Abstract
Few studies investigate factors that influence acquisition in nicotine self-administration (NSA), such as food training and training dose. Most have utilized peak doses along nicotine’s dose-response curve (15 and 30 μg/kg) that establish NSA in the majority of animals. To investigate the specific and combined effects of training and dose on NSA acquisition, separate and head-to head experiments using food training (FT) or spontaneous acquisition (SP) at multiple doses on the ascending limb of the dose-response curve were tested. First, rats underwent FT or SP under fixed ratio (FR1 and FR2) and progressive ratio (PR) schedules using 7.5–30 μg/kg nicotine. More rats acquired NSA with FT vs. SP at 3.75 μg/kg (56% vs. 38%) and 7.5 μg/kg (88% vs. 40%, p<0.05) and FT rats responded higher under PR. Based on these findings, rats then underwent identical NSA acquisition and PR (with and without nicotine), extinction and reinstatement induced by cue exposure and nicotine in a head-to-head comparison of FT and SP using 7.5 μg/kg. Acquisition differences were replicated: 100% FT and 60% SP rats met criteria (p<0.05). Without nicotine (cue only), no FT rats and 8% SP rats met criteria. FR and PR responding, extinction, and cue and nicotine-induced reinstatement did not differ between FT and SP. FT versus SP enhances acquisition at lower nicotine doses but does not alter subsequent behaviors. Lower doses can reinforce NSA and be used, in the absence of FT, to study influences on acquisition more closely modelling the initial phases of human smoking.
Keywords: Nicotine, self-administration, food training, rat, acquisition, progressive ratio
1. Introduction
Nicotine self-administration (NSA) is the benchmark animal model used to study smoking behaviour. Multiple stages of smoking behaviour can be investigated with this model, such as acquisition, maintenance, and cessation. Unlike other drugs of abuse such as cocaine and amphetamine, where self-administration is easily established, nicotine is considered a weak reinforcer, and additional experimental conditions are often required in order for animals to learn NSA. Manipulations such as food restriction, food training, training dose of nicotine and addition of auditory and visual cues are used to facilitate NSA [1, 2]. There is a significant body of literature that has investigated the role of secondary cue factors in NSA [3–5], which is important in determining the reinforcing efficacy of nicotine alone as well as its interaction with non-drug cues on this behavior. Few studies have investigated or reported the effect of other factors on acquisition. Determining the direct impact these factors have on acquisition is important because it will enhance our understanding of how nicotine reinforcement can lead to the initiation and persistence of the drug-taking behavior.
Food training has been standard practice for producing rapid acquisition of NSA, and using this procedure, the majority of animals acquire the behavior [1, 2, 6]. This prior training also creates a different pattern of responding during the acquisition period, as responding changes from food reinforcement to nicotine reinforcement. More recent self-administration studies have begun to train animals to self-administer nicotine without food (spontaneous acquisition) [7]. Without prior food training, animals respond on the nicotine-associated lever reinforced by nicotine from the start, which allows the pattern of responding during the acquisition period to be a reflection purely of nicotine reinforcement.
Studies that have used spontaneous acquisition in a limited access paradigm have used training doses of nicotine that fall along the peak of nicotine’s dose-response curve, 15 and 30 μg/kg [2, 7–12]. Few spontaneous acquisition studies with lower training doses exist in the literature, and these studies have used adolescents [13], female rats [14] or unlimited access paradigms [14, 15], which are all populations or procedures that possess or induce different reinforcement behavior in NSA. The one study that used training doses lower than 15 μg/kg in a limited access paradigm with male adult rats, which falls along the ascending limb of the dose-response curve, did not report acquisition information [16]. Only one study to date has investigated the effect of food training and spontaneous acquisition directly [11]. In this study they were able to compare the effect of food training and spontaneous acquisition on responding for nicotine, but animals were only trained on 30 μg/kg nicotine, which is commonly used to initiate NSA because the majority of animals will readily acquire the behavior. The high rate of acquisition at this unit dose makes it difficult to investigate factors that can influence nicotine’s ability to establish acquisition since this nicotine dose may produce a ceiling effect on NSA behavior.
Analysis of these factors, specifically food training and training infusion dose, is important in investigating the biological mechanisms underlying acquisition in vulnerable populations. By examining these factors in this stage of self-administration, the question can be addressed of whether there is a dose range that displays dose-dependent increases in acquisition and identification of doses which are submaximal, allowing for assessment of the influence of other factors. Also, the possibility that food training has an impact on nicotine-reinforced behaviours tested long after food training was complete would have important implications on the conclusions drawn from these tests.
We conducted multiple experiments in animals that were food trained or which underwent spontaneous acquisition at various doses along the ascending limb of the nicotine infusion dose-response curve. We found several interesting effects of food training and nicotine infusion dose. Based on these results we selected doses in order to do a direct head-to-head comparison where animals were either food trained or not (i.e. spontaneous acquisition) and went through acquisition, maintenance, the progressive ratio schedule, and nicotine seeking-behaviour (extinction followed by cue and nicotine induced reinstatement) to determine whether these differences were persistent. In addition, the potential reinforcing effect of the compound cue (light+ tone) employed in the nicotine studies, was also evaluated by assessing responding for this cue alone under both FR and PR schedules.
2. Materials and Methods
2.1. Subjects
Adult male Wistar rats (250–300 g) were obtained from Charles River Laboratories (Quebec, Canada) and individually housed in a temperature-controlled environment on a 12 hr reverse light/dark cycle (lights on at 1900 hr). Water was available ad libitum and for the duration of the experiment rats received 20–25 g of Purina rat chow daily after each operant session.
