Abstract
Approximately 20% of familial amyotrophic lateral sclerosis (FALS) cases are caused by mutant superoxide dismutase type 1 (mtSOD1). Although the mechanisms of mtSOD1-induced toxicity remain poorly understood, evidence suggests that accumulation of misfolded SOD1 is fundamental to its toxicity and the death of motor neurons. Misfolded mtSOD1 can accumulate inside the endoplasmic reticulum (ER), leading to ER stress, with activation of the unfolded protein response (UPR). We have previously carried out genetic studies focused on PERK (which is an eIF2α kinase that is rapidly activated in response to ER stress and leads to a repression in translation) and GADD34 (which participates in the dephosphorylation of eIF2α). We reported that mtSOD1 transgenic mice that are haploinsufficient for PERK have a significantly accelerated ALS disease, while mtSOD1 mice that are haploinsufficient for GADD34 have a remarkably ameliorated disease. Guanabenz, a centrally acting oral drug approved for the treatment of hypertension, enhances the PERK pathway by selectively inhibiting GADD34-mediated dephosphorylation of eIF2α. We have now treated G93A mtSOD1 transgenic mice with guanabenz and found a significant amelioration of disease with a delay in the onset and prolongation of the early phase of disease and survival. Guanabenz-treated G93A mice have less accumulation of mtSOD1 and an enhanced phosphorylation of eIF2α at endstage. This study further emphasizes the importance of the PERK pathway in the pathogenesis of FALS and as therapeutic targets in ALS, and identifies guanabenz as a candidate drug for the treatment of ALS patients.
Keywords: amyotrophic lateral sclerosis, superoxide dismutase type 1, unfolded protein response
Introduction
Approximately 10% of patients with amyotrophic lateral sclerosis (ALS) have an inherited disease known as familial ALS (FALS). The best-studied cause of FALS is mutant Cu/Zn superoxide dismutase (mtSOD1), which is associated with ∼20% of FALS cases. Compelling evidence indicates that mtSOD1 causes FALS because of a gain-of-function rather than a deficiency of SOD1 enzymatic activity (reviewed in (Renton et al., 2014)). Although the basis for toxicity of mtSOD1 is not clearly defined, it appears that aggregation of misfolded mtSOD1 is critically important to the pathogenesis of the disease. The finding that mutations in a number of genes that cause FALS function in protein degradation pathways provides additional support for the importance of protein misfolding in FALS. Of note, aggregation of misfolded proteins is also present in sporadic ALS, most typically TDP-43, as well as in a number of other neurodegenerative diseases.
A number of varied stresses can lead to the phosphorylation of serine 51 on eukaryotic translation initiator factor 2α (eIF2α), which is central to the integrated stress response (ISR). PKR-like ER-localized eIF2α kinase (PERK), which is activated by endoplasmic reticulum (ER) stress from misfolded or unfolded proteins in the ER, is one of four kinases that can carry out this phosphorylation, and thereby coordinate a cytoprotective response known as the ISR. PERK and two other sensors of ER stress, activating transcription factor 6 (ATF6) and inositol-requiring transmembrane kinase/endonuclease-α (IRE1), constitute important branches of the unfolded protein response (UPR). The UPR functions in protein quality control of the cell by refolding misfolded or unfolded proteins or translocating them from the ER to the cytosol for ER-associated degradation (ERAD) by the ubiquitin-proteasomal system. Although phosphorylated eIF2α (p-eIF2α) leads to repression of most protein synthesis, upregulation of ATF4 levels occurs. ATF4 expression leads to the expression of cytoprotective genes important in chaperone function, protein folding, redox homeostasis and protein degradation. ATF4 also activates transcription of growth arrest and DNA damage 34 (GADD34) protein, which is a stress-inducible regulatory subunit of holophosphatase that selectively dephosphorylates p-eIF2α, and CCAAT/enhancer-binding protein-homologous protein (CHOP), which has apoptotic activity. In the presence of excessive or sustained ER stress, activation of CHOP can change the normally protective ISR to one that leads to apoptosis. The other two branches of the UPR, ATF6 and IRE1, upregulate transcription of a number of genes, including those important in protein quality control.
