Abstract
Despite tremendous advances in the targeted therapy for various types of hematological malignancies with successful improvements in the survival rates, emerging resistance issues are startlingly high and novel therapeutic strategies are urgently needed. In addition, chemoprevention is currently becoming an elusive goal. Plant-derived natural products have garnered considerable attention in recent years due to the potential dual functions as chemotherapeutics and dietary chemoprevention. One of the particularly ubiquitous families is the polyphenolic flavonoids. Among them, baicalin and its aglycone baicalein have been widely investigated in hematological malignancies because both of them exhibit remarkable pharmacological properties. This review focuses on the recent achievements in drug discovery research associated with baicalin and baicalein for hematological malignancy therapies. The promising anticancer activities of these two flavonoids targeting diverse signaling pathways and their potential biological mechanisms in different types of hematological malignancies, as well as the combination strategy with baicalin or baicalein as chemotherapeutic adjuvants for recent therapies in these intractable diseases are discussed. Meanwhile, the biotransformation of baicalin and baicalein and the relevant approaches to improve their bioavailability are also summarized.
Keywords: flavonoids, baicalin, baicalein, hematological malignancies, cancer targets
1. Introduction
Hematological malignancies as a group of highly heterogeneous diseases affect the production and function of blood or blood-forming tissues including lymphatic system and bone marrow. These life threatening illnesses comprise various distinct disease types including leukemia, lymphoma, multiple myeloma, myeloproliferative neoplasms (MPN), myeloproliferative syndrome (MPS), and myelodysplastic syndromes (MDS) [1]. Each type has diverse incidence, prognosis, etiology, different features, particular treatment pathways and clinical outcomes [2].
The etiology of most of the hematological malignancies is not yet known. Radiation, exposure to chemicals and dusts, industrial exposures, viral infections, genetic predisposition and Down's syndrome are all associated with the increasing risk of one or several of these diseases. As the functions of blood or blood-forming tissues are closely connected through the immune system, when one of them is affected by a disease, the others will often be simultaneously affected. For instance, lymphoma is a disease of the lymph nodes; however, it often spreads to bone marrow, occasionally producing a paraprotein and affecting the blood.
Great advances have been made in early detection or in development of effective targeted and combination therapies for hematological malignancies in recent years. In spite of these advances having had favorable impact on survival, several types of hematological malignancies remain incurable [3]. In addition, although some patients having significant improvement in survival are now living longer with their disease, they are often suffering the associated symptoms. Furthermore, many patients ultimately develop resistance to currently available treatments. Meanwhile, the existing chemotherapeutic agents often have significant toxicities [4]. Because patients with hematological malignancies already suffer from compromised immune systems, the side effects of the chemotherapeutic agents are always intolerable. Not only that, the premium pricing of these promising drugs is becoming one of the major burdens for cancer patients [5]. Therefore, novel effective treatment options with less toxicity, higher tolerability, and more affordable price are urgently needed.
2.1. Dietary Polyphenolic Flavonoids
Natural products especially plant-derived traditional medicines have been utilized for the prevention and treatment of various human diseases since time immemorial [6,7]. About 65% of people around the world mainly rely on herbal medicines for their primary health care. There is an indisputable fact that more than 50% of the drugs currently in clinical application are of natural product origin. Utilization of plant-derived traditional medicines has a long history for cancer therapies. Jonathan Hartwell published his monumental work which contains over 3,000 plant species for the treatment of various cancers [8]. Despite the fact that the pharmaceutical industry appears to be shifting from the research direction on the natural product-inspired drug discovery, there is an increasing interest on the development of low toxic natural antioxidants as promising leads, especially those compounds that are available in extremely large quantities. Polyphenolic flavonoids, one unique family in dietary plants, are widely distributed in vegetables, fruits, and many traditional Chinese herbal medicines [9]. Flavonoids exhibit diverse biological properties including antifungal, antioxidant, antiallergic, antiinflammatory, anticancer, antiobesity, antidiabetic, and immune-modulating effects [10-12]. Recently, the remarkable multifunctional antiproliferative effects of flavonoids against various cancer cells including an array of different malignancies are intensively investigated. Accumulating evidence has demonstrated that flavonoids exhibit potential anticancer properties in vivo, resulting in several bioflavonoids in preclinical and clinical trials [13].
2.2. Scutellaria baicalensis, Baicalin and Baicalein
Scutellaria baicalensis (S. baicalensis, common name: Huang Qin in China, Figure 1A) as one of the fifty fundamental herbs in traditional Chinese herbal medicine is widely used for the prevention and treatment of various ailments including cardiovascular diseases, hypertension, bacterial infection, inflammation, and cancer [14]. More than 50 flavonoids have been purified and identified from S. baicalensis [15]. The major components (Figures 1B and 1C) are baicalin (baicalein-7-O-glucoside), and its aglycone baicalein (5,6,7-trihydroxyfavone) [16]. Accumulating evidence has demonstrated that both of them exhibit extensive pharmacological effects. Due to their relatively low toxicity and the abundance in the root of S. baicalensis, baicalin and baicalein became the most extensively researched components in recent years [16]. Over the past decade, a considerable amount of studies has demonstrated that baicalin and baicalein display potent anticancer effects in various types of hematological malignancies [17]. Significant progress has been made in identifying the precise targets and elucidating relevant biological mechanisms of baicalin and baicalein involved in the inhibition of hematological malignancies.
Figure 1.