2.2. Surgery
Rats were anaesthetized with Isofluorane and the local anaesthetic Marcaine (Hospira Healthcare, Montreal, QC, Canada) was applied to incision sites (0.1 ml, 0.125%, s.c.). Derapen (Wyeth Animal Health, Guelph, ON, Canada; 0.1 ml, s.c.) was used as an antibiotic and Anafen (Merial Canada, Baie D’Urfé, QC, Canada; 5 mg/kg, s.c.) was used as an analgesic. Catheters were implanted into the right jugular vein as previously described [7] and rats were given 7 days to recover before NSA. To maintain patency catheters were flushed daily with Heparin in a sterile saline solution (0.1 ml, 50 U/ml).
2.3. Apparatus
NSA was carried out in sixteen operant chambers that were operated by a computer-controlled interface system (Med Associates, St Albans, VT). Each operant chamber was set up with two levers on the same wall located 2.5 cm above a grid floor. Pressing on the active lever activated a high-speed microliter syringe pump which delivered nicotine (0.1 ml/kg in approximately 1 sec). Pressing on the inactive lever was recorded but did not have any programmed consequences. A white cue light and a tone generator were located above the active lever. A white cue light was also located above the inactive lever at the same height as the light above the active lever; however pressing on the inactive lever did not turn this light on. Both visual (40 sec) and auditory (2800 Hz, 1 sec) stimuli above the active lever were turned on when nicotine was delivered. After a nicotine infusion, there was a timeout period (40 sec) where lever presses were recorded but there were no consequences. Active lever presses reported in these experiments include all lever presses made during the session including those during the timeout period. On the opposite wall of the chamber was a house light which signalled the start of the self-administration session. Nicotine was delivered via a modified 22-gauge cannula connected to a fluid swivel with Tygon tubing protected by a metal spring, which was attached to the intravenous catheter during each session. The swivel was connected to the microliter syringe with Tygon tubing.
2.4. Drugs
Nicotine (Sigma-Aldrich, Oakville, ON, Canada) was prepared daily using sterile saline and pH was adjusted to 6.8–7.2. The unit doses for NSA were 3.75, 7.5, 15, and 30 μg/kg/infusion (expressed as base). For reinstatement tests, nicotine (0.15 and 0.3 mg/kg) was administered subcutaneously in a volume of 1 ml/kg. Thiopental (2–4 mg, i.v., 20 mg/ml) was used to test catheter patency at the end of each experiment phase.
2.5. Self-administration procedures
2.5.1. Nicotine self-administration in multiple separate cohorts
2.5.1.1. Animal cohorts that underwent acquisition of NSA and PR with prior food training
Animals underwent NSA with prior food training in two separate cohorts with identical procedures. The first cohort used 7.5, 15, and 30 μg/kg/infusion unit doses of nicotine and the second cohort used 3.75 μg/kg/infusion unit dose of nicotine (Figure 1a). Before surgery, rats (n = 12/nicotine infusion dose) underwent operant training for 45 mg sucrose pellets (Bioserv, Frenchtown, NJ) in operant chambers equipped with pellet magazines. All components of the operant chambers (active and inactive levers, house and cue lights) were set up in the same locations as they are for NSA, with the exception of the pellet magazines. Rats were food deprived for 24 hrs prior to the first food training session. Food training was conducted under a fixed ratio 1 (FR1) schedule of reinforcement in two 8 hr sessions with no audio or visual cues presented. Subsequently, responding for food was assessed in a 1 hr session in which rats were considered successfully trained once they received 100 pellets within the session.
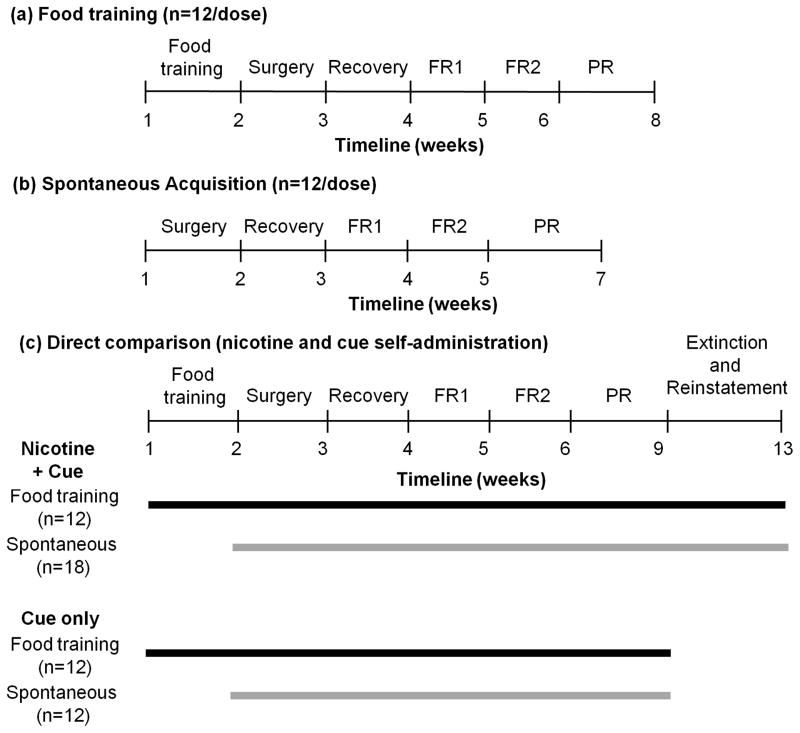
Fig. 1.
Study design for each self-administration experiment. Three separate experimental timelines were conducted: (a) Animals were food trained and had 1 hr sessions that were carried out Monday to Friday; (b) Animals underwent spontaneous acquisition and had 2 hr sessions that were performed daily; (c) Animals were either food trained or spontaneously acquired NSA or cue self-administration in a direct head-to-head comparison where they underwent 2 hr sessions that were performed daily. All other self-administration parameters were identical in all three experiments
Rats were assigned to one nicotine training dose throughout self-administration. Rats initiated 1 hr NSA sessions under an FR1 schedule for five days and then under an FR2 schedule for five days. Rats underwent each schedule Monday to Friday and had a two day period away from the operant boxes on the weekend days. Following FR training, rats then underwent a progressive ratio (PR) schedule of reinforcement. The sequence was determined using the exponential formula (5((0.2 × infusion number) − 5)), such that the required responses per infusion are as follows: 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 179, 219, 268, 328, 402, 492, 603 [7, 17, 18]. Self-administration parameters during the PR sessions were identical to FR training with the exception of session duration (2 hr) and continuous daily self-administration sessions (no days off). Animals achieved breakpoint (final ratio completed of active lever presses) when there was >20 minutes of inactivity on the active lever or the session ended.
