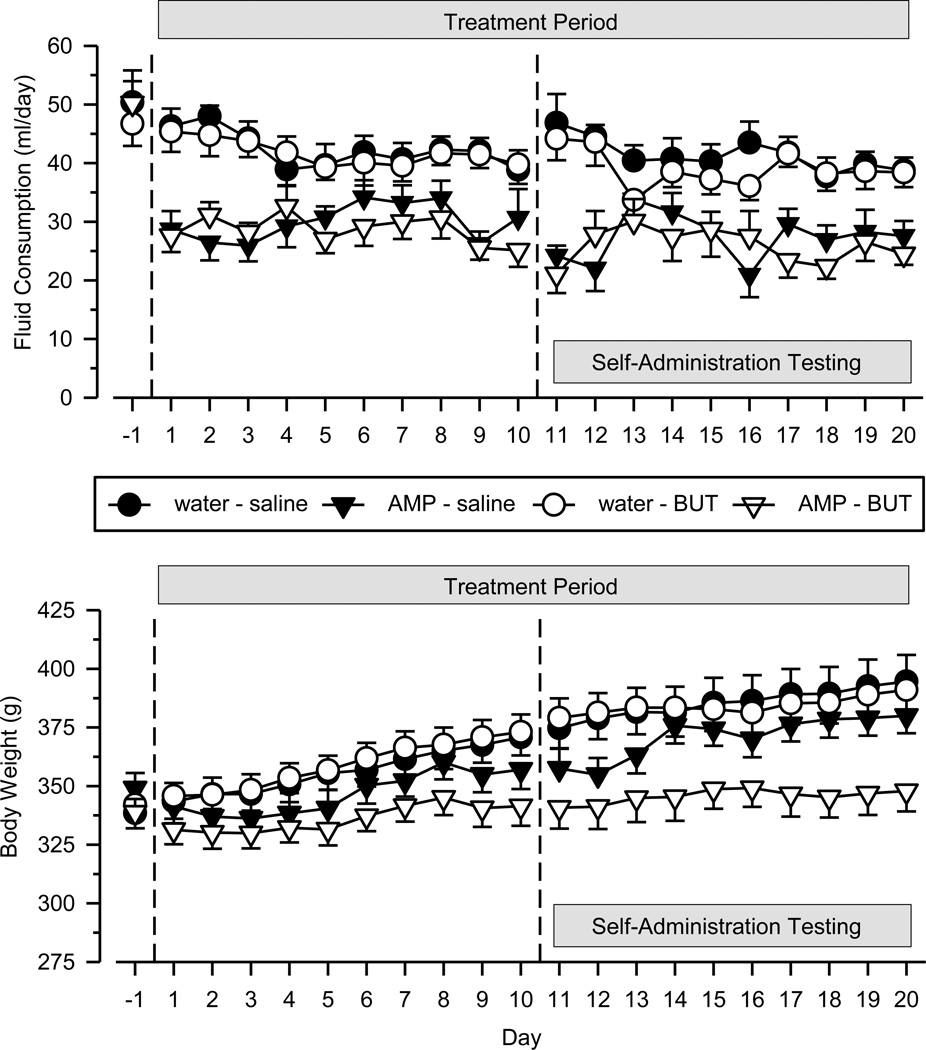
Rats consumed approximately 50 ml/day of fluid (tap water) prior to drug treatment, with no differences across groups (Figure 1). During the 20 days of drug treatment, fluid consumption fluctuated daily [main effect of day: F(19, 1026) = 2.196, p = .002] in a nonsystematic fashion across groups [group×day interaction: F(57, 1026) = 1.718, p = .001]. Despite these daily fluctuations, some consistent group differences were observed throughout the treatment period [main effect of group: F(3, 54) = 15.008, p < .001]. Specifically, rats drinking the amphetamine solution (AMP-SAL; AMP-BUT) consumed significantly less fluid (p < .01) than rats drinking ordinary tap water (WAT-BUT; WAT-SAL).
Figure 1.
