Abstract
Objective
To determine if increasing the hormone dose or eliminating the hormone-free interval improves key pharmacokinetic (PK) alterations caused by obesity during oral contraceptive (OC) use.
Study design
Obese (BMI ≥ 30 kg/m2), ovulatory, otherwise healthy, women received an OC containing 20 mcg ethinyl estradiol (EE)/100 mcg levonorgestrel (LNG) dosed cyclically (21 days active pills with 7-day placebo week) for two cycles and then were randomized for two additional cycles to: Continuous Cycling [CC, a dose neutral arm using the same OC with no hormone-free interval] or Increased Dose [ID, a dose escalation arm using an OC containing 30 mcg EE/150 mcg LNG cyclically]. During Cycle 2, 3, and 4, outpatient visits were performed to assess maximum serum concentration (Cmax), area under the curve (AUC0-∞), and time to steady state as well as pharmacodynamics. These key PK parameters were calculated and compared within groups between baseline and treatment cycles.
Results
A total of 31 women enrolled and completed the study (CC group n = 16; ID group n = 15). Demographics were similar between groups [mean BMI: CC 38kg/m2 (SD 5.1), ID 41kg/m2 (SD 7.6)]. At baseline, the key LNG PK parameters were no different between groups; average time to reach steady-state was 12 days in both groups; Cmax were CC: 3.82 ± 1.28 ng/mL and ID: 3.13 ± 0.87 ng/mL; and AUC0-∞ were CC: 267 ± 115 hr*ng/mL and ID: 199±75 hr*ng/mL. Following randomization, the CC group maintained steady-state serum levels whereas the ID group had a significantly higher Cmax (p< 0.001) but again required 12 days to achieve steady-state. However, AUC was not significantly different between CC (412 ± 255 hr*ng/mL) and ID (283 ± 130 hr*ng/mL). Forty-five percent (14/31) of the study population had evidence of an active follicle-like structure prior to randomization and afterwards this decreased to 9% (3/31).
Conclusion
Both increasing the OC dose and continuous dosing appear to counteract the impact of obesity on key OC PK parameters.
Keywords: BMI, drug levels, hormonal contraception, continuous-dosing
Introduction
Obesity impacts the pharmacokinetic (PK) parameters of contraceptive steroids; specifically, half-life, clearance, area under the curve, and time to achieve steady-state [1, 2]. These PK indices are considered critical indicators of drug therapeutic performance [3]. Whether the observed alterations in PK parameters observed in obese women translate into clinical evidence of failure (i.e. pregnancy) is controversial, but observed PK changes are concerning and may magnify the effects of poor pill compliance in this population [4]. Although it is tempting to simply recommend alternative forms of birth control to bypass both PK and compliance issues, oral contraceptives (OC) remain the most popular form of contraception [5] and a woman’s individual preference influences her compliance and continuation with any method [6]. Moreover, since obese women have been largely excluded from premarketing evaluations of the newest low dose formulations of OCs, we have insufficient information available to assess efficacy in this population.
Strategies to normalize PK parameters should improve effectiveness whether the mechanism of failure is altered drug metabolism or poor compliance. We have shown that the most likely ‘window for failure’ for obese OC users is with pill initiation or following the 7-day hormone-free interval due to a delay in achieving steady-state because of changes in contraceptive steroid clearance and not volume of distribution [1]. Following a 7-day hormone-free interval, women of normal BMI achieve a steady-state level of levonorgestrel within 5 days whereas obese women take twice as long [1]. We hypothesized that two readily available strategies: eliminating the hormone-free interval (continuous cycling) or using a higher dose pill cyclically (increased dose) might have the potential to counteract this obesity-related change in OC PK. This study was designed to determine if increasing the hormone dose or eliminating the hormone-free interval resolves the impact of obesity on PK and improves end-organ suppression.
Materials and Methods
A prospective randomized study was conducted at Oregon Health & Science University (OHSU) in Portland, Oregon from January 2010 to June 2011. The OHSU Institutional Review Board and OHSU Clinical & Translational Research Institute (OCTRI) approved the study protocol and all subjects underwent informed written consent.
Otherwise healthy, obese (BMI ≥30 kg/m2) reproductive-aged (18–35 years old) women, not currently using hormonal contraception but seeking to initiate combination oral contraceptives, were recruited. Inclusion and exclusion criteria included regular menstrual cycles, not actively seeking weight gain or loss, no evidence of anemia (hematocrit ≥ 36%), no contraindications to hormonal contraception, no use of tobacco or drugs known to interfere with the metabolism of sex steroids, and no overt clinical features of or prior treatment for metabolic disorders (i.e. polycystic ovarian syndrome, diabetes).