2.5.1.2. Animal cohorts that underwent spontaneous acquisition of NSA and PR
Animals underwent NSA without food training in three separate cohorts with identical procedures. The first cohort self-administered 3.75 μg/kg/infusion dose of nicotine, the second cohort self-administered 7.5 and 15 μg/kg/infusion doses, and the third cohort self-administered 30 μg/kg/infusion (Figure 1b). Rats initiated NSA under the same conditions as rats that were food trained with the following exceptions: (1) animals did not receive food training and self-administration sessions commenced one week following recovery from surgery, (2) session duration was 2 hr, and (3) sessions were conducted on a daily basis.
2.5.2. Nicotine self-administration in a head to head comparison of food training and spontaneous acquisition
2.5.2.1. Acquisition, PR and extinction and reinstatement of NSA in animals with and without food training
Animals were assigned to two groups: food trained and spontaneous acquisition (Figure 1c). Animals in the food trained group underwent operant training for sucrose pellets before surgery as described in section 2.5.1.1. After surgery and recovery, both food-trained and spontaneous groups initiated NSA under the same conditions described in spontaneous acquisition under the FR schedule of reinforcement. All animals were trained on the 7.5 μg/kg/infusion dose of nicotine. Acquisition criteria were identical to experiments previously conducted with prior food training and spontaneous acquisition. Following self-administration on the FR2 schedule, animals were switched to a PR schedule with the same training dose for five daily sessions. To determine whether each group responded differently for higher nicotine infusion doses, the infusion dose was increased to 15 μg/kg for five PR sessions and then 30 μg/kg for another five PR sessions. Animals returned to an FR2 schedule for 3 days and were tested for extinction and reinstatement. During the extinction phase, responding on the active lever did not elicit nicotine infusions or the presentation of auditory and visual cues previously associated with nicotine delivery. Animals underwent extinction sessions until they reached the extinction criteria, which consisted of <20 active lever presses in two consecutive sessions. Cue-induced reinstatement was conducted once animals met the extinction criteria. During this test, responding on the active lever resulted in the delivery of the compound cues (visual and auditory) previously associated with nicotine delivery. Additional extinction sessions were then carried until they met criteria again (two consecutive sessions <20 active lever presses) and reinstatement induced by priming injections of saline, 0.15 and 0.3 mg/kg of nicotine (given 10 minutes prior to the start of the session) were then evaluated using repeated measure design.
2.5.2.2. Acquisition of self-administration behavior in response to the compound cue
Acquisition of operant responding for the compound cue following food training and under spontaneous was examined in 2 separate group (n=12 each) of rats (Figure 1c). Both FT and SP animals underwent operant self-administration under identical conditions as the NSA procedures as described above in the FT and SP head-to-head experiment, with the exception that animals did not receive nicotine infusions. Animals underwent FR1, FR2, and PR for 5 days under each reinforcement schedule.
2.6. Statistical Analysis
Animals that acquired NSA had to meet the following criteria: (1) A ratio of at least 2:1 active compared to inactive lever presses; (2) at least 10 lever presses in the majority of sessions (at least 3 out of 5); and (3) at least 10 reinforcements in the majority of sessions (at least 3 out of 5). These criteria were assessed based on data under the FR2 schedule and are similar to that used in Shram et al. [7] where the criterion for minimum of 10 lever presses was based on the majority of all FR sessions.
Acquisition data were analyzed using Fisher’s Exact tests (2-sided). Self-administration data from animals that acquired the behaviour were analyzed using repeated measures analysis of variance (ANOVA) tests to compare between doses and training paradigms. Post-hoc analysis was conducted with Bonferroni tests. Analysis of data from the PR schedule was conducted using the log transformation of final ratio completed. Significance level for all comparisons was p = 0.05.
3. Results
3.1. Comparison of food trained animals to spontaneously trained animals in separate cohorts show dose-dependent differences in acquisition
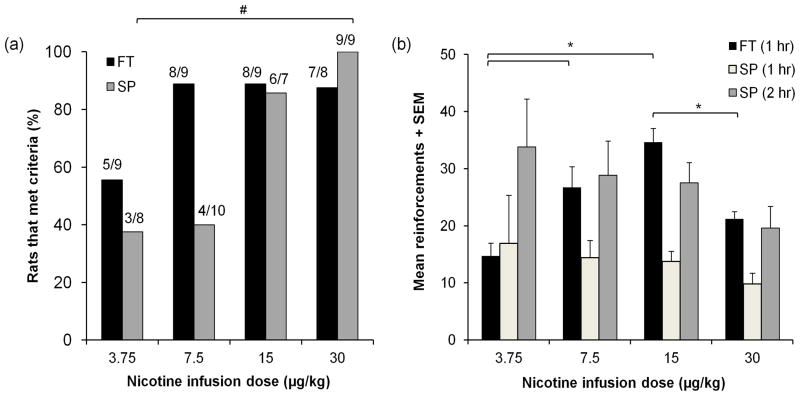
Animals from four separate cohorts with and without food training underwent acquisition of NSA under an FR schedule (Figures 1a and 1b). The proportion of animals in FT and SP that acquired NSA for each training dose was determined at the end of FR2 is shown in figure 2a. Food training resulted in a similar proportion of animals meeting acquisition criteria at unit doses of 7.5 μg/kg and higher. Only the lowest unit dose (3.75 μg/kg) tested resulted in a lower proportion of FT animals acquiring NSA compared to the other training doses (7.5 – 30 μg/kg). In contrast, over the same dose range in animals that underwent spontaneous acquisition, the proportion of animals that met criteria increased in a dose-dependent manner (Fisher’s Exact, p = 0.006). The two lowest nicotine doses (3.75 and 7.5 μg/kg) resulted in a smaller proportion of animals acquiring NSA, the second highest dose (15 μg/kg) showed a higher proportion of animals that acquired than these two doses, and the highest dose (30 μg/kg) resulted in all rats acquiring the behaviour. Acquisition curves between FT and SP for all nicotine infusion doses are available in the Supplementary Figure.