The presence of misfolded proteins in FALS and the recognition that to a certain extent mtSOD1 is secreted (Kikuchi et al., 2006; Turner et al., 2005; Urushitani et al., 2008; Urushitani et al., 2006) suggested that the UPR may be involved in the disease's pathogenesis (reviewed in (Matus et al., 2011; Nassif et al., 2010)). This possibility has garnered support from a number of studies that have demonstrated markers of the UPR and ISR in tissues from FALS models (Kikuchi et al., 2006; Saxena et al., 2009; Wate et al, 2005) as well as from patients with FALS and sporadic ALS (Ilieva et al., 2007).
We have used a genetic approach to investigate the role of the UPR and ISR in mtSOD1-induced FALS transgenic mice. We initially focused on the PERK pathway because it reacts early to ER stress and has a substantial effect on global protein synthesis. Our data showed that mtSOD1 transgenic mice with a diminished capacity to respond to ER stress (PERK haploinsufficiency) display an accelerated disease (Wang et al., 2011), while mtSOD1 transgenic mice with an enhanced capacity to respond to ER stress (GADD34 mutation) show a marked amelioration in disease (Wang et al., 2014). These results demonstrated the significant way that the UPR and ISR can impact ALS, and highlighted the potential of these pathways as a therapeutic target for this disorder.
The remarkable prolongation of survival that was achieved in mtSOD1 mice with a GADD34 mutation prompted us to explore the possibility of using a drug to mimic the effect of the mutated GADD34. In fact, salubrinal, which inhibits the dephosphorylation of eIF2α, has been found to prolong survival of G93A mice by 25-30 days when daily dosages are given starting at day 30 (Saxena et al., 2009); however, salubrinal is somewhat toxic. Recent studies have demonstrated that guanabenz, an FDA-approved centrally-acting oral antihypertensive drug inhibits stress-induced dephosphorylation of eIF2α by binding to GADD34 and thereby inhibiting the formation of the phosphatase complex (Tsaytler et al., 2011). This activity is presumably the basis for its reported effectiveness in promoting clearance of scrapie prion protein (PrPsc) (a misfolded protein) in cell culture and in prolonging survival of PrPsc transgenic mice (Tribouillard-Tanvier et al., 2008). Our studies show that guanabenz ameliorates disease in FALS transgenic mice, and therefore is a candidate for the treatment of ALS patients.
Materials and Methods
Mice and breeding
All the animal experiments were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals, and the approval of the Institutional Animal Care and Use Committee of The University of Chicago. G93A transgenic mice on a C57BL/6J background have been previously described (Wooley et al., 2005). All the mice were received from Jackson Labs (Bar Harbor, ME; strain 004435) at approximately seven weeks of age. These mice were bred and genotyped by PCR using the following primers for the detection of SOD1 (Forward, 5′-CATCAGCCCTAATCCATCTGA-3′; Reverse, 5′-CGCGACTAACAATCAAAGTGA-3′) gene. Both male and female mice were used for the studies since no difference in their clinical phenotype was found in a previous study (Wooley et al., 2005) as well as the present one.
Guanabenz (cat#. 193657, MP Biomedicals, LLC, Solon, OH) was prepared and dissolved in saline before each injection. G93A transgenic mice received intraperitoneal (IP) injections in less than 100 μl of guanabenz (8mg/kg) or saline vehicle for each time 3 days a week starting at 60 days of age.
Assessment of disease phenotype
Clinical assessment. Mice were weighed every two days and clinically assessed as previously described in studies of FALS transgenic mice (Boillee et al., 2006): onset of disease was defined as peak weight before a decline; early phase of disease was the period from peak weight until loss of 10% of maximal weight; late phase of disease was the time from 10% loss in weight until death (when a mouse was unable to right itself within 20 s after being put on its back). In experiments monitoring disease parameters and survival, the guanabenz-treated mice were compared with littermate G93A mice. Non-transgenic littermate mice were also used as additional controls in other experiments.