In addition to baseline demographic information, several obesity biomarkers including weight, height, and body composition measurements by air displacement plethysmography were collected. A blood sample was obtained to measure progesterone (P) levels during the luteal phase of the pretreatment cycle to confirm ovulation. A value of ≥ 3 ng/mL was required for enrollment.
All qualifying study subjects were placed on a combination monophasic birth control pill containing 20 mcg ethinyl estradiol/100 mcg levonorgestrel (Aviane; Teva; Israel) at the onset of menses following the pre-treatment cycle. The medication was dosed in a cyclic fashion (21 days active pill with a 7-day hormone-free interval) for a total of two treatment cycles [7]. Randomization to study groups occurred after the completion of these two baseline cycles. Women were then randomized to one of two arms for another two cycles: Continuous Cycle [CC, a dose neutral arm using the same OC with no hormone-free interval] or Increased Dose [ID, a dose escalation arm using an OC containing 30 mcg EE/150 mcg LNG cyclically] (Portia; Barr Laboratories; USA). Subjects were randomized to treatment group by the OHSU Research Pharmacy using a predetermined computer-generated randomization scheme and were allocated using sequentially numbered, opaque, sealed envelopes. Once randomized, women and study staff were not blinded to group allocation. The randomization scheme was provided to the primary investigator after enrollment and data entry were completed.
Women were instructed to take each pill at 9:00 AM daily. Self-reported compliance with the medication was recorded on a calendar (compliant cycle ≤ two late and/or missed pills during 1 cycle) and confirmed based on OC serum levels (nonuser: all LNG values < 0.16 ng/mL, inconsistent user: two or more values < 1.0 ng/mL, consistent user: no more than one value < 1.0 ng/mL) [8].
To determine baseline PK parameters of EE and LNG, serial serum samples were collected during a clinical research inpatient stay beginning on Cycle 1, Day 21 [last day of active pills, 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 hours], and continuing with daily samples on Cycle 1, Day 22, 23, 24, and 27 (e.g. hormone-free interval in Cycle 1). Outpatient Serum samples were obtained to compute the time to reach steady-state, maximum serum concentration (Cmax), and area under the curve (AUC0-∞); twice weekly during Cycle 2,3, and 4 as well as daily samples in Cycle 3 on Day 22, 23, and 24. Additional serum samples were obtained for FSH, LH, estradiol (E2) and P at these visits. Exact serum sampling time in relationship to pill ingestion was accounted for in the PK analysis. At each of these visits, a vaginal probe ultrasound (GE LOGIQ 400 Proseries ultrasound, 7.5-MHZ) was performed to monitor growth of ovarian follicles. The total number of antral follicles (≥4mm) was documented and the largest follicle on each ovary was measured in two dimensions. Cervical mucus was obtained from the endocervix using a Select endocervical aspirator (Select Medical Systems Inc., Williston, VT) and scored according to World Health Organization guidelines (0–4 poor, 5–8 fair, 9–12 good) [9].
Serum samples were assayed for LNG and EE by radioimmunoassay (RIA) with preceding organic solvent extraction and Celite column partition chromatography at the Reproductive Endocrine Research Laboratory, University of Southern California, Los Angeles, CA. These purification steps remove potential interfering conjugated and unconjugated metabolites of EE, NET and endogenous steroids. Use of highly specific antisera in the RIAs adds to their specificity. The sensitivities of these RIAs are 0.05 ng/mL for LNG and 15 pg/mL for EE. Intra-assay and inter-assay coefficients of variation are 4.4% and 8.9% for the LNG RIA and 6.9% and 11% for the EE RIA, respectively. FSH, LH, estradiol (E2) and P assays were performed at the Endocrine Technology Services Core Laboratory (ETSL) at the Oregon National Primate Research Center (ONPRC, Beaverton, Oregon) using an automated Immulite 2000 chemiluminescent immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL 60015, USA). The assay sensitivity of E2, P, FSH, and LH assays are 20 pg/mL, 0.2 ng/mL, 0.1 mIU/mL, and 0.1 mIU/mL respectively.