Fluid consumption (upper panel) and body weight (bottom panel) over the 20-day treatment period. Fluid consumption is depicted as ml/day; body weight is depicted as grams (g). Rats were treated for 10 consecutive days (Days 1–10) before beginning self-administration testing (Days 11–20). Data points above “−1” reflect fluid consumption and body weight on the day immediately preceding treatment. Vertical lines surrounding data points depict the SEM; where not indicated, the SEM fell within the data point.
Rats weighed approximately 340 g prior to drug treatment, with no differences across groups (Figure 1). Body weights increased linearly in all groups across the 20 days of drug treatment [main effect of day: F(19, 1026) = 64.801, p < .001], but the rate of increase differed across groups [group×day interaction: F(57, 1026) = 3.201, p < .001]. Some consistent group differences were observed throughout the treatment period [main effect of group: F(3, 54) = 6.391, p = .001], with rats treated with both amphetamine and butorphanol (AMP-BUT) weighing significantly less (p < .01) than rats in the other three groups.
3.2 Drug Self-Administration
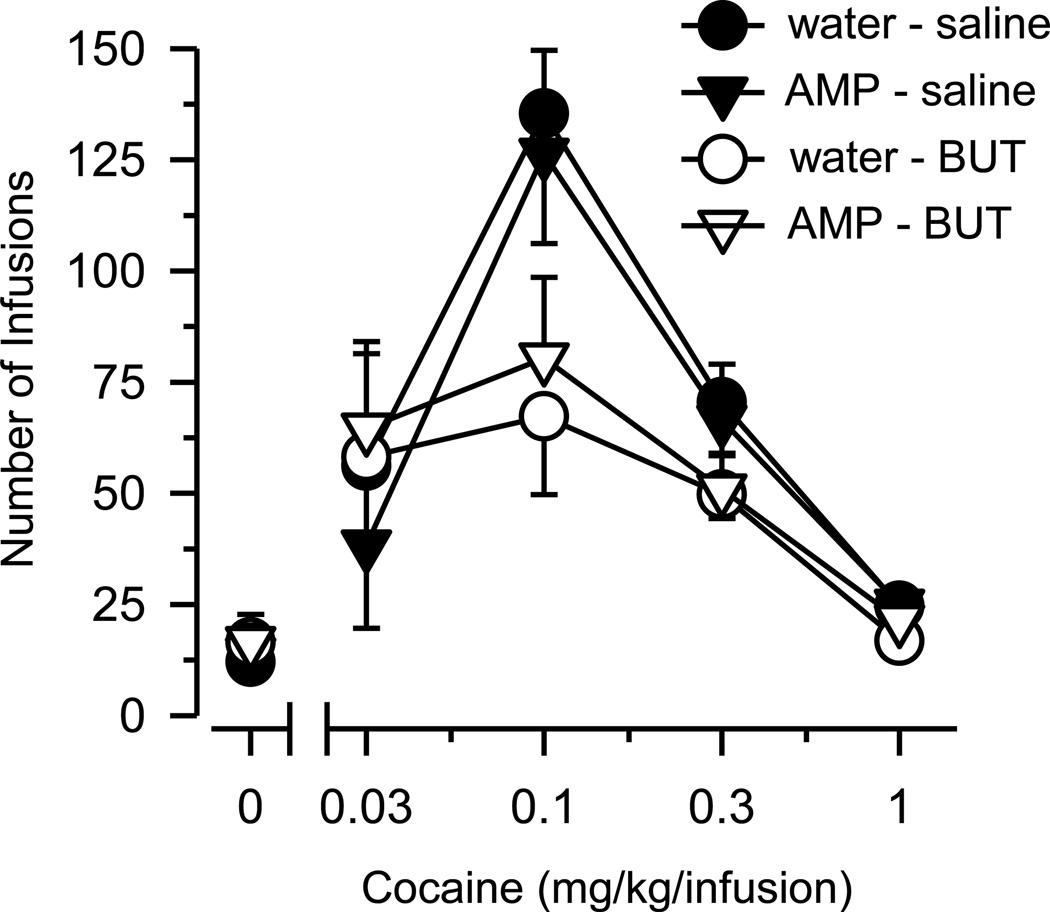
On the FR schedule of reinforcement (Figure 2), cocaine self-administration was characterized by an inverted, U-shaped, dose-effect curve in all groups [main effect of dose: F(3, 141) = 26.933, p < .001]. Differences between groups were apparent at 0.1 mg/kg cocaine, the dose that maintained the highest level of responding in all groups [group×dose interaction: F(9, 141) = 2.214, p = .024]. At this dose, both groups treated with butorphanol (WAT-BUT; AMP-BUT) self-administered significantly less cocaine (p < .01) than both groups treated with saline (WAT-SAL; AMP-SAL). No group differences were apparent at any other dose.