Pathology and immunohistochemical evaluation
The guanabenz-treated and untreated mice were compared with respect to neuropathology in the spinal cord, as previously described (Wang et al., 2014). Sections from the anterior horn of the lumbar spinal cord were processed for Nissl staining to assess motor neurons (MNs), and immunohistochemically stained with glial fibrillary acid protein (GFAP) antibody (1:1,000, cat#.MAB360, Chemicon, Temecula, CA), and Iba1 antibody (1:2,000, cat#. 019-19741, Wako, Richmond, VA) to assess astrocytes and activated microglia respectively, as previously described (Wang et al., 2010). SOD1 aggregation was determined by immunohistochemical staining using a rabbit antibody that recognizes the carboxyl end of mouse and human SOD1 (Deng et al., 2006), as previously described (Wang et al., 2014; Wang et al., 2009).
Western blot
For experiments involving mtSOD1, the lumber spinal cord (15mg) from each mouse was collected and homogenized in 200μl homogenizing buffer (50mM HEPES, 1mM EDTA, 100mM iodoacetamide and 2.5% SDS). The supernatant of spinal cord homogenates were obtained following a high speed centrifugation and processed in one of the two following ways, as described (Wang et al., 2014): (i) The homogenates (10μg of total protein) were boiled in 2×SDS loading buffer that did not contain β-mercaptoethanol and then loaded on to 15% SDS polyacrylamide gels for western blot analysis. A human-specific rabbit anti-SOD1 antibody was used to detect both monomeric and high molecular weight forms of mtSOD1, and anti-β-tubulin antibody (1:500, Developmental Studies Hybridoma Bank) was used to stain for β-tubulin as a control for the amount of protein loaded. (ii) The homogenates were diluted 1:5 (2μg of total protein) followed by boiling in loading buffer that contained β-mercaptoethanol and then loaded on to 15% SDS polyacrylamide gels for western blot analysis. A human-specific rabbit anti-SOD1 antibody was used to detect total SOD1, and mouse anti-β-tubulin antibody was used to stain for β-tubulin as a control for the amount of protein loaded. After washing, the rabbit antibody stained blots were incubated for 2 hours at room temperature with horseradish peroxidase (HRP)-linked anti-rabbit IgG (1:3000, Cell Signaling), while the mouse antibody stained blots were incubated with HRP-linked anti-mouse IgG (1:6000, Santa Cruz) followed by washing and then detection with an ECL-Plus detection kit (Amersham, NJ).
For experiments involving p-eIF2α, the lumbar spinal cord tissue from each mouse was collected and homogenized in 200μl homogenizing buffer (20mM Tris, pH 8.0, 150mM NaCl, 1mM EDTA, 0.5% Triton X-100, 0.5% SDS) containing a protease inhibitor cocktail. The homogenate was then centrifuged 20,000g for 15 minutes. 20 μg of total protein from the supernatant was loaded on to 10% or 15% SDS polyacrylamide gels for western blot analysis. Immunostaining was carried out with overnight incubation at 4°C with anti-rabbit p-eIF2α antibody (1:1,000, cat#.3597, Cell Signaling Technology) and mouse β-tubulin serum (to stain for β-tubulin as a control for the amount of protein loaded). After washing, the western blots were processed as described above. The signal intensity of p-eIF2α was estimated by densitometry using Image J (National Institute of Mental Health, Bethesda, MD, USA).
Statistics
The data were statistically analyzed using a t-test.
Results
Guanabenz delays the onset and prolongs survival of G93A transgenic mice
In order to test the effect of guanabenz on G93A transgenic mice, we compared disease in mice treated with drug or vehicle. Because of concern that daily IP inoculations might aggravate breathing problems in older G93A mice, we opted for treatment at IP injections three times a week with 8mg/kg of guanabenz.
When compared with saline treatment (n= 15), guanabenz treatment (n= 22) starting at 60 days of age significantly delayed disease onset (134.8±6.1 vs. 120.2±6.8 days, P<0.001) (Fig 1A), delayed the time to the beginning of the early phase of disease (155.0±8.7 vs. 134.8±5.6, P<0.001) (Fig. 1B), and prolonged survival (171.2±10.1 vs. 150.4±5.6 days, P <0.001) (Fig. 1C) of G93A mice. The early phase of disease was prolonged from 14.6±3.5 to 20.2±4.8 days (P <0.001) (Fig. 1D), but there was no significant change in the late phase of disease (15.6±2.1 vs.16.2±2.9 days, P= 0.45) (Fig. 1E).