PK parameter analysis
Baseline LNG and EE PK data were analyzed separately by noncompartmental methods using WinNonLin (v 5.2; Pharsight, Moutain View, CA). LNG and EE Cmax and time to maximum concentration (Tmax) were observed values. AUC was calculated from time zero to 168 hours (AUC0-t) using the linear trapezoidal rule and then extrapolated to infinity (AUC0-∞). Drug half-life (t1/2), oral clearance (CL/F), and volume of distribution (VD/F) were estimated using standard pharmacokinetic calculations (t1/2= 0.693/λz, CL= dose/AUC0-∞; VD= CL//λz) where λz is the terminal elimination rate constant. Additionally, the time to reach steady-state is an observed value derived from plotting the LNG and EE plasma concentration (Cycles 2 and 4). During Cycle 4, AUC between days 20 and 26 (AUC20–26) was computed using the linear trapezoidal rule.
Sample size
We assumed that the interventions should normalize the LNG AUC (the major component in calculating t1/2, CL, and time to steady-state) in an obese woman to that of a normal BMI woman. Based on our previous data, 15 subjects per group would provide 99% power to detect a difference of 112 pg hr/mL in LNG AUC [1] between treatment arms with an alpha=0.05. To accommodate for drop out we planned to enroll 32 subjects in total.
Statistical Analysis
Descriptive statistics were generated and compared for demographics and baseline PK parameters between treatment groups. Key PK parameters were then compared within groups between Cycle 2 (baseline) and Cycle 4 (on treatment). Secondary outcomes included comparisons of end-organ activity within groups between baseline and treatment. Increased end-organ activity or ‘active follicle-like structure’ was defined as a follicle of ≥10 mm, cervical mucus scores ≥5, E2>75 pg/mL and/or P > 3 ng/mL. Based upon prior observations of obese and normal weight women followed in a similar protocol, the reference LNG AUC for normal BMI women from our prior work is 112.1 ± 84.3 h*ng/mL[1].
Results
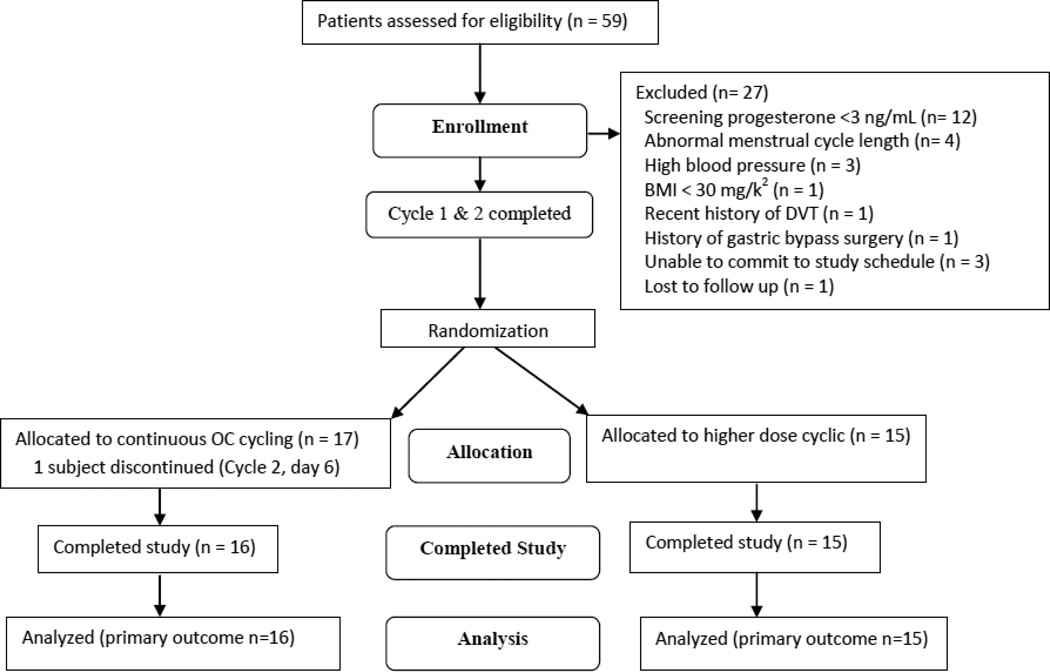
Thirty-two subjects signed informed consent (CC n = 16; ID n = 15) and met eligibility criteria (1 subject discontinued Cycle 2, day 6) (Figure 1). No differences in demographics were found between groups except that more of the CC group had experienced a prior pregnancy (Table 1). There were no serious adverse events or pregnancies during the study period. All participants were found to be compliant with study medication serum levels (e.g. no subject had an on-treatment LNG level of < 1.0 ng/mL) except for one subject during Cycle 4 only who met criteria for an inconsistent user (CC group, BMI 38.5 kg/m2) via drug trough levels.