Fig. 2.
Acquisition of NSA is dependent on both prior food training and training dose but once animals have acquired, with different patterns of responding over nicotine doses in FT and SP. (a) In FT groups, animals acquired NSA (approximately 90%) for all nicotine infusion doses except for 3.75 μg/kg while in SP groups animals acquired NSA dose-dependently, with a greater proportion of animals acquiring with increasing infusion dose. Fractions at top of each bar indicate the number of animals that acquired compared to total number of animals. (b) Mean total reinforcements in animals that acquired produce an inverted “u” shape curve in FT (1 hr) and a descending curve in SP (1 hr and 2 hr shown) with increasing nicotine training doses. Error bars are expressed as standard error of the mean (SEM). #p < 0.05 between nicotine doses within SP, *p < 0.05 between nicotine doses within FT and SP groups
Mean nicotine reinforcements for FT (1 hr sessions) and SP (2 hr sessions) rats that met acquisition criteria for all nicotine infusion doses are shown in figure 2b. Mean reinforcements obtained within the first hour of the 2 hr session for SP rats are shown along with total mean reinforcements obtained from the whole 2 hr session. Mean reinforcements in FT animals showed an inverted U-shape dose-response curve where peak reinforcements were earned under the 15 μg/kg training dose (F[3, 24] = 8.797, p < 0.001). Mean reinforcements in SP animals decreased with increasing nicotine dose, but there was no significant difference between the training doses under this paradigm.
The breakpoints attained during PR over 2 hour NSA sessions were also compared between FT and SP animals at three training doses: 7.5, 15, and 30 μg/kg. FT animals had higher breakpoints (calculated as mean final ratio completed) compared to SP at 7.5 μg/kg (31 ± 11 (SEM) vs. 9 ± 7), 15 μg/kg (121 ± 30 vs. 50 ± 17), and 30 μg/kg (94 ± 16 vs. 54 ± 6) (F[1, 36] = 9.155, p = 0.005), where there was a significant difference in the final ratio completed between FT and SP at 30 μg/kg (p = 0.019). Mean inactive lever presses in FT compared to SP were 22 ± 8 and 6 ± 4 for 7.5 μg/kg, 129 ± 24 and 32 ± 18 for 15 μg/kg, and 77 ± 14 and 41 ± 6 for 30 μg/kg.
3.2. Direct head-to-head comparison of FT vs. SP with identical study parameters
3.2.1. FT results in a greater proportion of rats meeting acquisition criteria compared to SP rats
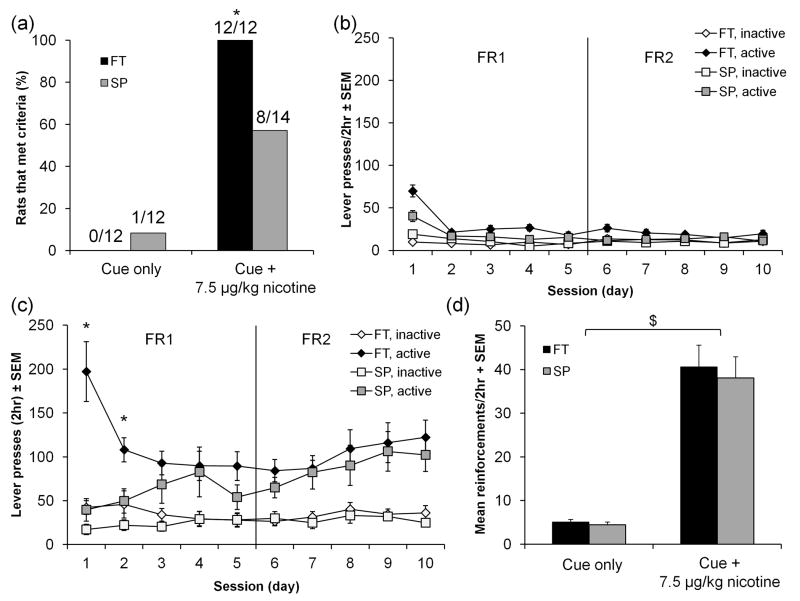
The design for this experiment is shown in figure 1c. Acquisition of NSA under SP or following FT was carried out using the 7.5 μg/kg training dose with a 2-hour session duration. This dose and session duration was selected it demonstrated the largest differences in acquisition between FT and SP animals in 3.1 (Figure 2a). Here we show again that a higher proportion of FT animals met the acquisition criteria compared to SP animals (Fisher’s Exact, p = 0.02) (Figure 3a). In the cue only condition, no FT animals and one of twelve SP animals met acquisition criteria.
Fig. 3.
A greater proportion of FT animals acquired NSA compared to SP animals but after acquisition FT animals did not respond differently from SP animals. (a) All FT animals compared to approximately 60% of SP animals acquired NSA at a dose of 7.5 μg/kg nicotine unit dose; no FT animals compared to 8% of SP animals acquired in the cue only SA. (b) Mean active lever presses in FT and SP animals that underwent cue SA (all animals). Active lever presses were not different under the cue only condition. (c) Mean active and inactive lever presses in FT and SP animals that underwent NSA (acquired animals). Active lever presses were different between FT and SP with nicotine reinforcement in the first 2 sessions. (d) Mean nicotine reinforcements between FT and SP animals were not different for either cue only (all animals) or nicotine SA (acquired animals; FT [n=12] and SP [n=8]). Mean reinforcements between cue only and nicotine SA were significantly different. (*) p < 0.05, FT vs. SP, ($) p < 0.001, cue vs. nicotine. Error bars are expressed as standard error of the mean (SEM).