Figure 1.
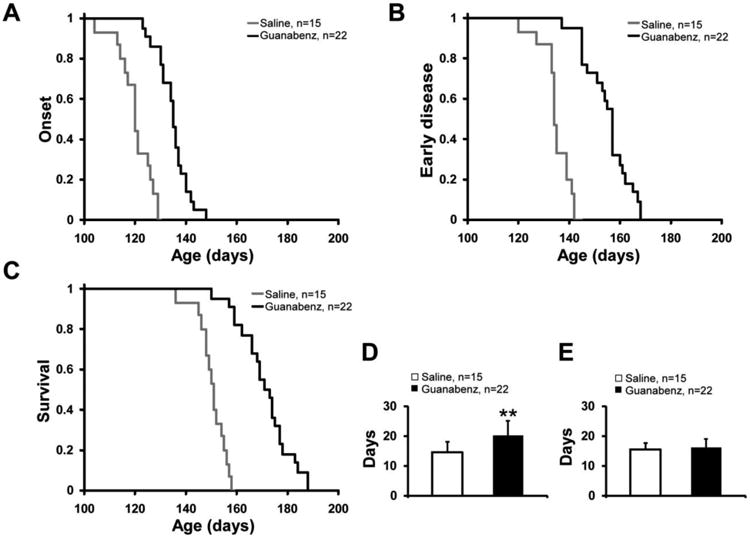
Plots of disease onset (A), the end of early disease (B), and survival (C) as well as diagrams of the duration (with standard deviation) of the early phase (D) and late phase (E) of disease in guanabenz-treated (n=22) vs. untreated G93A transgenic mice (n=15). In the Y axis of A-C, 1 refers to the total number of animals. ** P < 0.001.
Guanabenz-treated G93A transgenic mice have delayed pathology compared to similarly-aged G93A transgenic mice
In order to compare the pathology of guanabenz-treated and untreated G93A transgenic mice, we examined the anterior horn of the lumbar spinal cord from drug and vehicle treated transgenic mice as well as non-transgenic littermate control mice. Both guanabenz-treated and untreated G93A transgenic mice exhibited MN loss (i.e., fewer Nissl staining cells), astrocytosis (more prominent GFAP-positive cells), and microgliosis (more abundant Iba-positive cells) when compared to non-transgenic controls (Fig. 2A, B). However, when compared to untreated G93A mice at 150 days, guanabenz-treated G93A mice had less pathology (Fig. 2A), with less MN loss, astrocytosis, and microgliosis (P<0.01, n= 4, Fig. 2B). At the end stage of disease, at ∼170 days, guanabenz-treated G93A mice (n= 4) had similar pathological changes to those seen in untreated G93A mice (n= 4) at endstage (Fig. 2A, B).
Figure 2.
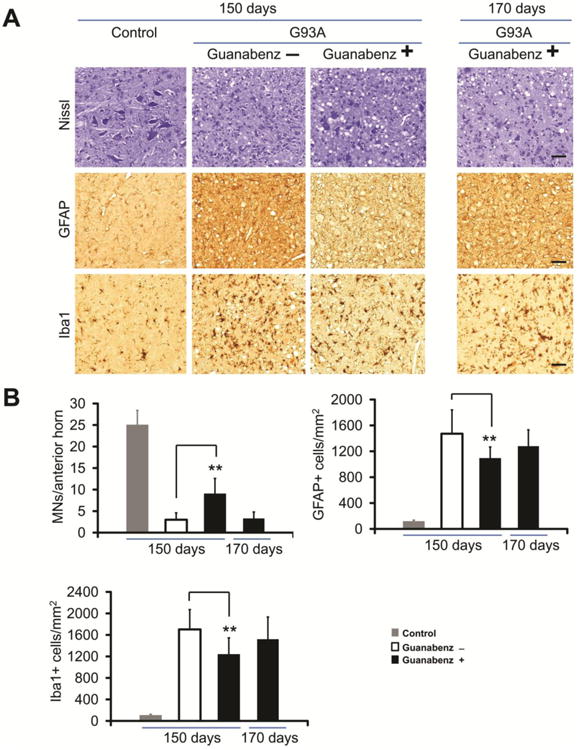
Immunohistochemical studies of the anterior horn of the lumbar spinal cord of guanabenz-treated G93A transgenic mice, untreated G93A transgenic mice, and non-transgenic littermate mice at varying ages. Representative sections of the anterior horn of the lumbar spinal cord were stained with: (A) Nissl for MNs, anti-GFAP antibody for astrocytes, and anti-Iba1 antibody for microglia. (B) Bar diagrams show mean ± standard deviation calculated by counting cells in 15 to 20 sections from the lumbar spinal cord anterior horn of 4 mice from each of the following groups (guanabenz-treated G93A mice, untreated G93A mice, and littermate non-transgenic mice) at each age. The scale bar = 100μm. ** P < 0.001. The data were statistically analyzed using a t-test.