Figure 1.
Study flowchart (CONSORT)
Table 1.
Baseline demographics and pharmacokinetic parameters (mean ± standard deviation) of LNG and EE prior to randomization to study arms.
| CC group | ID group | |||
|---|---|---|---|---|
| Age | 30 (SD 4) | 27 (SD 4) | ||
| Race Non-Hispanic, Caucasian Other |
14 3 |
15 0 |
||
| Ever pregnant? (yes)** | 12 | 3 | ||
| BMI | 38.0 kg/m2 (SD 5.1) | 41.1 kg/m2 (SD 7.6) | ||
| % body fat | 45.5 (SD 3.5) | 47.3 (SD 4.1) | ||
| PK Parameters | LNG | EE | LNG | EE |
| Tmax (hr) | 1.86 ± 0.85 | 1.91 ± 1.10 | 2.28 ± 1.39 | 1.61 ± 1.10 |
| Cmax (ng/mL) | 3.82 ± 1.28 | 0.12 ± 0.04 | 3.13 ± 0.87 | 0.10 ± 0.03 |
| AUC(0-∞) (hr*ng/mL) | 267 ± 115 | 10.4 ± 4.33 | 199 ± 75.0 | 10.6 ± 4.49 |
| T1/2 (hr) | 70.7 ± 50.4 | 158 ± 81.1 | 59.4 ± 26.6 | 185 ± 99.6 |
| Vd/F (L) | 43.5 ± 29.2 | 454 ± 186 | 46.7 ± 23.4 | 482 ± 121 |
| CL/F (L/hr) | 0.47 ± 0.28 | 2.29 ± 0.99 | 0.57 ± 0.19 | 2.17 ± 0.85 |
denotes p value < 0.05
Pharmacokinetics and pharmacodynamics
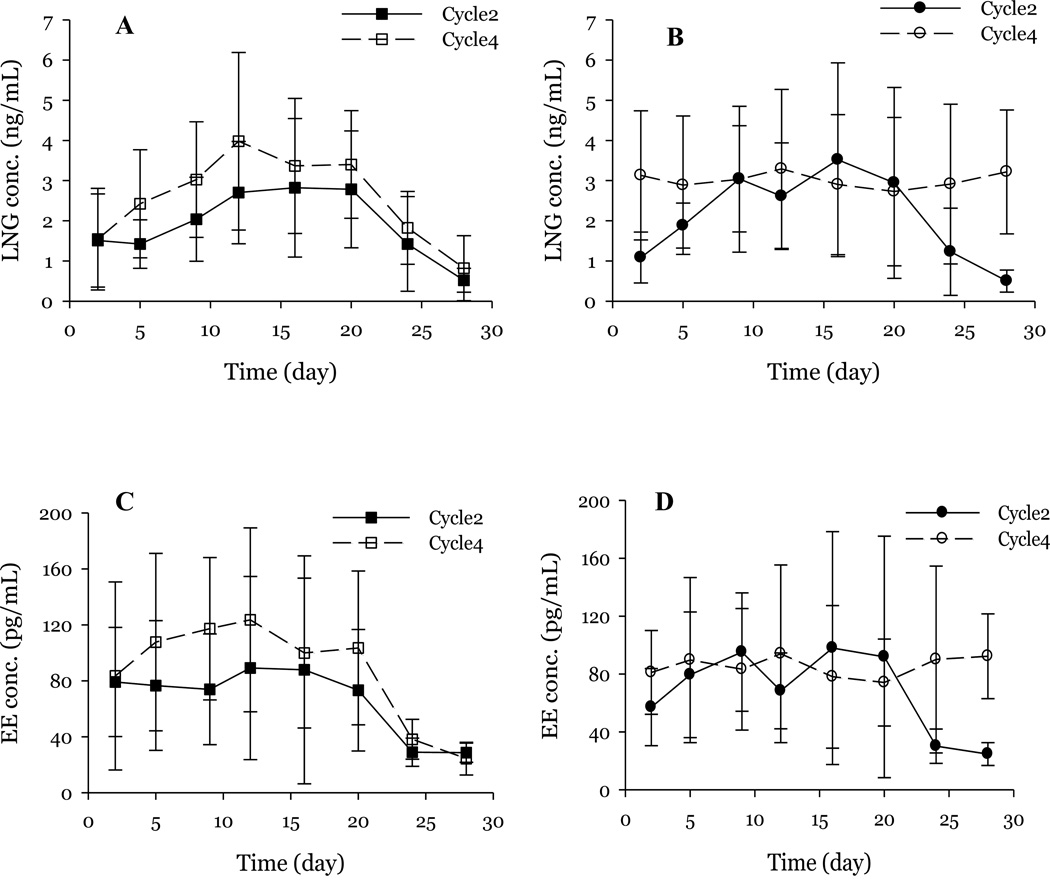
There were no significant differences in the baseline cycle LNG or EE PK parameters between groups prior to randomization (Table 1). The observed average time to reach steady-state of LNG at baseline was 12 days in both groups and the steady-state concentrations at baseline were similar (CC: 3.82 ± 1.28 ng/mL; ID: 3.13 ± 0.87 ng/mL). Following randomization, the CC group maintained a steady plasma LNG concentration (3.01 ± 0.19 ng/mL) (Figure 2). On the other hand, women initiating the ID group still required 12.3 days (SD 2.7) to reach the new steady-state (Figure 2). Consistent with increased drug delivery, this new steady-state was significantly higher in the ID group (3.58 ± 0.35 ng/mL, p < 0.001) than in the lower dose CC group. However, the calculated AUC(20–26) was similar between the two treatment groups (CC: 412 ± 255 hr*ng/mL; ID: 283 ± 130 hr*ng/mL). As expected, the AUC within groups were significantly higher post-randomization as compared to baseline (CC: p < 0.001; ID: p <0.01). A comparison of AUC during days 0 and 26, demonstrated no difference between groups (CC: 1716 ± 930 ng.hr/mL; ID: 1632 ± 643 ng.hr/mL). In summary Figure 2 illustrates that while the increased dose results in higher concentrations, the time to reach steady state is not affected. In contrast, the continuous dosing eliminates the drop in drug levels associated with the hormone free interval.