3.2.2. Self-administration behavior is not different between FT and SP rats
Figure 3b shows the pattern of active lever responding between FT and SP animals for both nicotine and cues only. During NSA, FT animals responded higher in the first few sessions which decreased to mean levels throughout the rest of FR1 while SP animals show increasing lever presses over the same sessions. FT active lever presses were significantly different from SP (F[1,18] = 5.154, p = 0.036) in the first (p = 0.004) and second (p = 0.011) sessions. There was no difference between FT and SP responding in FR2 and in either FR1 or FR2 in the cue only condition. There are no significant differences in the number of nicotine reinforcements or total nicotine intake between FT and SP rats that met acquisition criteria by the end of FR2 (Figure 3c, d). All FT animals met acquisition criteria while only a portion (8 out of 14) of SP animals met criteria; therefore the data presented for FT is based on mean values from all FT animals but the data presented for SP is based on mean values from SP animals that met criteria. The 6 animals did not meet criteria mainly because responding on both active and inactive levers was the same (mean = 45 ± 20 (SEM), both active and inactive lever presses, FR2). Four of these animals also displayed low and decreasing active lever presses as sessions continued (data not shown). Only one animal that responded for the cue alone met acquisition criteria so to compare responding for the cue alone vs. nicotine infusions, mean reinforcements (cue or nicotine) for all FT and SP animals are reported in figure 3c. The number of reinforcements obtained in responding for cue alone in FT and SP animals was not significantly different (F[1,22] = 0.79, p = 0.38 for FR1 and F[1,22] = 2.09, p = 0.16 for FR2). In fact the number of reinforcement obtained for cue alone is about 12% of the number of reinforcements obtained in comparison to animals responding for nicotine.
3.2.3. Final ratio completed in PR was not different between FT and SP rats
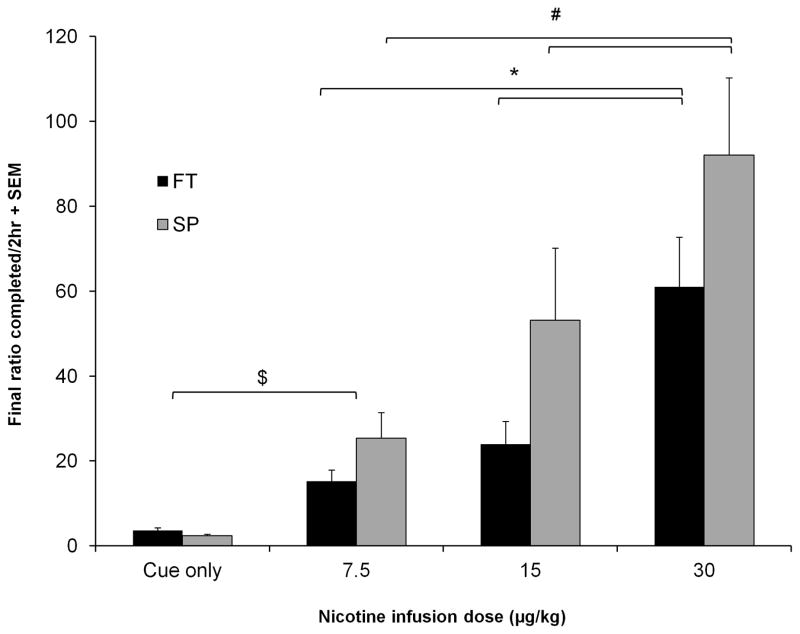
FT and SP rats that acquired NSA underwent PR sessions after FR2 sessions were completed. A lack of catheter patency tested at the end of FR2 reduced the number of animals to 8 for FT and 8 for SP. The mean final completed ratio for FT and SP rats under PR at each nicotine dose are presented in figure 4. Similar to the results for FR, final ratios completed for the cue only condition include data from all animals. The majority of animals in FT and SP groups reached the operational criterion for breaking point (>20 inactivity resulting in shutdown of the session) at 7.5 μg/kg but they did not at the 15 and 30 μg/kg doses. The data at these higher doses are comparable between FT and SP because both groups did not reach this shutdown point. Repeated measures ANOVA with training paradigm as a between subjects factor for animals that underwent NSA revealed no difference between FT and SP animals (p = 0.132). There was a significant difference between doses (F[2,28] = 35.411, p < 0.001) where final ratios completed were significantly different between all doses: 7.5 μg/kg vs. 15 μg/kg (p = 0.027), 7.5 μg/kg vs. 30 μg/kg (p < 0.001), and 15 μg/kg vs. 30 μg/kg (p < 0.001). There was also little responding for cue alone in either FT or SP animals. In the cue only condition (Figure 4) the final ratio completed was 3 for FT and 2 for SP animals, while the final ratio completed at the lowest nicotine infusion dose (7.5 μg/kg) was significantly higher than the cue only condition (15 for FT and 25 for SP animals; cue vs. nicotine, F[1,39] = 51.7, p < 0.001). Separate analysis of FT and SP animals revealed dose-dependent increases in the final ratios completed for both FT animals (F[2, 14] = 22.813, p < 0.001) and SP animals (F[2, 14] = 14.415, p < 0.001) that self-administered nicotine (Figure 4). Post-hoc pairwise comparisons with Bonferroni correction show that the final ratio completed between 7.5 μg/kg vs. 30 μg/kg and 15 μg/kg vs. 30 μg/kg was significantly different (p < 0.001 and p = 0.004, respectively) in FT animals. The final ratios completed between 7.5 μg/kg vs. 15 μg/kg trended toward significance (p = 0.060) and was significantly different between 7.5 μg/kg vs. 30 μg/kg and 15 μg/kg vs. 30 μg/kg (p = 0.015 and p = 0.019, respectively) in SP animals.