Guanabenz-treated G93A transgenic mice have less accumulation of mtSOD1 than similarly-aged untreated G93A transgenic mice
Abundant mtSOD1 aggregates were present in cells with MN morphology and in cell processes in immunohistochemically-stained sections of the anterior horn of the lumbar spinal cord of both guanabenz-treated and untreated G93A mice at 150 days (Fig. 3A). The presence of mtSOD1 aggregates was confirmed in western blots of lumbar spinal cord homogenates that were boiled in the absence of β-mercaptoethanol and then subjected to SDS-PAGE followed by immunostaining with human-specific anti-SOD1 antibody. Fig. 3B shows western blots that were overexposed to show high molecular weight forms of mtSOD1. High molecular weight forms of mtSOD1 were present in both untreated as well as guanabenz-treated G93A transgenic mice starting at 130 days of age, the earliest time tested.
Figure 3.
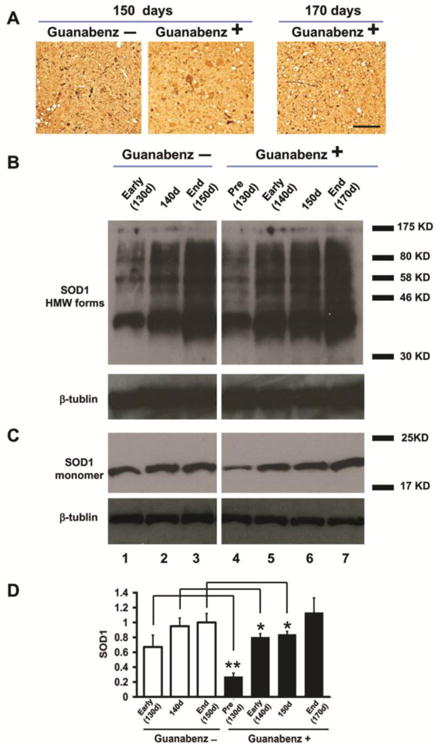
G93A mtSOD1 in the lumbar spinal cord of guanabenz-treated G93A mice, untreated G93A transgenic mice, and untreated littermate non-transgenic mice. In this figure and the next, “Pre” refers to before disease onset, “Early” refers to the early phase of disease, and “End” refers to end stage; the approximate ages of the mice are noted. (A) Representative sections of the anterior horn of the lumbar spinal cord were stained with anti-human SOD1 antibody to identify aggregates. (B) Representative western blot of homogenate of the lumbar spinal cord of guanabenz-treated G93A and untreated G93A mice sacrificed at different times and stages of disease, and processed as described in Materials and Methods. The samples were treated without β-mercaptoethanol before boiling and then loaded on 15% SDS polyacrylamide gels, electrophoresed, blotted, followed by immunostained with a human-specific anti-SOD1 antibody (to detect the monomeric and high molecular weight [HMW] forms) and an anti-β-tubulin antibody. (C) Representative western blot of homogenates of the lumbar spinal cord of guanabenz-treated G93A mice and untreated G93A mice that were harvested and processed as described above, except the samples were diluted 1:5 and treated with β-mercaptoethanol before boiling to identify total SOD1. (E) Bar diagram shows total SOD1 in the lumbar spinal cord of guanabenz-treated and untreated mice at different ages and stages of disease. Each bar, which represents data from 4 animals, shows the mean±standard deviation of the ratio of SOD1 signal to β-tubulin signal, displayed as a fraction of the value of the untreated G93A mice at 150 days, which is set at 1. There is a statistically significant lower amount of total SOD1 in the lumbar spinal cord of guanabenz-treated G93A mice at 130 days (** P < 0.005, n=4), 140 days (*P < 0.05, n=4), and 150 days (*P < 0.05, n=4) compared to similarly aged untreated G93A mice (n=4 for each age). The data were statistically analyzed using a t-test.