Figure 2.
Cocaine self-administration on a fixed ratio (FR1) schedule of reinforcement. Vertical axis depicts number of infusions obtained during 2-hr test session. Horizontal axis depicts dose of cocaine in mg/kg/infusion. Data points above “0” depict the effects of saline. Vertical lines surrounding data points depict the SEM; where not indicated, the SEM fell within the data point.
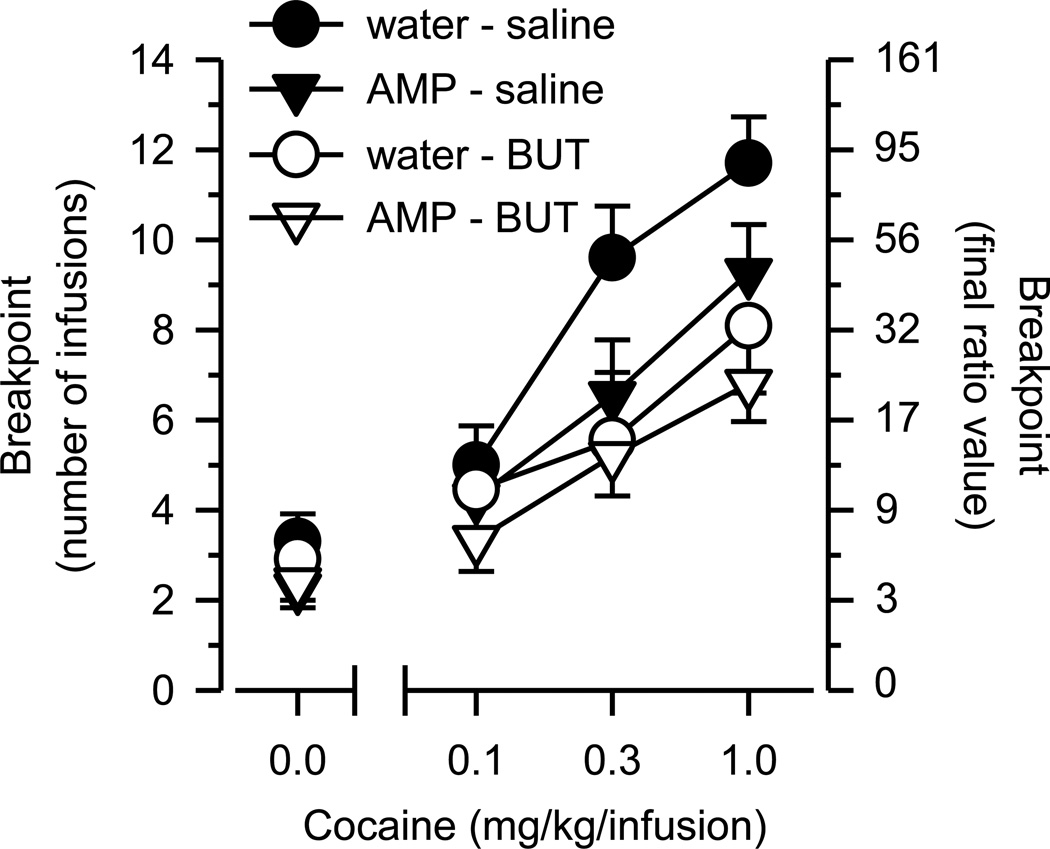
On the PR schedule of reinforcement (Figure 3), breakpoints increased linearly as a function of dose [main effect of dose: F(2, 94) = 34.926, p < .001] and differed significantly across groups [main effect of group: F(3, 47) = 3.145, p = .034]. Rats receiving both amphetamine and butorphanol (AMP-BUT) self-administered significantly less cocaine (p < .01) than rats receiving only vehicle controls (WAT-SAL). Cocaine self-administration was numerically less in both groups receiving only one of the two treatment drugs (AMP-SAL; WAT-BUT), but these groups did not differ significantly from the vehicle-control group (WAT-SAL).