Fig. 4.
Final ratios completed under the PR schedule were not different between FT and SP animals. This was observed in both cue only (all animals [n=12]) and nicotine infusion self-administration conditions (shown for acquired animals; FT and SP [n=8]). Within training paradigm, significant dose-dependent differences in final ratio completed were found. Mean values for each dose group were calculated using mean values of the last 3 sessions for each animal. Mean inactive lever presses (± SEM) for the last 3 sessions in FT animals were 15 ± 3 for 7.5 μg/kg, 16 ±5 for 15 μg/kg, and 41 ± 10 for 30 μg/kg; in SP animals they were 19 ± 7 for 7.5 μg/kg, 30 ± 10 for 15 μg/kg, and 48 ± 12 for 30 μg/kg. Error bars are expressed as standard error of the mean (SEM). p<0.05 for nicotine doses within FT (*) and SP (#) groups, p < 0.001 between cue only vs. nicotine SA ($)
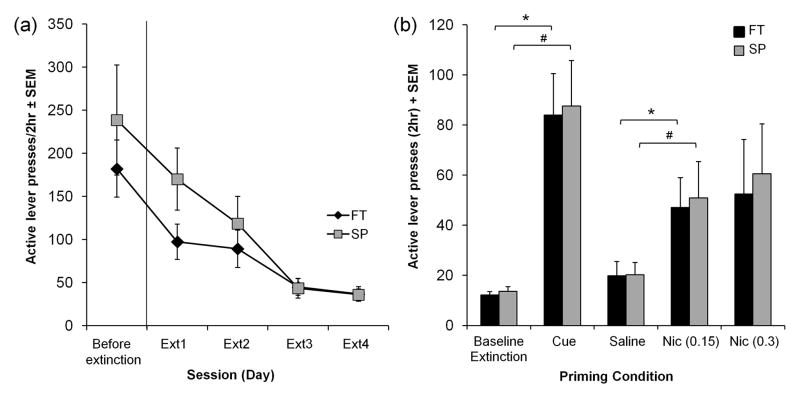
3.2.4. Extinction and reinstatement was not different between FT versus SP rats
Figure 5a shows active lever presses for all rats during the last FR2 session where they received nicotine and then first four extinction sessions. The first four extinction sessions are shown because from extinction session 5 and onwards, rats began to meet extinction criteria and were subsequently tested for reinstatement. All animals underwent extinction and reinstatement, therefore extinction data was analyzed with a repeated measure ANOVA. There was a significant effect of Session (F[1.6, 26.5] = 25.296, p<0.001), as responding decreased over days, but neither the effect of training condition (F[1, 17] = 1.15, p = 0.3) nor the Session x Training interaction (F[1.6, 26.5] = 3.467, p = 0.056) were significant. The number of sessions required to reach extinction criteria was also assessed between FT and SP rats, where FT rats averaged 10 ± 1 sessions and SP rats averaged 14 ± 3 sessions (t[9.533] = −1.155, p = 0.276).
Fig. 5.
There was no difference in extinction and reinstatement between FT and SP animals (n=8 per group). (a) Mean active lever presses during extinction sessions with no cues were similar between FT and SP animals. The first four extinction sessions are shown. Mean inactive lever presses (± SEM) for these four extinction sessions are 17 ± 5, 20 ± 6, 10 ± 3, 9 ± 3 for FT and 18 ± 5, 18 ± 4, 14 ± 5, 12 ± 5 for SP. (b) Mean active lever presses at baseline (the last two extinction sessions for each animal), and during the cue, saline, and two nicotine priming sessions are shown. Mean responding was similar for cue- and nicotine-induced reinstatement (Nic: 0.15 and 0.3 mg/kg/sc) in both training groups. Mean inactive lever presses (± SEM) in FT were 6 ± 2 for baseline, 11 ± 3 for cue, 8 ± 2 for saline, 10 ± 3 for Nic (0.15 mg/kg) and 18 ± 6 for Nic (0.3 mg/kg). Mean inactive lever presses (± SEM) in SP were 3 ± 1 for baseline, 8 ± 2 for cue, 5 ± 2 for saline, 22 ± 4 for Nic (0.15 mg/kg) and 20 ± 7 for Nic (0.3 mg/kg). Error bars are expressed as standard error of the mean (SEM). p<0.05, cue vs. baseline extinction and saline vs. 0.15 mg/kg nicotine for FT (*) and SP (#) rats
Figure 5b shows that both FT and SP rats reinstated nicotine-seeking behaviour in response to exposure to the compound cue previously associated with nicotine infusions. Repeated measures ANOVA showed that active lever presses in response to cues were significantly higher than during extinction (F[1,17] = 34.671, p < 0.001). Training paradigms as a between subjects factor in repeated measures ANOVA showed there was no difference between FT and SP rats.
Animals then underwent further extinction sessions until they met extinction criteria and nicotine-induced reinstatement was tested. Responding after a saline s.c. injection, a baseline for nicotine priming injections, did not differ between FT and SP animals.
Responding after nicotine priming s.c. injections (saline, 0.15 and 0.3 mg/kg) were analyzed by repeated measures ANOVA, where there was a significant difference in responding after the 3 s.c. injections (F[2, 34] = 3.54, p = 0.026). Post-hoc pairwise comparisons with Bonferroni correction revealed that responding after the 0.15 mg/kg nicotine priming dose was significantly higher than responding after saline (p = 0.02). Training paradigm was a between subjects factor and there was no difference between FT and SP animals (Figure 5b).