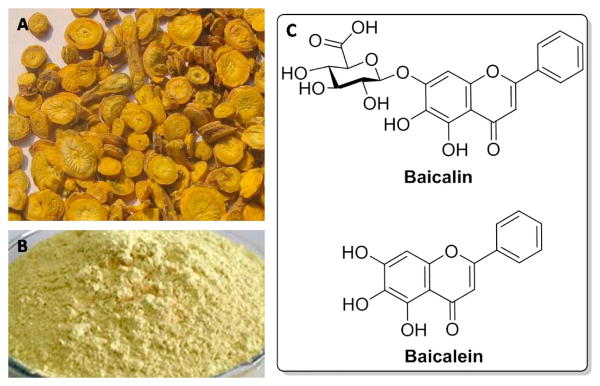
(A) The roots of Scutellaria baicalensis; (B) The powder of baicalin; (C) Chemical structures of baicalin and baicalein.
In this concise review, we seek to summarize the recent achievements in drug discovery research on baicalin and baicalein in hematological malignancies and discuss the associated diverse signaling pathways and the potential biological mechanisms. In addition, we also discuss the combination strategy with baicalin or baicalein as chemotherapeutic adjuvants for recent therapies in these intractable diseases, and summarize their biotransformation and the relevant approaches to improve their bioavailability.
2.3. Biotransformation pathways and bioavailability
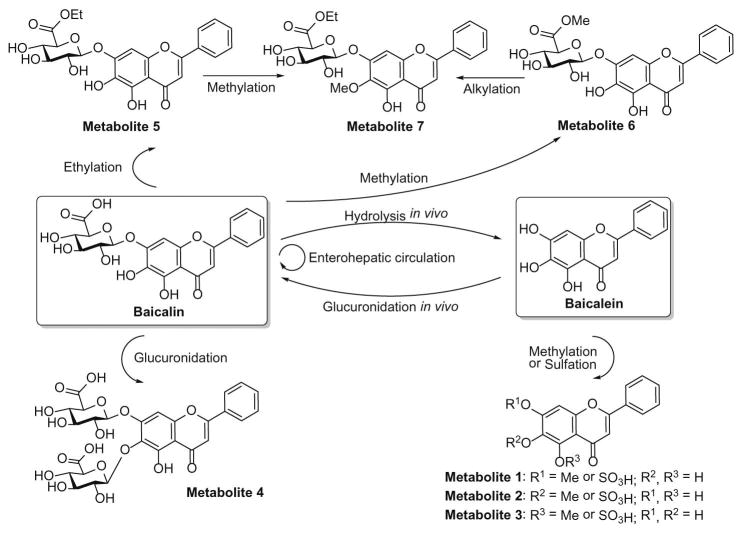
Baicalin belongs to Class IV of Biopharmaceutical Classification System (BCS) due to the extremely low hydrophilicity (solubility 0.052 mg/mL in water) and lipophilicity (Papp = 0.037×10−6 cm/s) [18]. Baicalein is highly permeable (Papp = 1.7×10−5 cm/s) but poorly water soluble, which is classified as a Class II compound according to BCS [19,20]. The poor solubility results in both baicalin and baicalein very low bioavailability [21]. Extensive studies have been conducted to explore the in vivo processes of these two drugs. The serum profiles and pharmacokinetics of orally administered baicalein and baicalin were compared. Baicalin was absorbed more slowly and had lower Cmax than baicalein [22]. There exists wide complicated biotransformation of baicalin and baicalein in vivo (Figure 2). As a natural glycoside, baicalin possesses more favorable aqueous solubility than baicalein. However, baicalin is difficult to be absorbed as its parent form due to the poor lipophilicity. When baicalin was orally administered, only a small portion was absorbed as its original form by the body, and most was hydrolyzed to baicalein by intestinal bacterial [23]. The recovered baicalein was then extensively subjected to Phase 2 metabolism, and glucuronides and/or sulfates of baicalein were exclusively presented in the plasma. Notably, the circulating baicalin is not the administered parent drug but one of the conjugated metabolites of baicalein after oral administration of baicalin. The circulating baicalin reenters the gastrointestinal tract via the biliary excretion mechanism [24]and undergoes enterohepatic circulation [21]. After oral administration of baicalein, it is subjected to the extensive first-pass metabolism in liver and small intestine [25,26], and therefore, glucuronides/sulfates of baicalein including baicalin are predominant in the plasma [22]. The biotransformation of baicalin and baicalein and the enterohepatic circulation of baicalin can keep a balance in the systemic levels. With the oral administration of baicalin and baicalein, dominantly circulating in the plasma are the glucuronides/sulfates of baicalein, and therefore, the conjugated metabolites are actually responsible for the in vivo effects. Because baicalin itself is one of the conjugated metabolites after oral administration of baicalin or baicalein, the activity of baicalin reported in in vitro studies can only partially explain the in vivo effects of baicalin and baicalein [22].
Figure 2. The potential biotransformation pathways of baicalin and baicalein in vivo.
Baicalin is hydrolyzed to baicalein by intestinal bacterial and is absorbed as baicalein by the body. The recovered baicalein is extensively subjected to Phase 2 metabolism. Baicalin is one of the conjugated metabolites after oral administration of baicalein. The circulating baicalin reenters the gastrointestinal tract via the biliary excretion mechanism and undergoes enterohepatic circulation.
A sound knowledge of the pharmacokinetic characteristics of baicalin and baicalein enables scientists to further optimize use of these agents. Various formulations have been developed to improve the oral bioavailability of baicalin and baicalein. Baicalein nanocrystal [27], baicalein-hydroxypropyl-β-cyclodextrin inclusion complex [28], baicalein self-microemulsifying drug delivery system [29], and baicalein solid dispersion [30]have been developed to improve dissolution and oral bioavailability of baicalein. Some nano-based formulations such as solid nanocrystals [31], nanoemulsions [32], and solid lipid nanoparticles [33]have been designed for increasing baicalin's solubility and absorption rate and improving its oral bioavailability. Besides, change administration route of baicalein may be employed to avoid the first-pass effect of the gastrointestinal tract or liver and enhance its bioavailability. For example, pulmonary administration of baicalein nanocrystal can obtain similar pharmacokinetic parameters to intravenous injection of baicalein solution [27].