Figure 2.
LNG and EE concentrations at baseline while using 20mcg EE/100mcg LNG (closed squares/circles) and following randomization (open squares/circles). Panel A: LNG; Higher dose cyclic study group; Panel B LNG; Continuous OC study group; Panel C: EE; Higher dose cyclic study group; Panel D: EE; Continuous OC study group. While the increased dose results in higher concentrations, the time to reach steady state is not affected. In contrast, the continuous dosing eliminates the drop in drug levels associated with the HFI.
EE plasma concentrations reached steady-state earlier than that of LNG in both groups at baseline, with most women reaching steady-state by day 5. The steady-state concentrations at baseline were similar between groups (CC: 0.12 ± 0.04 ng/mL; ID: 0.10 ± 0.03 ng/mL) (Table 1). As expected, plasma concentrations declined during the hormone-free interval during Cycle 2 (days 21–28). However, unlike the slow decline observed for LNG, EE concentrations declined rapidly and reached baseline values by day 24. Consistent with the LNG data, upon randomization to treatment arms, the steady-state concentrations of EE in the ID group (111 ± 11.2 pg/ml) were significantly higher than that of low-dose continuous OC regimen (82.3 ± 8.6 pg/ml; p<0.001) (Figure 2) and the time to steady-state remained unchanged.
End-organ activity is described in Table 2. Over half of the study population had evidence of a follicular development (defined as E2>75 pg/mL + maximum follicular diameter >10 during the baseline cycle while on the cyclic low dose regimen (20 mcg EE/100 mcg LNG), and one subject demonstrated an elevation of P4 suggesting ovulation. Following randomization, both study arms experienced a decrease in follicular activity (Table 2). Further delineation of the end-organ activity in women with potential pre-ovulatory follicles (≥18mm) is shown in Table 3. Fewer subjects demonstrated follicle growth following randomization.
Table 2.