Figure 3.
Cocaine self-administration on a progressive ratio (PR) schedule of reinforcement. Left axis depicts breakpoint as number of infusions obtained; right axis depicts breakpoint as final ratio value completed. Horizontal axis depicts dose of cocaine in mg/kg/infusion. Data points above “0” depict the effects of saline. Vertical lines surrounding data points depict the SEM; where not indicated, the SEM fell within the data point.
4.0 Discussion
In this study, chronic treatment with butorphanol significantly decreased responding maintained by cocaine on an FR schedule of reinforcement. Chronic treatment with either butorphanol or amphetamine produced nonsignificant decreases in breakpoints maintained by cocaine on a PR schedule of reinforcement; however, combined treatment with both drugs produced statistically significant decreases in breakpoints under the same set of conditions. These data suggest that a combined opioid-stimulant pharmacotherapy may be more effective than a single-drug monotherapy at reducing cocaine self-administration when response requirements are high (or progressively increase, such as on a PR schedule), but would not offer an advantage when response requirements are low (such as on an FR1 schedule).
As expected, adding amphetamine to the subjects’ only source of drinking water significantly decreased fluid consumption by approximately 40% in the amphetamine groups (AMP-SAL, AMP-BUT). Despite this decrease in fluid consumption, rats in both groups still consumed pharmacologically active doses of amphetamine. Rats treated with amphetamine and saline (AMP-SAL) consumed an average of 4.0 mg/kg/day, and rats treated with amphetamine and butorphanol (AMP-BUT) consumed an average of 4.2 mg/kg/day. Both of these doses are within the dose range reported in previous studies in which amphetamine was administered orally in drinking water and pharmacologically active effects were observed (Monder, 1981; Kanarek and Marks-Kaufman, 1988; Jänicke et al., 1990). No changes in fluid consumption were observed in rats treated with butorphanol alone (WAT-BUT).
Although both groups of rats treated with amphetamine exhibited similar amounts of fluid consumption, only rats treated with both amphetamine and butorphanol (AMP-BUT) exhibited significant differences in body weight relative to vehicle control rats (WAT-SAL). Rats treated with both amphetamine and butorphanol weighed approximately 45 g less than control rats at the end of the treatment period, gaining less than 10 g over the 20-day treatment interval. Given that similar effects were not observed with rats treated with amphetamine alone (AMP-BUT) or with rats treated with butorphanol alone (WAT-BUT), these data suggest that the weight suppression was a consequence of the interaction between these drugs. It is important to note that no rat lost more than 4% of its baseline body weight during the treatment period, and no rat was removed from the study because of illness or other adverse event.
Only a small number of studies have combined opioids and psychomotor stimulants in tests of drug self-administration, but those studies that have examined opioid-stimulant combinations have reported positive effects. Mello and Negus (2001) examined the ability of indatraline, a dopamine reuptake inhibitor, and buprenorphine, a mixed mu agonist/kappa antagonist, to reduce speedball (cocaine + heroin) self-administration and food-maintained responding in rhesus monkeys. Doses of indatraline and buprenorphine that did not alter responding when administered alone reduced speedball self-administration when administered in combination. Importantly, reductions in speedball self-administration were sustained over time, whereas reductions in food-maintained responding returned to baseline levels within a few days. In a follow-up study, these same investigators reported that combinations of d-amphetamine and buprenorphine reduced speedball self-administration at doses that did not alter responding maintained by food (Mello and Negus, 2007). Similar to the previous study, the results suggested superiority of the drug combination when both therapeutic efficacy (reductions in drug self-administration) and adverse effects (disruptions in food-maintained responding) were taken into consideration.