2.4. Leukemia
Leukemia is a cancer of the bone marrow or blood where the blood cells – mostly leukocytes abnormally proliferate. It is estimated that over 52,380 new cases will be diagnosed and over 24,090 patients will die from leukemia in 2014 in America. The research over the past few decades yielded remarkable improvements in chemotherapy for patients with leukemia. However, drug resistance and serious toxicity remain to be major challenges. Natural antioxidants have gained an increasing interest due to their potential to be developed as low toxic antileukemic compounds [34]. Accumulating studies support that flavonoids isolated from Scutellaria baicalensis exhibit promising antileukemic activity [35-39]. Numerous reports have shown that baicalin and baicalein as the major components display remarkable antiradical, antioxidative and antileukemic effects [40,41]. Romanouskaya et al. chose 11 flavonoids and investigated their antiproliferative effects on chronic myeloid leukemia K562 cells and healthy peripheral blood mononuclear cells [42]. Among 11 flavonoids, baicalein displayed the specific cytotoxic effect on leukemia cells. Dong et al. revealed that baicalein could arrest K562 cells in the S phase and selectively induce apoptosis via upregulating caspase-3 and Fas [43]. In addition, Wang et al. found that baicalin and baicalein might target endogenous CBF1-dependent Notch signal pathway to affect tumorigenesis of K562 cells [44].
Baicalein was also found to inhibit the proliferation of human T-lymphoid leukemia cells (CEM cells) with an IC50 value of 4.7 μM [45]. Baicalein significantly inhibited the protein tyrosine kinase in a dose-dependent manner and reduced the expression of PDGF-A mRNA. All these data indicate that baicalein may affect cell proliferation by direct inhibition of growth-related signal, reduction of mRNA expression of PDGF-A, and inhibition of protein tyrosine kinase. Lin et al. also reported that baicalein is capable of suppressing the holoenzyme activity of CK2 with an IC50 of 2.54 μM, indicating that baicalein may target protein kinase CK2 [46].
Despite several studies revealing the possible biological targets of baicalin and baicalein, the signaling pathways are substantially distinct in different cancer cell lines, and partial signaling pathways may be regulated successively or simultaneously. In CCRF-CEM leukemic cells, baicalin exhibits a potent anticancer effect with an IC50 value of 10.6 μg/mL [47]. The apoptosis of CCRF-CEM induced by baicalin is Bcl-2-dependent, but not p53-dependent pathway. Chen et al. further investigated the biological mechanisms of baicalein in inducing CCRF-CEM apoptosis [48]. They found that baicalein could induce cleavage of caspases-9 and -3 and PARP, and decrease the expression of IAP family proteins, XIAP, and survivin. They also found that baicalein might trigger a convergence of the intrinsic and extrinsic apoptotic pathways via the death receptor-caspase 8-tBid signaling cascade.
The anticancer activity and the mechanism of action of baicalein in human promyelocytic leukemia HL-60 cells have been studied by several research groups. Li et al. demonstrated that baicalein activates caspase-3 and induces the cleavage of PARP and DNA fragmentation [49]. They further demonstrated that baicalein can affect N-acetylation of 2-aminofluorene in HL-60 cells, indicating that baicalein can affect the N-acetyltransferase (NAT) activity in vitro [50]. Wang et al. confirmed that baicalein is capable of inducing apoptosis in HL-60 cells [51]. By finding that baicalein can induce the cleavage of Bid protein, release of cytochrome c from mitochondria into cytosol, activate caspase-3, -8 and -9, and elevate the intracellular hydrogen peroxide level, they concluded that baicalein might trigger the apoptotic death program through ROS-mediated mitochondrial dysfunction pathway. Chow et al. also studied the anticancer effects of baicalein in HL-60 [52]. However, they found that apoptosis induced by baicalein is independent of the production of ROS. Intriguingly, Zheng et al. demonstrated that baicalin displays promising anticancer effect in drug-resistant HL-60/ADR cells through PI3K/Akt signaling pathway [53]. Taken together, all these results encourage further investigation of baicalin and baicalein as potential drug candidates for patients with leukemia.
2.5. Lymphoma
Lymphomas as one kind of the intractable cancers are developed from the uncontrolled proliferation of cells in the lymphatic system. The lymphatic cells are able to overcrowd, invade, and destroy lymphoid tissues and metastasize to other organs. Several studies have demonstrated that S. baicalensis, baicalin and baicalein display antiproliferative and apoptotic effects against lymphoma [17,54,55]. Huang et al. reported that baicalin is capable of suppressing the growth of CA46 Burkitt lymphoma cells via induction of apoptosis with an IC50 value of 10 μM [56]. In addition, baicalin almost completely inhibits colony formation at 10 μM. CA46 cells undergo apoptosis in response to baicalin induced apoptosis via up-regulating the cleaved forms of caspase-9, caspase-3, and PARP. Furthermore, baicalin is capable of downregulating antiapoptotic and upregulating apoptotic components of the PI3K/Akt signaling pathway [56].