End-organ activity at baseline (Cycle 2) on a cyclic low dose regimen (20mcg EE/100mcg LNG) and following randomization to either a continuous low dose regimen or cyclic dosing with an increased dose of an OC (30mcg EE/150mcg LNG).
| Study group | CC group | ID group | ||
|---|---|---|---|---|
| Cycle | Cycle 2 | Cycle 4 | Cycle 2 | Cycle 4 |
| Maximum follicular diameter* | ||||
| ≥8 mm | 13 | 8 | 10 | 8 |
| ≥10 mm | 11 | 4 | 8 | 4 |
| ≥13 mm | 9 | 3 | 7 | 3 |
| ≥18 mm | 8 | 2 | 5 | 1 |
| Peak cervical mucus score | ||||
| 0–4 | 9 | 8 | 8 | 11 |
| 5–8 | 6 | 6 | 7 | 3 |
| 9–12 | 1 | 2 | 0 | 1 |
| E2 > 75pg/mL | 10 | 2 | 6 | 3 |
| Progesterone >3ng/mL | 1 | 0 | 0 | 0 |
| Active follicle-like structure** | 9 | 2 | 5 | 1 |
Numbers are cumulative
E2>75pg/mL + Maximum follicular diameter >10
Table 3.
End-organ activity in subjects* with follicles ≥18mm in either Cycle 2 and/or Cycle 4. Maximum value for each parameter during the Cycle.
| Study Group | Cycle 2 (Baseline) | Cycle 4 (after randomization) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follicle size (mm) |
E2 >75pg/mL |
P ng/mL |
LH mIU/mL |
FSH mIU/mL |
Cervical mucus score |
Follicle size (mm) |
E2 >75pg/mL |
P ng/mL |
LH mIU/mL |
FSH mIU/mL |
Cervical mucus score |
|
| Cyclic | 27 | 192 | 0.22 | 8.39 | 8.19 | 5 | 7.60 | 28 | 0.19 | 6.77 | 6.47 | 5 |
| Cyclic | 50.9 | 619 | 2.23 | 10.62 | 5.80 | 6 | 13 | 55 | 0.63 | 8.08 | 8.87 | 3 |
| Cyclic | 9.14 | 58 | 0.77 | 12.07 | 7.85 | 6 | 25.54 | 160 | 0.80 | 4.97 | 6.06 | 9 |
| Cyclic | 18.1 | 87 | 0.38 | 8.95 | 15.35 | 4 | 8.50 | 70 | 0.35 | 7.14 | 5.87 | 4 |
| Cyclic | 31.6 | 679 | 0.83 | 13.54 | 5.18 | 5 | 9 | 55 | 0.50 | 7.30 | 5.51 | 4 |
| Cyclic | 18.25 | 105 | 0.47 | 11.41 | 8.24 | 3 | 10.40 | 53 | 0.55 | 5.28 | 5.63 | 4 |
| Continuous | 31.4 | 452 | 1.10 | 10.42 | 7.47 | 5 | 8 | 41 | 1.11 | 13.05 | 7.91 | 5 |
| Continuous | 26.2 | 481 | 0.29 | 5.95 | 8.63 | 2 | 15.80 | 78 | 0.39 | 9.34 | 8.38 | 6 |
| Continuous | 36.3 | 855 | 0.22 | 10.92 | 10.70 | 3 | 9.32 | 27 | 0.18 | 8.59 | 8.24 | 3 |
| Continuous | 37 | 581 | 0.74 | 8.09 | 4.15 | 5 | 12.00 | 29 | 0.59 | 5.53 | 5.02 | 5 |
| Continuous | 23.88 | 283 | 0.69 | 8.54 | 7.56 | 4 | 6.30 | 16 | 0.60 | 3.78 | 5.95 | 1 |
| Continuous | 36.4 | 750 | 0.58 | 7.64 | 10.40 | 6 | 31.90 | 615 | 0.54 | 14.33 | 9.22 | 4 |
| Continuous | 28.2 | 125 | 5.17 | 4.94 | 7.67 | 4 | 6.04 | 47 | 0.42 | 2.61 | 4.27 | 3 |
| Continuous | 20.34 | 145 | 0.37 | 10.48 | 9.95 | 5 | 21.84 | 44 | 0.40 | 9.02 | 12.24 | 3 |
each line represents a single subject’s values
Discussion
We studied two strategies for correcting an observed PK abnormality – prolonged time to reach steady state concentrations when starting the pill or following the hormone-free interval - in obese OC users. Continuous dosing with a very low dose pill (20mcg EE/100mcg LNG) eliminated the delay in time to reach steady-state while a slightly higher dosage pill (30mcg EE/150mcg LNG) used cyclically does not. Subjects using the 30mcg EE/150mcg LNG pill achieved significantly higher LNG and EE plasma steady-state levels, and by day 5 these levels were similar to those found in a reference group of normal weight women using a 20-mcg EE/100mcg LNG pill [1, 7] (Figure 2). In other words, increased dosing did not reduce the time to reach steady-state whereas continuous dosing, by the nature of the dosing scheme, is at steady-state throughout the dosing regimen. The measure of drug exposure (AUC20–26) was similar between the two treatment arms and significantly higher than at baseline which probably accounted for the improved suppression of end-organ activity (Table 3). At baseline, numerous women in both groups had evidence of end-organ activity with over half having follicles 8 mm or greater; both treatment regimens demonstrated greater suppression of the hypothalamic-pituitary-ovarian axis.