Human laboratory and clinical studies evaluating potential opioid-stimulant combinations are still in their infancy. Currently, only a couple of studies have evaluated an opioid-stimulant combination on measures of cocaine-seeking behavior and compared the effects of the drug combination to one drug administered alone. Grabowski et al. (2004) examined the effects of methadone alone versus methadone + d-amphetamine on cocaine and opioid use in an outpatient population of cocaine and heroin abusers. The study compared two escalating doses of amphetamine (15/30 or 30/60 mg) and placebo, each in combination with a maintenance dose of methadone (1.1 mg/kg). In the 24-week study, individuals receiving the 30/60 mg dose of amphetamine had significantly fewer cocaine-positive urine screens than the other two groups, suggesting that amphetamine dose-dependently increases the effects of methadone on measures of cocaine self-administration. Retention rates were similar in all three groups and no significant adverse events were reported. In a more recent study, Greenwald et al. (2010) examined the ability of sustained-release d-amphetamine to reduce cocaine, hydromorphone, and speedball (cocaine + hydromorphone) self-administration in buprenorphine-maintained polydrug abusers. In an inpatient facility, volunteers were maintained on buprenorphine and treated with placebo or amphetamine for 3 days using a within-subjects design. After stabilization on either placebo or amphetamine, participants sampled four drug combinations in a counter-balanced order (placebo, cocaine, hydromorphone, or speedball) while subjective and physiological effects were measured. Later, participants responded on a PR schedule of reinforcement to earn either drug or money. Relative to placebo treatment, amphetamine significantly reduced breakpoints maintained by cocaine (but not hydromorphone or speedball) and choice for cocaine. Amphetamine also significantly reduced the positive subjective effects of cocaine but did not increase cocaine-induced cardiovascular effects. Collectively, these findings suggest that a combined opioid-stimulant pharmacotherapy may be effective at treating cocaine use disorders in clinical populations. Furthermore, given the mu agonist profile of the opioids tested (methadone and hydromorphone), these findings also suggest an important role of the mu opioid receptor in mediating these effects.
Several limitations of the present study must be acknowledged. First, only a single dose of butorphanol and a single concentration of amphetamine were tested. The desire to test each drug independently and include a vehicle control condition limited the number of groups we could test. Similarly, the desire to give the drugs for multiple weeks and test responding on two schedules of reinforcement limited the duration of testing. Although the doses were selected to maximize the possibility of obtaining a positive signal, the single dose manipulations made it difficult to compare the efficacy of each drug alone. Furthermore, the use of only a single dose makes it difficult to determine whether the effects of the drug combination were unique to the combination or simply an effect that would be expected if higher doses of either drug were administered alone. Another limitation of the study was the use of asymmetrical routes of administration for the two drugs. We chose the routes out of pharmacokinetic considerations of how these drugs are available for use in humans, with amphetamine available as an extendedrelease oral formulation and butorphanol available as a parenteral injection and nasal spray. This also complicates direct comparisons between the drugs, because butorphanol was likely tested at the time of peak effectiveness (but see data from Lynch et al., 1998 indicating that butorphanol is effective for up to 24 hours) whereas this was unlikely the case with amphetamine. An additional limitation concerns the final number of subjects. Although 51 rats were used for the statistical analysis, the desire to test all possible between-group comparisons meant that only differences with very large effect sizes could be detected after correcting for multiple comparisons. Thus, it is possible that we missed smaller but meaningful differences between groups because of type II error. Finally, no control tests were conducted on other measures of positively reinforced behavior. This is important because vertical shifts in the dose-effect curve do not necessarily indicate a change in the hedonic effects of a drug (see Zernig et al., 2004), and may be due to nonselective changes in consummatory behavior. This latter possibility cannot be dismissed because significant decreases in breakpoints were only observed under conditions that also decreased fluid consumption and body weight.
In conclusion, the present findings offer modest support for the use of dual opioid-stimulant combinations in the treatment of cocaine use disorders. Although no advantage of the dual treatment was seen on responding maintained by cocaine on an FR schedule, only rats treated with both butorphanol and amphetamine exhibited a significant decrease in cocaine-maintained breakpoints on the PR schedule. These data suggest that pharmacotherapies for cocaine abuse that target both the endogenous opioid and monoamine systems may offer advantages over single-drug monotherapies under conditions in which the response requirement or behavioral cost of cocaine is high.
Research Highlights.