2.6. Myeloma
Although the current cancer treatments have progressed rapidly, myeloma remains incurable because most patients may eventually relapse or become refractory to available therapies [57]. In order to search more effective adjuvant therapies for the treatment of myeloma, Ma et al. investigated the effects of several traditional Chinese herbal medicines on the proliferation and apoptosis of myeloma cells [58]. They found that Huang-Lian-Jie-Du-Tang (HLJDT) inhibits the proliferation of either myeloma cell lines or primary myeloma cells, especially MPC-1-immature myeloma cells, and induces myeloma cell apoptosis through reduction of mitochondrial membrane potential and activation of caspase-9 and caspase-3. The Scutellaria radix was confirmed to be responsible for the suppressive effect of HLJDT on myeloma cell proliferation. Baicalein as one of the major components in Scutellaria radix took the lead in the antiproliferation effects in comparison with baicalin or wogonin. Biological mechanistic studies revealed that baicalein inhibits the phosphorylation of IkB-α, and decreases the expression of the IL-6 and XIAP genes.
Lin et al. examined the active effects of Scutellaria extract and its main flavonoids constituents on the proportion of side population cells in human multiple myeloma (MM) cell line RPMI 8226 cells [59]. Gu et al. further revealed that baicalein inhibits RPMI 8226 cells with an IC50 value of 168.5 μM [60]. They found that baicalein can significantly decrease the expression level of ABCG2 protein. The molecular docking investigation demonstrated that baicalein and fumitremorgin C may share similar binding sites in TM domain of ABCG2, indicating baicalein decreases the proportion of side population through inhibition of ABCG2 protein in RPMI 8226 cells. Li et al. found that baicalein inhibits proliferation and induces apoptosis of RPMI 8226 cells and causes G0/G1-phase arrest [61]. They also confirmed that baicalein is able to inhibit 12-LOX protein expression.
IL-6 is one of the most important growth factors for myeloma cells. IL-6 promotes the proliferation and survival of MM cells through the phosphorylated proteins, including STAT3, Akt, and MAPK. Chemical components that suppress the phosphorylation of signaling proteins might have a potential therapeutic effects for the treatment of MM. Liu et al. investigated the therapeutic effects of baicalein in four myeloma cell lines, namely U266, NOP2, AMO1, and ILKM2, and demonstrated that baicalein suppresses proliferation and induces apoptosis of myeloma cells by blocking IkB-α degradation, resulting in down-regulation of IL-6 and XIAP gene expression [62]. They also found that baicalein not only inhibits IL-6-mediated phosphorylation of signaling proteins, such as Akt, Jak, MAPK, and STAT3, but also inhibits the expression of their target genes, such as Bcl-XL. In addition, baicalein exhibits a stronger inhibitory effect on Erk(1/2) phosphorylation. In contrast, baicalin has no significant effect on IL-6-mediated protein phosphorylation. All these results suggest that baicalein is a potent inhibitor of protein phosphorylation induced by IL-6. Xu et al. further revealed that baicalein simultaneously inhibits β-catenin protein level to resist the effect of IL-6 on inducing MM cell proliferation, and results in decrease of β-catenin, c-myc, cyclinD1 and integrin β7 mRNA levels [63]. They also found that baicalein decreases migration ability of MM cells in a dose-dependent manner by SDF-1.
The representative in vitro studies of baicalin and baicalein on various hematological cancer cell lines are summarized in Table 1. Despite these findings have demonstrated the significant biological promise, further mechanistic investigations of baicalin and baicalein are imperative to provide helpful insights for the advancement into the potential clinical trials.
Table 1. Summary of In Vitro Studies with Baicalin and Baicalein on Various Hematological Cancer Cell Lines.
| Origin | Cell lines | Biological effects | Molecular mechanisms | Ref. |
|---|---|---|---|---|
| Leukemia | CEM | Baicalein inhibits CEM cell proliferation with an IC50 value of 4.7 μM | direct inhibition of growth-related signal, protein tyrosine kinase, and reduction of mRNA expression of PDGF-A | [45] |
| CCRF-CEM | Baicalein triggers mitochondria-mediated apoptosis | Baicalein induces mitochondria-dependent cleavage of caspases-9 and -3 and PARP with concomitant decreases in IAP family proteins, survivin, and XIAP | [48] | |
| CCRF-CEM | Baicalin exhibits remarkable anticancer effect with an IC50 value of 10.6 μg/mL | Baicalin induces apoptosis by Bcl-2-dependent, but not p53-dependent pathway | [47] | |
| K562 | Baicalein exhibits the specific cytotoxic effect on leukemia cells | not disclosed | [42] | |
| K562 | Baicalein arrests K562 cells in the S phase and selectively induces apoptosis | upregulating caspase-3 and Fas | [43] | |
| K562 | Baicalin and baicalein affects tumorigenesis of K562 cells | induction of Notch signal pathway | [44] | |
| HL-60 | Baicalein induces in vitro apoptosis | Baicalein activates caspase-3, resulting in the cleavage of PARP and DNA fragmentation; Baicalein affects the NAT activity | [49,50] | |
| HL-60 | Baicalein is capable of inducing apoptosis | Baicalein induces the cleavage of Bid protein, cytochrome c release from mitochondria into cytosol, and activation of | [51] | |
| caspase-3, -8 and -9; Baicalein causes elevation of intracellular hydrogen peroxide level; through ROS-mediated mitochondrial dysfunction pathway | ||||
| HL-60 | Baicalein induces apoptosis, suppresses mitochondrial dysfunction | Baicalein-induced apoptosis is independent of the production of ROS, activation of PKC and JNKs participated in TPA's prevention | [52] | |
| HL-60/ADR | Baicalin induces apoptosis | PI3K/Akt signaling pathway | [53] | |
| Lymphoma | CA46 | Baicalin is capable of suppressing the growth of CA46 cells via induction of apoptosis with an IC50 value of 10 μM | up-regulate the cleaved forms of caspase-9, caspase-3, and PARP; down-regulate anti-apoptotic and up-regulate apoptotic components of the PI3K/Akt signaling pathway signaling pathway | [56] |
| Myeloma | U266, NOP-2, AMO1, ILKM2 | Baicalein induces apoptosis | Baicalein inhibits the phosphorylation of IkB-alpha, followed by decreased expression of the IL-6 and XIAP genes and activation of caspase-9 and caspase-3 | [58] |