While this study is not powered to determine contraceptive failure (i.e. pregnancy), PK parameters are critical to drug performance and safety [10]. Hormonal contraceptive methods are dosed on a one-size-fits-all model which means that most users are exposed to a higher level than they might need but when does this model start to fail? At this point in time, we do not have a good understanding regarding what magnitude of change in the PK parameters is necessary to significantly impact OC efficacy or possibly impact safety. Both of the interventions appear to improve PK parameters and resulting PD outcomes but the CC arm accomplishes this with no increase in estrogen dose which theoretically could be safer in a population with an already elevated baseline risk of thromboembolic events.
Recently, Westhoff et.al proposed that the observed PK alterations in obese women have no clinical relevance as the plasma concentrations of LNG are well above the minimum therapeutic concentration [11]. However, the commonly referred to therapeutic concentration for LNG (0.4 ng/ml), is non-empirically derived from normal BMI women treated with an LNG-only formulation (Norplant® implant) [12]. Unlike Norplant, OCs include EE, a potent inducer of sex hormone-binding globulin (SHBG) which strongly binds LNG [13–15]. While LNG-only methods decrease plasma SHBG levels by ~50% from baseline, OCs increase SHBG by ~50% [13–15]. The role of obesity is complicated, as obesity is associated with a decrease in SHBG levels but also with alterations in drug transport [16]. As a result, the concentrations of unbound or active LNG are probably different between obese OC users and normal BMI women treated with an LNG-only method. Thus, we speculate that the ‘therapeutic’ target tissue concentration for LNG with combined OCs in obese users is not being achieved. Moreover, tissue concentrations may be different in obese women in important end organ sites such as the hypothalamus and in the cervix.
Studies of LNG-based emergency contraception (EC) provide some additional insights. EC is a single-dose treatment; reliant on achieving a certain peak level (influenced by drug clearance) at a critical time directly prior to ovulation. Recently, LNG-based emergency contraceptives have been shown to be less effective in obese women as compared to women of normal BMI (OR4.41, 95% CI 2.05–9.44) [17].
Strengths of our study included the proven treatment compliance of subjects documented through serum levels of LNG with only one inconsistent user identified. We reanalyzed our data excluding this participant and this had no impact on any of our key PK findings. Our study population also represented a broader range of obese BMI participants than previous publications [1, 11]. Furthermore, the interventions utilized in this study have scientific merit; large observational studies of shortening the hormone-free interval in a normal BMI population demonstrate increased OC efficacy [18] as well as smaller studies monitoring end-organ activity showing greater inhibition of the hypothalamic-pituitary-ovarian axis [19, 20]. We also obtained pharmacodynamic correlates of our PK profiles. However, potential weaknesses include the lack of a control group (normal BMI) and a small sample size that may not reflect important differences between different obesity phenotypes and surrogate endpoints (e.g. cervical mucus, ovulation) rather than pregnancy. Additionally, the CC group were of higher parity which could reflect a greater propensity for pregnancy; yet both groups had proven ovulatory cycles prior to study entry and both groups had similar PK and PD findings prior to group randomization, making it more likely that this was a personal choice rather than a physiologic difference.
Obesity clearly alters the PK of contraceptive steroid hormones but currently we have no way to predict who is at greatest risk for OC failure, as the degree or magnitude of obesity does not appear to correlate linearly with the amount of PK alteration [1, 7]. Epidemiologic and PK data support obesity-related effects on OC efficacy. Our findings provide some concrete and readily available clinical strategies for optimizing PK of OCs in obese users which theoretically should aid in maintaining contraceptive efficacy for these women. Unfortunately, neither strategy is useful for resolving the ‘window’ of opportunity for failure at the time of OC initiation. Continued work should be focused on identifying women who might be at greatest risk for contraceptive failure and identifying strategies that improve contraceptive efficacy, no what matter their weight is.