4. Discussion
Consistent with earlier studies with nicotine [11] and other drugs of abuse [19–23], the result of the present study confirm and further extend the finding that prior training with another reinforcer (food) can facilitate acquisition of NSA. One significant finding from the present work is that prior FT resulted in a greater proportion of animals achieving the criteria for NSA in comparison to spontaneous acquisition. The increase in acquisition of NSA by prior food training is dependent on the training dose employed, with a more pronounced effect at low training doses (3.75–7.5 μg/kg) but little influence at higher infusion doses of nicotine. Of note, this occurred both when the duration of training sessions differed or was identical at the 7.5 μg/kg infusion dose where acquisition differences were the greatest between FT and SP rats, suggesting that the increases acquisition were specific to food training at this lower nicotine infusion dose, while food training did not have an effect on higher nicotine doses. Rats that acquired NSA at the lowest infusion dose tested, 3.75 μg/kg, showed higher mean reinforcements with FT compared to SP, which further supports the idea that FT has a greater influence on NSA at low infusion doses. Also of relevance is that food training did not influence the acquisition of responding for the visual and auditory cues that are paired with NSA, as most rats did not meet criteria for acquisition in either the FT or SP groups responding for the cues alone and responding was not different between these two groups. A direct comparison of the effect of food training and spontaneous acquisition on NSA at an infusion dose of 30 μg/kg has been examined earlier using both lever press and nose poking [11]. Food training facilitated acquisition of NSA but the number of infusions subsequently obtained at FR5 was not different from that of spontaneously acquired animals. Shoaib et al. [8] and Shram et al. [7] have also reported dose-dependent increases in the proportion of rats that acquired NSA; however the lowest dose used in both of these studies was 15μg/kg. In our study, we provide information on the dose-response curve for acquisition and show the effect of food training on multiple infusion doses. We demonstrate that the proportion of rats that acquire NSA at the lower infusion doses (3.75 and 7.5 μg/kg) is increased by prior food training, illustrating a shift in dose response between food training and spontaneous acquisition. Consistent with this, dose-dependent increases in acquisition have been observed for cocaine and amphetamine using doses along the ascending limb of their dose-response curves [24, 25]. On the other end of the spectrum, food training does not affect acquisition at the higher nicotine infusion doses as most rats acquired NSA without food training, indicating that food training is not necessary to induce stable and reliable NSA at these higher doses of nicotine.
Nicotine has dual reinforcing effects whereby nicotine has a direct reinforcing effect on its own, as well as a reinforcement enhancing effect [3–5]. Stimuli such as visual cues [5, 26, 27] or white noise [16] can function as weak reinforcers as animals will lever press for them. Importantly, nicotine has been shown to enhance such responding. Our control study indicated that rats did not acquire responding for the compound cue (light+tone) alone, a combination which was employed in the nicotine studies both under spontaneous acquisition and following food training. While this data cannot differentiate between the direct reinforcing and the reinforcement enhancing effect of nicotine, it rules out the possibility that responding for nicotine under our experimental conditions, particularly for the low infusion doses of nicotine, is due to the reinforcing effect of the compound cue alone. In a recent study, Peartree et al. [12] showed that rats can acquire NSA using infusion doses ranging from 15–60 μg/kg without prior food training or stimuli associated with nicotine delivery. Food trained animals that acquired NSA achieved similar total mean nicotine reinforcements and thus self-administered comparable levels of nicotine. These data are in agreement with previous findings showing that food training does not influence the number of infusions or amount of nicotine intake after animals acquired stable NSA [11].
Food trained animals appeared to have greater motivation to obtain nicotine compared to spontaneously acquired animals when they were compared in separate experiments. It is possible that food training could have made the cues more salient or created a food-chamber association. Food training can increase responding for nicotine and even saline in the presence of cues [11, 28]. However, cues were not present during food training and we found no differences between FT and SP animals’ motivation to self-administer nicotine in the head to head experiment. There was no difference in responding between FT and SP animals exposed to the cue only condition, which suggests that food training alone cannot explain the observations made in these experiments. Also, in the head to head experiment, FT animals show a reduction in active lever presses during FR1 that stabilize in FR2, which suggests that any association with food may have been gone by the end of this schedule. Food trained animals had less time to self-administer nicotine in the separate experiments so it is possible that session duration in addition to food training resulted in more motivation to respond and thus reach higher final ratios completed during the PR sessions. It is interesting to note that the opposite was found in the mean final ratios for SP animals: Mean final ratios were higher in SP than in FT animals in the head to head experiment, which are the opposite findings in the separate experiment, although this did not reach statistical significance. While all animals at the 7.5μg/kg infusion dose reached their breakpoint prior to the end of the 2 hour session, some rats did not at the two higher doses (15 and 30 5μg/kg). Our PR sessions were 2 hours long so it is possible that longer PR sessions might yield higher final ratios. Nevertheless, our PR findings from the separate and head to head experiments are still comparable because they were collected under the same session length.
The effect of FT on PR responding in self-administration has not been previously investigated. Other factors, such as pre-treatment with the self-administered drug prior to acquisition, can increase PR breakpoints (final ratio completed) for amphetamine [29, 30]. Two studies that directly compared food training to spontaneous acquisition only tested extinction and reinstatement in nicotine and cocaine-trained animals [11, 23]. Clemens et al. [11] found no effect of FT on extinction but observed higher responding to the compound cue and nicotine (0.3 mg/kg/s.c.) in animals that were food trained. Bongiovanni and See [23] also did not observe an effect of food training on cocaine extinction or reinstatement. Our study indicated that there were no differences in extinction and reinstatement between animals that were food or spontaneously trained which is in agreement with these previous findings.
We initially expected food training to impact cue-induced reinstatement as Clemens et al. [11] reported greater reinstatement to a visual cue in animals that were food trained compared to animals that spontaneously acquired. It is possible that our low training dose was not as reinforcing as the dose used in Clemens et al. [11] study, which is higher and commonly used to establish NSA (30 μg/kg). Food training did not affect cue reinstatement for cocaine in Bongiovanni and See [23], however they only tested one infusion dose; together this suggests that the effect of food training on cue-induced reinstatement may be training dose dependent. These results indicate that once acquisition has been established and animals are tested for motivation and drug-seeking behaviour, prior food training might not influence responding when low training doses of nicotine are used.