| MM cells | Baicalein inhibits proliferation and migration | Its molecular mechanisms are associated with inhibition of proliferation related genes β-catenin, c-myc, cyclin D1 and integrin β7 expression | [63] | |
| RPMI 8226 | Baicalein inhibits proliferation and induces apoptosis and causes G0/G1-phase arrest | Baicalein inhibits 12-LOX protein expression | [61] | |
| RPMI 8226 | Baicalein inhibits RPMI 8226 cells with an IC50 value of 168.5 μM | Baicalein significantly decreases the expression level of ABCG2 protein; Baicalein and fumitremorgin C might share similar binding sites in TM domain of ABCG2 | [59,60] | |
| U266, NOP2, AMO1, | Baicalein suppresses proliferation and induces apoptosis of various | blocking IkB-α degradation followed by down-regulating IL-6 and XIAP gene expression; Baicalein not only inhibits | [62] | |
| ILKM2 | myeloma cells | IL-6-mediated phosphorylation of signaling proteins, such as Jak, STAT3, MAPK, and Akt, but also inhibits the expression of their target genes, such as Bcl-XL; Baicalein exhibits a potent inhibitory effect on Erk(1/2) phosphorylation |
2.7. Combination strategy
Some cancers remain susceptible to other drugs even though they are resistant to one drug. Based on this view, the concept of combination therapy is well recognized in oncology. As discussed above, baicalin and baicalein have demonstrated the potential therapeutic effects for hematological malignancies treatment. Because the mode of actions of baicalin and baicalein are different from that of conventional cytotoxic agents, combination regimens may show particular promise in early trials.
Combination of baicalein and vincristine yields a synergistic antileukemic efficacy [48]. This combination strategy is applicable to future clinical trials in the treatment of pediatric leukemia because baicalein has beneficial effects in alleviating the vomiting, nausea, and skin rashes caused by chemotherapy. Hexamethylene bisacetamide (HMBA) and baicalin both exert potent antileukemic activity. Ren et al. indentified that the combination of HMBA and baicalin cooperatively inhibits the proliferation of AML cell lines [64]. The combination treatment triggers apoptosis through the intrinsic pathway, as well as through the extrinsic pathway, which involves loss of matrixmetalloproteinase, decreased Bcl-2/Bax ratio and Bcl-XL/Bax ratio, and activation of caspase-8, caspase-9, and Fas. Notably, combination of baicalin and HMBA shows little toxic effect on healthy peripheral blood mononuclear cells. Otsuyama et al. revealed that the synergistic growth suppression of dexamethasone and baicalein in myeloma cells may be useful for myeloma therapy [65]. Interestingly, this combinatory treatment may overcome the dexamethasone resistance in either primary myeloma cells or myeloma cell lines. Baicalein combined with dexamethasone can induce the activation of PPARb and glucocorticoid receptor (GR), which synergistically suppresses the transcriptional activity of nuclear NF-kB. Recently, Zhang et al. reported that baicalein in combination with lenalidomide exhibits synergistic effect via inducing apoptosis of myeloma cells [66]. Further clinical trials are anticipated to rapidly evaluate the utility of combinatory treatments in relapsed and refractory hematological disorders.
3. Conclusions and future perspectives
There is an increasing interest concerning the application of natural antioxidants as low toxic anticancer agents. Some flavonoids from dietary sources have shown to exhibit antioxidative activity, coronary heart disease prevention, free-radical scavenging capacity, and anticancer activity. S. baicalensis has been clinically used as a major component in traditional Chinese medicines to treat various diseases including hematologic malignancies. Notably, baicalin and baicalein are the main bioactive constituents in S. baicalensis and have been subjected to extensive investigations as potential candidates for the treatment of cancers. This review summarizes the recent progress of studies on baicalin and baicalein in hematological malignancies. Evidence from various in vitro studies suggests that both natural products can indeed suppress multiple molecular targets, induce cell cycle arrest and differentiation on various hematological cancer cell lines. However, very few in vivo experiments were conducted to exploit their efficacy for the treatment of hematological malignancies. Our preliminary in vivo studies revealed that baicalin can suppress the tumor growth of CA46 cell xenografts in nude mice at a dose of 60 mg/kg (unpublished data).
Over the past decade, accumulating evidence from pharmacokinetics studies suggests that baicalin and baicalein are orally bioavailable despite the limited capability. Further pharmaceutical research indicates that development of appropriate delivery systems can significantly enhance their bioavailability and efficacy. Despite aforementioned advances, more detailed investigations are needed to extensively understand their exact mechanisms of biological effects against different hematological malignancies. In addition, with respect to their limited bioavailability and low toxicity, extensive in vivo studies are required to fully exploit their efficacy. Furthermore, as S. baicalensis has already been clinically utilized as the traditional medicines and the nutritional supplements, additional clinical trials of baicalin and baicalein are warranted to prove their possibility as potential drug candidates for chemoprevention and treatment of hematological malignancies in clinic. It is worthy to note that chemical modification of baicalein results in several promising compounds in preclinical studies [67-69]. Due to the chemical complexity, structural optimization of baicalin and baicalein remains sparse. Nevertheless, overcoming such challenges represents a promising research direction for further explorations to yield better anticancer agents and provides a great opportunity to identify novel drug targets for treatment of hematological malignancies.
Highlights.
Baicalin and baicalein display therapeutic potentials for hematological malignancies.
They are associated with diverse cancer targets and signaling pathways.
Potential biological mechanisms in various hematological malignancies are discussed.
Biotransformation and approaches to improve their bioavailability are summarized.