Because of difficulty in quantitating and then comparing the immunohistochemically-stained aggregates and high molecular weight forms in samples from the guanabenz-treated and untreated G93A mice, we compared total mtSOD1 by treating the lumbar spinal cord samples with β-mercaptoethanol prior to SDS-PAGE, and therefore making all the SOD1 monomeric. A representative western blot (Fig. 3C) shows that there is a decrease in the amount of total SOD1 in the lumbar spinal cord homogenates of guanabenz-treated G93A compared to similarly aged untreated G93A mice. Quantitation of the SOD1 showed that the differences were statistically significant at 130 days (P<0.005, n= 4), 140 days (P<0.05, n= 4), and 150 days (P< 0.05, n= 4) (Fig. 3D).
Guanabenz-treated G93A transgenic mice have enhanced phosphorylation of eIF2α at endstage compared to untreated G93A transgenic mice
Phosphorylation of eIF2α was observed at 130 days, the earliest time tested, and progressively increased in both the guanabenz-treated and untreated G93A mice. Although the amount of p-eIF2α at 130, 140, and 150 days in guanabenz-treated G93A mice was lower than that seen in similarly aged untreated G93A mice, the differences were not significant. Of note, the amount of p-eIF2α at end stage at ∼170 days in guanabenz-treated G93A mice was statistically significantly increased when compared to the amount seen in untreated G93A mice at end stage at 150 days (P<0.02, n= 4) (Fig. 4B).
Figure 4.
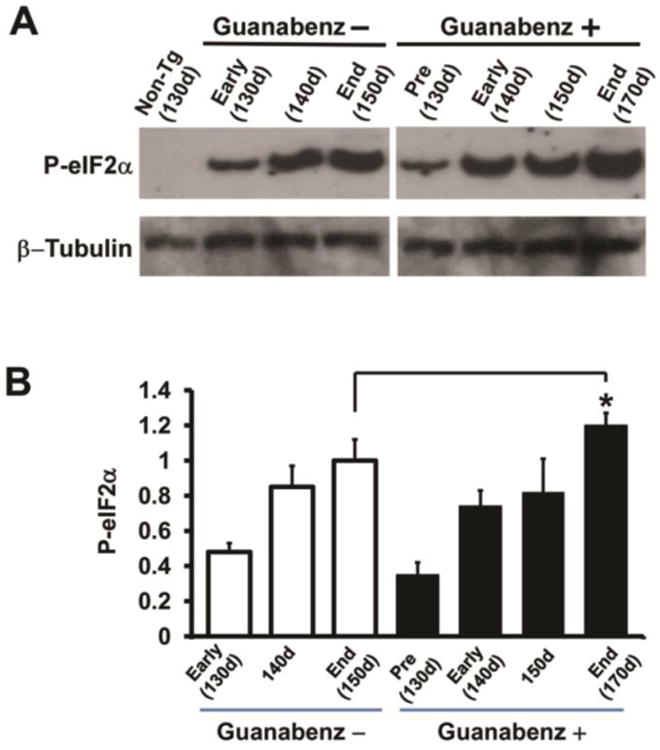
Levels of phosphorylated eIF2α mice in the lumbar spinal cord of guanabenz-treated and untreated G93A mice and untreated littermate non-transgenic mice. (A) Representative western blots from differently aged guanabenz-treated G93A mice, untreated G93A mice, and control non-transgenic littermate mice immunostained with antibodies to p-eIF2α. Anti-β tubulin antibody was used as a loading control. (B) Bar diagrams showing quantitation of p-eIF2α from western blots of homogenates of the lumbar spinal cord from guanabenz-treated G93A mice (n=4) and untreated G93A mice (n=4) mice at end stage of disease. The mean±standard deviation is shown. The value of p-eIF2α detected in untreated G93A mice at 150 days mice was arbitrarily set as 1. There is a statistically significant higher amount of p-eIF2α in the lumbar spinal cord of guanabenz-treated G93A mice at endstage (170 days) (** P < 0.05, n=4) compared to untreated G93A mice at endstage (150 days). The data were statistically analyzed using a t-test.