The present findings were limited in that data from separate experiments and could not be directly compared. There were also parametrical differences between food trained and spontaneously acquired animals in addition to the food training manipulation, such as different animal cohorts, different session lengths and the number of consecutive sessions conducted under FR training. In contrast, in the head to head experiment, once a dose which showed differences in acquisition was identified, all animals received it, allowing for direct determination of the effects of food training on acquisition and drug-seeking behavior. We also acknowledge that the findings reported are limited to the acquisition criteria used, as there are no standard criteria for nicotine self-administration and criteria can vary between studies in the literature. It is important to note, however, that when we compare animals that did not meet our criteria compared to animals that did, the acquisition curves do not show increasing trends in responding or a preference for the active lever, two criteria which are characteristic of SA acquisition. This gives us some confidence in our criteria, at least for comparing our findings from the separate cohorts and the head to head experiment.
Our results demonstrate that there is an ascending dose-response curve for acquisition of NSA and that nicotine infusion doses lower than doses at the peak of the curve commonly used in NSA (15–30 μg/kg) can elicit self-administration behaviour in rats. These findings are important to consider when investigating acquisition in animal models of vulnerable populations recognized in humans, such as in animals selected for impulsivity or on other traits, where acquisition differences can be tested at infusion doses where only a portion of animals would acquire [31, 32]. Training on peak doses establishes self-administration in most animals, which cannot provide information about the factors that would promote acquisition due to ceiling effects on the proportions of animals that acquire self-administration. The number of animals that acquired NSA at the lowest infusion dose tested (3.75 μg/kg) was low but these individual animals did respond for nicotine and met criteria regardless of FT or SP training, which along with the number that acquired NSA at 7.5 μg/kg, shows that (1) NSA can be established at very low nicotine doses (< 15 μg/kg) and (2) biological factors are involved in the vulnerability to nicotine dependence. Lower nicotine training doses may be more relevant to the human condition, as they are closer in level of nicotine intake concentration to puffs of a cigarette rather than levels occurring following the smoking of an entire cigarette [16, 33]. This is important when interested in acquisition as the infusion doses commonly used to establish NSA (15 and 30 μg/kg) are closer to the nicotine content of one whole cigarette, so small unit doses, analogous to doses from individual puffs of a cigarette, may better model human acquisition of smoking, particularly among children and/adolescents.
Taken together, these results show that the combination of food training and training dose can influence different aspects of nicotine intake and seeking. When investigating acquisition, food training can have a significant impact at nicotine training doses that fall along the ascending limb of nicotine’s dose-response curve. Also, nicotine’s dose-response curve is relatively flat but with spontaneous acquisition dose-dependent increases were observed at these training doses. These differences in acquisition at lower doses can be used to more accurately address nicotine’s reinforcing efficacy without the effect of prior reinforcers and to determine the influence of other factors on the parameters of acquisition, such as the number that acquire NSA and the rate of acquisition. When investigating motivation and drug-seeking behaviour, food training does not appear to affect these behaviours, at least when using training doses of nicotine lower than the peak doses for establishing NSA. Therefore, both food training and nicotine training dose are important factors to consider in the design of NSA experiments as they can have separate and combined impacts on the behavioral outcomes of these studies.
Supplementary Material
Highlights.
A nicotine dose-response curve acquisition without prior food training was reported
Influence of food training on acquisition greater at low nicotine doses
Low nicotine doses were nicotine self-administered following food training
Food training increases the proportion of rats that acquire self-administration
Food training did not influence subsequent behaviors after acquisition
Acknowledgments
We acknowledge the support of an Endowed Chair in Addictions (RFT), CIHR grant MOP97751, NIH NIDA R21 DA029160, CAMH, Canada Foundation for Innovation (#20289 and #16014), and the CAMH Foundation and the Ontario Ministry of Research and Innovation.
Abbreviations
- NSA
nicotine self-administration
- i.v
intravenous
- s.c
subcutaneous
- FR
fixed ratio
- PR
progressive ratio
- FT
food trained
- SP
spontaneous acquisition
Footnotes
Conflicts of interest
Dr. Tyndale has acted as a consultant to several pharmaceutical companies, primarily concerning smoking cessation medications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 2.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 3.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–30. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 4.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 6.Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–4. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- 7.Shram MJ, Li Z, Le AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl) 2008;197:45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- 8.Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- 9.Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A) Neuropharmacology. 2008;55:712–6. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P. Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2009;19:487–98. doi: 10.1016/j.euroneuro.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Clemens KJ, Caille S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- 12.Peartree NA, Sanabria F, Thiel KJ, Weber SM, Cheung THC, Neisewander JL. A new criterion for acquisition of nicotine self-administration in rats. Drug Alcohol Depend. 2012;124:63–9. doi: 10.1016/j.drugalcdep.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Matta SG, Sharp BM. Acquisition of Nicotine Self-Administration in Adolescent Rats Given Prolonged Access to the Drug. Neuropsychopharmacology. 2006;32:700–9. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 14.Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. British Journal of Pharmacology. 1984;83:49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- 16.Sorge RE, Clarke PBS. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther. 2009;330:633–40. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- 17.Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–48. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- 18.Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, et al. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–42. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- 19.Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–58. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- 20.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 21.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–9. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- 22.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–6. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–33. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther. 1978;204:547–57. [PubMed] [Google Scholar]
- 25.Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–14. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- 26.Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- 27.Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behav Pharmacol. 2012;23:781–9. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12:1355–66. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- 29.Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–22. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- 30.Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 31.Granö N, Virtanen M, Vahtera J, Elovainio M, Kivimäki M. Impulsivity as a predictor of smoking and alcohol consumption. Personality and Individual Differences. 2004;37:1693–700. [Google Scholar]
- 32.Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.