Combination with baicalin or baicalein offers promise as chemotherapeutic adjuvants.
Acknowledgments
This work was supported by the Technology Development Foundation of Fuzhou University (Project Numbers 2013-XQ-8 and 2013-XQ-9), National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, grants P50 CA097007, P30 DA028821, R21 MH093844 from the National Institutes of Health, R. A. Welch Foundation Chemistry and Biology Collaborative Grant from the Gulf Coast Consortia (GCC), John Sealy Memorial Endowment Fund, Institute for Translational Sciences (ITS), and the Center for Addiction Research (CAR) at UTMB.
Abbreviations
- PDGF-A
platelet-derived growth factor-A
- CK2
casein kinase 2
- Bcl-2
B-cell lymphoma-2
- PARP
poly-(ADP-ribose) polymerase
- IAP
inhibitor of apoptosis protein
- XIAP
X-linked inhibitor of apoptosis protein
- 8-tBid
caspase-8-cleaved Bid
- CBF1
C-repeat binding factor 1
- NAT
N-acetyltransferase
- ROS
reactive oxygen species
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- Akt
protein kinase B
- HLJDT
Huang-Lian-Jie-Du-Tang
- MM
multiple myeloma
- TM
transmembrane
- ABCG2
ATP-binding cassette sub-family G member 2
- 12-LOX
12-lipoxygenase
- STAT3
signal transducer and activator of transcription 3
- MAPK
Mitogen-activated protein kinases
- IL-6
interleukin-6
- Jak
Janus kinase
- Bcl-XL
B-cell lymphoma-extra large
- Erk(1/2)
extracellular-signal-regulated kinase (1/2)
- SDF-1
stromal cell-derived factor 1
- HMBA
hexamethylene bisacetamide
- AML
acute myeloid leukemia
- Bax
Bcl-2-associated X protein
- NF-κB
nuclear factor-κB
- GR
glucocorticoid receptor
- BCS
biopharmaceutical classification system
Footnotes
Conflict of interest: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravandi F, Talpaz M, Estrov Z. Modulation of cellular signaling pathways: prospects for targeted therapy in hematological malignancies. Clin Cancer Res. 2003;9:535–550. [PubMed] [Google Scholar]
- 2.Zvara A, Hackler L, Jr, Nagy ZB, Micsik T, Puskas LG. New molecular methods for classification, diagnosis and therapy prediction of hematological malignancies. Pathol Oncol Res. 2002;8:231–240. doi: 10.1007/BF03036737. [DOI] [PubMed] [Google Scholar]
- 3.Shi M, Xiao R, Woda BA, Yu H. Five important advances in hematopathology. Arch Pathol Lab Med. 2014;138:410–419. doi: 10.5858/ARPA.2012-0645-RA. [DOI] [PubMed] [Google Scholar]
- 4.Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, Garrido C. Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia. 2000;14:1833–1849. doi: 10.1038/sj.leu.2401902. [DOI] [PubMed] [Google Scholar]
- 5.L. Experts in Chronic Myeloid. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 7.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 8.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer - an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–377. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- 11.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Mrazek AA, Wang X, Ding C, Ding Y, Porro LJ, Liu H, Chao C, Hellmich MR, Zhou J. Design, synthesis, and characterization of novel apigenin analogues that suppress pancreatic stellate cell proliferation in vitro and associated pancreatic fibrosis in vivo. Bioorg Med Chem. 2014;22:3393–3404. doi: 10.1016/j.bmc.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XW, Li WF, Li WW, Ren KH, Fan CM, Chen YY, Shen YL. Protective effects of the aqueous extract of Scutellaria baicalensis against acrolein-induced oxidative stress in cultured human umbilical vein endothelial cells. Pharm Biol. 2011;49:256–261. doi: 10.3109/13880209.2010.501803. [DOI] [PubMed] [Google Scholar]
- 15.Gasiorowski K, Lamer-Zarawska E, Leszek J, Parvathaneni K, Yendluri BB, Blach-Olszewska Z, Aliev G. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS Neurol Disord Drug Targets. 2011;10:184–191. doi: 10.2174/187152711794480384. [DOI] [PubMed] [Google Scholar]
- 16.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai T, Muller CI, Desmond JC, Imai Y, Heber D, Koeffler HP. Scutellaria baicalensis, a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res. 2007;31:523–530. doi: 10.1016/j.leukres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Long X, Yuan F, Chen L, Pan S, Liu Y, Stowell Y, Li X. Combined use of phospholipid complexes and self-emulsifying microemulsions for improving the oral absorption of a BCS class IV compound, baicalin. Acta Pharm Sin B. 2014;4:217–226. doi: 10.1016/j.apsb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Lin G, Zuo Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm Res. 2007;24:81–89. doi: 10.1007/s11095-006-9126-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Luo R, Chen Y, Ke X, Hu D, Han M. Application of carrier and plasticizer to improve the dissolution and bioavailability of poorly water-soluble baicalein by hot melt extrusion. AAPS PharmSciTech. 2014;15:560–568. doi: 10.1208/s12249-013-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing J, Chen X, Zhong D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005;78:140–146. doi: 10.1016/j.lfs.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 22.Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol. 2003;55:205–209. doi: 10.1211/002235702522. [DOI] [PubMed] [Google Scholar]