Discussion
A number of studies have found markers of ER stress and the UPR in mtSOD-1 induced cell death in tissues from FALS transgenic mice and from patients with FALS and sporadic ALS (reviewed in (Walker and Atkin, 2011)). Furthermore, studies have shown that mtSOD1 interacts or associates with a number of proteins that are involved in ER stress, including derlin-1 (Nishitoh et al., 2008), binding immunoglobulin protein (Wate et al., 2005), and protein-disulfide isomerase (Atkin et al., 2006). These studies have suggested that the UPR and ISR are overwhelmed in FALS leading to toxicity and apoptosis, and that bolstering these pathways may ameliorate disease.
Central to the ISR is PERK, which responds quickly to ER stress by phosphorylating eIF2α leading to a suppression of translation. Despite the decrease in protein synthesis, some key mRNAs are translated, such as ATF4, which is a transcriptional activator that leads to expression of a number of cytoprotective genes. eIF2α is dephosphorylated under stress conditions by a holophosphatase composed of a catalytic subunit, protein phosphatase 1 (PP1), and a regulatory subunit, GADD34, so that the enzymatic activity selectively targets p-eIF2α.
In order to investigate the role of ER stress and the ISR in mtSOD 1-induced FALS, we initially employed a genetic approach, making use of G85R mtSOD1 transgenic mice crossed with mice that had mutations in key enzymes in the PERK pathway. In a first series of experiments, we found that compared to G85R/PERK+/+, G85R/PERK+/− mice demonstrated an earlier disease onset, shortened survival, and earlier neuropathological changes in the spinal cord (Wang et al., 2011). These results demonstrated the critical nature of the PERK pathway in mtSOD 1-induced FALS and prompted us to then test whether bolstering the UPR and ISR by augmenting the phosphorylation of eIF2α would ameliorate disease in mtSOD1 mice. For the latter studies, we crossed G85R mice with GADD34+/ΔC mice, which have a mutation in one allele of GADD34 (Wang et al., 2014). We anticipated that compared to G85R mice, G85R/GADD34+/ΔC mice would have a decrease in misfolded proteins because enhanced eIF2α phosphorylation would augment translation repression and also increase expression of cytoprotective genes as a result of ATF4 upregulation. When compared to G85R mice, G85R/GADD34+/ΔC mice had a marked delay in onset, a longer duration of the early phase of disease, and very significantly prolonged survival. To the best of our knowledge, the enhanced survival of G85R/GADD34+/ΔC mice has only been exceeded in one report, which involved a knockdown of mtSOD1 expression in oligodendrocyte precursor cells and oligodendrocytes of G37R transgenic mice (Kang et al., 2013). The marked amelioration of mtSOD1-induced disease as a result of GADD34 mutation prompted us to explore the possibility of testing drugs that mimic the effect of the mutation on mtSOD1 transgenic mice.