Implications.
Obesity adversely affects the pharmacokinetics of very low dose oral contraceptive pills. Although the impact of these changes on oral contraceptive efficacy is still under debate, pharmacokinetic parameters can be normalized in obese users by continuous dosing or increasing to a low dose pill.
Acknowledgements
Dr. Edelman: consultant for Gynuity Health Projects, Genzyme, and Agile Therapeutics. Nexplanon trainer for Merck. Author for UptoDate (Royalties received). She receives research funding from the National Institute of Health, and the Bill & Melinda Gates Foundation. These potential conflicts of interest have been reviewed and managed by OHSU. Dr. Jensen has received payments for consulting from Bayer Healthcare, Merck, Agile Therapeutics, HRA Pharma, and the Population Council, and for giving talks for Bayer and Merck. He has also received research funding from Abbott Pharmaceuticals, Bayer, the Population Council, the National Institute of Health, and the Bill & Melinda Gates Foundation. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Dr. Stanczyk is presently on the advisory board for Merck & Co., Inc. and Agile Therapeutics. Also, in the last few years he has consulted for Shionogi Inc. and Bayer Healthcare.
Financial Support: The authors acknowledge the grant support from National Institutes of Health (R01 HD061582-01 NICHD; 2K12HD043488 NICHD), the OHSU Oregon Clinical & Translational Research Institute (NIH NCRR 1 UL1 RR024120), and the Bioanalytical Shared Resource/Pharmacokinetics Core at OHSU
The authors would like to thank the Women’s Health Research Unit, the Department of Obstetrics & Gynecology, and the Oregon Clinical & Translational Research Institute at Oregon Health & Science University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Disclosures: Ms. McInnis and Drs. Munar and Cherala have no disclosures.
References
- 1.Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–127. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314–323. doi: 10.1016/j.contraception.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Rahman SM, Kauffman RE. The integration of pharmacokinetics and pharmacodynamics: understanding dose-response. Annu Rev Pharmacol Toxicol. 2004;44:111–136. doi: 10.1146/annurev.pharmtox.44.101802.121347. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2011 doi: 10.1016/j.contraception.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed 9/28/2012];Contraceptive use in the United States. 2012 Jul; (at http://www.guttmacher.org/pubs/fb_contr_use.html.)
- 6.Pinter B. Continuation and compliance of contraceptive use. Eur J Contracept Reprod Health Care. 2002;7:178–183. [PubMed] [Google Scholar]
- 7.Edelman AB, Cherala G, Munar MY, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception. 2012;87:220–226. doi: 10.1016/j.contraception.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westhoff CL, Torgal AH, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116:275–283. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 9.Organization WH. WHO laboratory manual for the examination of human sperm and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 10.Nicolau DP. Predicting antibacterial response from pharmacodynamic and pharmacokinetic profiles. Infection. 2001;29(Suppl 2):11–15. [PubMed] [Google Scholar]
- 11.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–480. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croxatto HB. NORPLANT: levonorgestrel-releasing contraceptive implant. Ann Med. 1993;25:155–160. doi: 10.3109/07853899309164160. [DOI] [PubMed] [Google Scholar]
- 13.Fotherby K. Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:203–215. doi: 10.2165/00003088-199528030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fotherby K. Pharmacokinetics of gestagens: some problems. Am J Obstet Gynecol. 1990;163:323–328. doi: 10.1016/0002-9378(90)90576-s. [DOI] [PubMed] [Google Scholar]
- 15.Sambol NC, Harper CC, Kim L, Liu CY, Darney P, Raine TR. Pharmacokinetics of single-dose levonorgestrel in adolescents. Contraception. 2006;74:104–109. doi: 10.1016/j.contraception.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S64–S70. doi: 10.1038/sj.ijo.0801281. [DOI] [PubMed] [Google Scholar]
- 17.Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117:33–40. doi: 10.1097/AOG.0b013e31820095a2. [DOI] [PubMed] [Google Scholar]
- 19.Willis SA, Kuehl TJ, Spiekerman AM, Sulak PJ. Greater inhibition of pituitary-ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100–103. doi: 10.1016/j.contraception.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.van Heusden AM, Fauser BCJM. Residual ovarian activity during oral steroid contraception. Hum Reprod Update. 2002;8:345–358. doi: 10.1093/humupd/8.4.345. [DOI] [PubMed] [Google Scholar]