- 23.Akao T, Kawabata K, Yanagisawa E, Ishihara K, Mizuhara Y, Wakui Y, Sakashita Y, Kobashi K. Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J Pharm Pharmacol. 2000;52:1563–1568. doi: 10.1211/0022357001777621. [DOI] [PubMed] [Google Scholar]
- 24.Taiming L, Xuehua J. Investigation of the absorption mechanisms of baicalin and baicalein in rats. J Pharm Sci. 2006;95:1326–1333. doi: 10.1002/jps.20593. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Li C, Lin G, Krajcsi P, Zuo Z. Hepatic metabolism and disposition of baicalein via the coupling of conjugation enzymes and transporters-in vitro and in vivo evidences. AAPS J. 2011;13:378–389. doi: 10.1208/s12248-011-9277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Lin G, Chang Q, Zuo Z. Role of intestinal first-pass metabolism of baicalein in its absorption process. Pharm Res. 2005;22:1050–1058. doi: 10.1007/s11095-005-5303-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420:180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Qiu L, Gao J, Jin Y. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-beta-cyclodextrin. Int J Pharm. 2006;312:137–143. doi: 10.1016/j.ijpharm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Tian R, Hu W, Jia Y, Jiang H, Zhang J, Zhang L. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83:1532–1539. doi: 10.1016/j.fitote.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 30.He X, Pei L, Tong HH, Zheng Y. Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with Pluronic F68 to improve dissolution and oral bioavailability. AAPS PharmSciTech. 2011;12:104–113. doi: 10.1208/s12249-010-9560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue PF, Li Y, Wan J, Wang Y, Yang M, Zhu WF, Wang CH, Yuan HL. Process optimization and evaluation of novel baicalin solid nanocrystals. Int J Nanomedicine. 2013;8:2961–2973. doi: 10.2147/IJN.S44924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–3779. doi: 10.2147/IJN.S51578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, Zhao X, Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47:497–505. doi: 10.1016/j.ejps.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 34.De Martino L, D'Arena G, Filosa R, Peduto A, Zeppa R, De Feo V. Natural compounds in anti-leukaemic therapy: a review. Mini Rev Med Chem. 2011;11:492–502. doi: 10.2174/138955711795843284. [DOI] [PubMed] [Google Scholar]
- 35.Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, Nishio K, Yamamoto H, Tasaka K, Mimura A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–274. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Huang ST, Wang CY, Yang RC, Chu CJ, Wu HT, Pang JH. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine. 2010;17:47–54. doi: 10.1016/j.phymed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Ozmen A, Madlener S, Bauer S, Krasteva S, Vonach C, Giessrigl B, Gridling M, Viola K, Stark N, Saiko P, Michel B, Fritzer-Szekeres M, Szekeres T, Askin-Celik T, Krenn L, Krupitza G. In vitro anti-leukemic activity of the ethno-pharmacological plant Scutellaria orientalis ssp. carica endemic to western Turkey. Phytomedicine. 2010;17:55–62. doi: 10.1016/j.phymed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285–292. doi: 10.1002/pros.10024. [DOI] [PubMed] [Google Scholar]
- 39.Chen ZT, Dong Q, Zhang L. Study on effect of qingkailing injection and its active principle in inducing cell apoptosis in human acute promyelocytic leukemia. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21:840–842. [PubMed] [Google Scholar]
- 40.Ciesielska E, Gwardys A, Metodiewa D. Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. Anticancer Res. 2002;22:2885–2891. [PubMed] [Google Scholar]
- 41.Ciesielska E, Wolszczak M, Gulanowski B, Szulawska A, Kochman A, Metodiewa D. In vitro antileukemic, antioxidant and prooxidant activities of Antoksyd S (C/E/XXI): a comparison with baicalin and baicalein. In Vivo. 2004;18:497–503. [PubMed] [Google Scholar]
- 42.Romanouskaya TV, Grinev VV. Cytotoxic effect of flavonoids on leukemia cells and normal cells of human blood. Bull Exp Biol Med. 2009;148:57–59. doi: 10.1007/s10517-009-0633-9. [DOI] [PubMed] [Google Scholar]
- 43.Dong QH, Zheng S, Xu RZ, Lu QH. Baicalein selectively induce apoptosis in human leukemia K562 cells. Yao Xue Xue Bao. 2003;38:817–820. [PubMed] [Google Scholar]
- 44.Wang AM, Ku HH, Liang YC, Chen YC, Hwu YM, Yeh TS. The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J Cell Biochem. 2009;106:682–692. doi: 10.1002/jcb.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang HC, Hsieh LM, Chen HW, Lin YS, Chen JS. Effects of baicalein and esculetin on transduction signals and growth factors expression in T-lymphoid leukemia cells. Eur J Pharmacol. 1994;268:73–78. doi: 10.1016/0922-4106(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 46.Lin XC, Liu XG, Chen XW, Chen WZ, Liang NC. Inhibitory effect and its kinetic analysis of baicalein on recombinant human protein kinase CK2 holoenzyme. Ai Zheng. 2004;23:874–878. [PubMed] [Google Scholar]
- 47.Shieh DE, Cheng HY, Yen MH, Chiang LC, Lin CC. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am J Chin Med. 2006;34:245–261. doi: 10.1142/S0192415X06003801. [DOI] [PubMed] [Google Scholar]