The effects of an opioid-stimulant combination were examined on cocaine intake
Rats were treated with butorphanol, amphetamine, the drug combination, or vehicle
Butorphanol and the drug combination reduced cocaine intake on an FR schedule
The drug combination reduced cocaine intake on a PR schedule
These data support the use of an opioid-stimulant therapy under some conditions
Acknowledgements
The authors thank Amy Sullivan for expert animal care and technical assistance, and the National Institute on Drug Abuse for supplying the study drugs.
This study was supported by the National Institutes of Health (NIDA Grants DA027485, DA014255, and DA031725 to MAS). Additional support was provided by the Howard Hughes Medical Institute (Grant 52006292), the Duke Endowment, and Davidson College. The funding organizations had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous D-amphetamine treatment in rats. Psychopharmacology. 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology. 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not 'speedball'-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Jänicke UA, Jänicke B, Schulze G, Coper H. Learning abilities of rats in multiple T-mazes of two degrees of complexity under the influence of d-amphetamine. Pharmacol Biochem Behav. 1990;36:923–932. doi: 10.1016/0091-3057(90)90101-m. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R. Dietary modulation of oral amphetamine intake in rats. Physiol Behav. 1988;44:501–505. doi: 10.1016/0031-9384(88)90312-5. [DOI] [PubMed] [Google Scholar]
- Kuzmin AV, Gerrits MA, Zvartau EE, van Ree JM. Influence of buprenorphine, butorphanol and nalbuphine on the initiation of intravenous cocaine self-administration in drug naive mice. Eur Neuropsychopharmacol. 2000;10:447–454. doi: 10.1016/s0924-977x(00)00117-6. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Heaser WA, Carroll ME. Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp Clin Psychopharmacol. 1998;6:255–263. doi: 10.1037//1064-1297.6.3.255. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Drieze J, Mendelson JH. The effects of nalbuphine and butorphanol treatment on cocaine and food self-administration by rhesus monkeys. Neuropsychopharmacology. 1993;8:45–55. doi: 10.1038/npp.1993.6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of indatraline and buprenorphine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology. 2001;25:104–117. doi: 10.1016/S0893-133X(00)00247-5. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of d-amphetamine and buprenorphine combinations on speedball (cocaine+heroin) self-administration by rhesus monkeys. Neuropsychopharmacology. 2007;32:1985–1994. doi: 10.1038/sj.npp.1301319. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of d-amphetamine and buprenorphine combinations on speedball (cocaine+heroin) self-administration by rhesus monkeys. Neuropsychopharmacology. 2007;32:1985–1994. doi: 10.1038/sj.npp.1301319. [DOI] [PubMed] [Google Scholar]
- Monder H. Effects of prenatal amphetamine exposure on the development of behavior in rats. Psychopharmacology. 1981;75:75–78. doi: 10.1007/BF00433506. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Council on Drug Abuse. Minutes of the 105th Meeting of the National Advisory Council on Drug Abuse; May 5, 2010; Bethesda, MD. 2010. [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of mu-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. 2002. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of Modafinil and d-amphetamine for the Treatment of Cocaine Dependence: A Preliminary Investigation. Front Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Barrett AC, Picker MJ. Antinociceptive effects of opioids following acute and chronic administration of butorphanol: influence of stimulus intensity and relative efficacy at the mu receptor. Psychopharmacology. 1999;143:261–269. doi: 10.1007/s002130050945. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Goldberg SR. Exposure to delta-9-tetrahydrocannabinol (THC) increases subsequent heroin taking but not heroin's reinforcing efficacy: a self-administration study in rats. Neuropsychopharmacology. 2004;29:1301–1311. doi: 10.1038/sj.npp.1300431. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. 2014;7:363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [SAMHSA] Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings (SAMHSA, NSDUH Series H-46, HHS Publication No. SMA 13-4795) Rockville, MD: 2013. [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4:146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Skjoldager P, Woods JH. Effects of buprenorphine and other opioid agonists and antagonists on alfentanil- and cocaine-reinforced responding in rhesus monkeys. J Pharmacol Exp Ther. 1992;261:311–317. [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate "sensitization" to "drug wanting"? Psychopharmacology. 2004;171:349–351. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]