- 48.Chen YJ, Wu CS, Shieh JJ, Wu JH, Chen HY, Chung TW, Chen YK, Lin CC. Baicalein Triggers Mitochondria-Mediated Apoptosis and Enhances the Antileukemic Effect of Vincristine in Childhood Acute Lymphoblastic Leukemia CCRF-CEM Cells. Evid Based Complement Alternat Med. 2013;2013:124747. doi: 10.1155/2013/124747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YC, Tyan YS, Kuo HM, Chang WC, Hsia TC, Chung JG. Baicalein induced in vitro apoptosis undergo caspases activity in human promyelocytic leukemia HL-60 cells. Food Chem Toxicol. 2004;42:37–43. doi: 10.1016/j.fct.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Li YC, Tyan YS, Lee YM, Tsao TY, Chuang JY, Kuo HM, Hsia TC, Yang JH, Chung JG. N-acetyltransferase is involved in baicalein-induced N-acetylation of 2-aminofluorene and DNA-2-aminofluorene adduct formation in human leukemia HL-60 cells. In Vivo. 2005;19:399–405. [PubMed] [Google Scholar]
- 51.Wang J, Yu Y, Hashimoto F, Sakata Y, Fujii M, Hou DX. Baicalein induces apoptosis through ROS-mediated mitochondrial dysfunction pathway in HL-60 cells. Int J Mol Med. 2004;14:627–632. [PubMed] [Google Scholar]
- 52.Chow JM, Shen SC, Wu CY, Chen YC. 12-o-Tetradecanoylphorbol 13-acetate prevents baicalein-induced apoptosis via activation of protein kinase C and JNKs in human leukemia cells. Apoptosis. 2006;11:1999–2011. doi: 10.1007/s10495-006-0085-x. [DOI] [PubMed] [Google Scholar]
- 53.Zheng J, Hu JD, Chen YY, Chen BY, Huang Y, Zheng ZH, Liu TB. Baicalin induces apoptosis in leukemia HL-60/ADR cells via possible down-regulation of the PI3K/Akt signaling pathway. Asian Pac J Cancer Prev. 2012;13:1119–1124. doi: 10.7314/apjcp.2012.13.4.1119. [DOI] [PubMed] [Google Scholar]
- 54.Shih YT, Wu DC, Liu CM, Yang YC, Chen IJ, Lo YC. San-Huang-Xie-Xin-Tang inhibits Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. J Ethnopharmacol. 2007;112:537–544. doi: 10.1016/j.jep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Salini S, Chubicka T, Sasidharan N, Sindhu ER, Babu TD. Cytotoxic and antioxidant properties of selected Scutellaria species from the Western Ghats of Peninsular India. Pharm Biol. 2013;51:152–159. doi: 10.3109/13880209.2012.715170. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng Z, Chen Y. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. J Exp Clin Cancer Res. 2012;31:48. doi: 10.1186/1756-9966-31-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castelli R, Gualtierotti R, Orofino N, Losurdo A, Gandolfi S, Cugno M. Current and Emerging Treatment Options for Patients with Relapsed Myeloma. Clin Med Insights Oncol. 2013;7:209–219. doi: 10.4137/CMO.S8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, Miyamoto K, Kawano MM. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105:3312–3318. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 59.Lin MG, Liu LP, Li CY, Zhang M, Chen Y, Qin J, Gu YY, Li Z, Wu XL, Mo SL. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac J Cancer Prev. 2013;14:7179–7186. doi: 10.7314/apjcp.2013.14.12.7179. [DOI] [PubMed] [Google Scholar]
- 60.Gu YY, Liu LP, Qin J, Zhang M, Chen Y, Wang D, Li Z, Tang JZ, Mo SL. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia. 2014;94:21–28. doi: 10.1016/j.fitote.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Li QB, You Y, Chen ZC, Lu J, Shao J, Zou P. Role of Baicalein in the regulation of proliferation and apoptosis in human myeloma RPMI8226 cells. Chin Med J (Engl) 2006;119:948–952. [PubMed] [Google Scholar]
- 62.Liu S, Ma Z, Cai H, Li Q, Rong W, Kawano M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur J Haematol. 2010;84:137–144. doi: 10.1111/j.1600-0609.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 63.Xu CP, Cai HL, He L, Ma Z, Liu SQ. Effect of baicalein on proliferation and migration in multiple myeloma cell lines RPMI 8226 and U266 cells. Zhonghua Xue Ye Xue Za Zhi. 2012;33:938–943. [PubMed] [Google Scholar]
- 64.Ren X, Zhang Y, Li C, Wang H, Jiang Z, Zhang Z, Guo Q, Song G, Bi K, Jiang G. Enhancement of baicalin by hexamethylene bisacetamide on the induction of apoptosis contributes to simultaneous activation of the intrinsic and extrinsic apoptotic pathways in human leukemia cells. Oncol Rep. 2013;30:2071–2080. doi: 10.3892/or.2013.2684. [DOI] [PubMed] [Google Scholar]
- 65.Otsuyama KI, Ma Z, Abroun S, Amin J, Shamsasenjan K, Asaoku H, Kawano MM. PPARbeta-mediated growth suppression of baicalein and dexamethasone in human myeloma cells. Leukemia. 2007;21:187–190. doi: 10.1038/sj.leu.2404462. [DOI] [PubMed] [Google Scholar]
- 66.Zhang RB, He L, Huang Z, Ma Z, Liu SQ. Synergistic effect and mechanism of baicalein in combination with lenalidomide-induced apoptosis of myeloma cells. Zhonghua Xue Ye Xue Za Zhi. 2013;34:546–547. doi: 10.3760/cma.j.issn.0253-2727.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Ding D, Zhang B, Meng T, Ma Y, Wang X, Peng H, Shen J. Novel synthetic baicalein derivatives caused apoptosis and activated AMP-activated protein kinase in human tumor cells. Org Biomol Chem. 2011;9:7287–7291. doi: 10.1039/c1ob06094e. [DOI] [PubMed] [Google Scholar]
- 68.Donald G, Hertzer K, Eibl G. Baicalein--an intriguing therapeutic phytochemical in pancreatic cancer. Curr Drug Targets. 2012;13:1772–1776. doi: 10.2174/138945012804545470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo R, Wang J, Zhao L, Lu N, You Q, Guo Q, Li Z. Synthesis and biological evaluation of baicalein derivatives as potent antitumor agents. Bioorg Med Chem Lett. 2014;24:1334–1338. doi: 10.1016/j.bmcl.2014.01.053. [DOI] [PubMed] [Google Scholar]