The present investigation showed that guanabenz significantly delays disease onset, prolongs the duration of the early phase of disease, and extends survival of G93A mice. There was also a delay in the neuropathological changes in guanabenz-treated compared to untreated G93A mice. Although we were unable to compare the specific amount of mtSOD1 aggregates in guanabenz-treated mice with that found in untreated G93A mice, we did find a decrease in total mtSOD1 in the lumbar spinal cord of guanabenz-treated G93A mice when compared with similarly aged untreated G93A mice. We presume that the decrease in total mtSOD1 in guanabenz-treated G93A mice is a result of enhanced phosphorylation of eIF2α caused by the inhibition of GADD34. The enhanced phosphorylation of eIF2α presumably led to less total mtSOD1 because of a decrease in the synthesis of mtSOD1 or a reduction/clearance in mtSOD1 aggregation because of increased expression of cytoprotective genes. However, the possibility that guanabenz ameliorates disease by direct or indirect modulation of pathways other than the ISR/UPR cannot be excluded. Seemingly at odds with our explanation that guanabenz leads to enhanced phosphorylation of eIF2α was our finding that the amount of p-eIF2α was unchanged in guanabenz-treated G93A mice when compared to untreated G03A mice during the early stages of disease. It was only when we compared endstage guanabenz-treated mice with endstage untreated mice that we found an increase in p-eIF2α. This result is similar to our findings in G85R/GADD34+/ΔC mice, where there were significantly lower total amounts of p-eIF2α compared to similarly aged G85R mice. We suspect that enhanced phosphorylation of eIF2α does in fact occur as a result of both guanabenz treatment as well as GADD34 mutation, but that early in disease it is a rapid and transient response triggering a relative decrease in ER stress and unfolded proteins, and therefore resulting in a lower level of PERK activation and eIF2α phosphorylation. This transiently enhanced level of p-eIF2α with a resultant enhanced suppression of translation, including mtSOD1 and reduction/clearance in mtSOD1 aggregation, is presumably the reason that guanabenz-treated G93A mice have less total mtSOD1 than similarly-aged untreated G93A mice. When ER stress overwhelms the UPR, eIF2α phosphorylation is increased, as in endstage guanabenz-treated G93A mice (and G85R/GADD34+/ΔC mice).
Although the increased survival of guanabenz-treated G93A mice compared to untreated G93A mice was statistically significant, guanabenz did not have as robust an ameliorative effect on mtSOD1-induced disease as seen with GADD34 mutation. There may be several reasons for differences in the effect of guanabenz-treated mtSOD1 mice compared to mtSOD1 mice with a GADD34 mutation: guanabenz was administered to G93A transgenic mice while the GADD34 mutation was in G85R transgenic mice – and the G93A transgenic mouse is a much more aggressive model with a much younger age of onset than the G85R transgenic mouse; guanabenz treatment was started when the mice were 60 days of age, while the GADD34 knockdown was present from fertilization. Furthermore, we IP inoculated G93A transgenic mice every other day because of concerns for toxicity to the mtSOD1 mice following daily IP inoculations. Since the half life of guanabenz is 6 hours (Meacham et al., 1980), it is likely that this treatment regimen was not optimal.
The above studies provide a potential rationale for treatment of mtSOD1-induced FALS patients with guanabenz. Guanabenz may also be effective in sporadic ALS because of the following findings. Abnormalities in the structure of wild type SOD1 that resemble those of mtSOD1 have been described in sporadic ALS (Bosco et al., 2010; Ezzi et al., 2007; Grad et al., 2014; Gruzman et al, 2007; Haidet-Phillips et al., 2011; Kabashi et al., 2007; Pokrishevsky et al., 2012). Furthermore, there is aggregation of various proteins such as TDP-43 and FUS in sporadic ALS, suggesting that ER stress and the UPR may have a role in the pathogenesis of sporadic ALS in addition to FALS. In fact, markers of ER stress have been found in autopsy tissue from patients with sporadic ALS (Ilieva et al., 2007). Of interest, recent studies demonstrated that guanabenz reduces toxicity resulting from the expression of ALS-associated mtTDP-43 and mtFUS in C. elegans and zebrafish (Vaccaro et al., 2013). Therefore, the UPR and ISR are likely to play a key role in the pathogenesis of sporadic ALS as well as FALS.
Guanabenz is an attractive candidate for treatment of ALS for a number of reasons. It is FDA-approved, oral, and blood-brain-barrier permeable, with extensive pharmacodynamic and pharmacokinetic data available. Also, of note, the target of guanabenz treatment, the UPR and ISR, which are targeted by guanabenz, are new directions for ALS treatment. There remain, however, some concerns about this therapeutic direction. Although our studies demonstrate that enhancing the UPR and ISR in mtSOD1-induced ALS is beneficial, a recent publication reported that an inhibitor of PERK alleviates toxicity in Drosophila and rat primary cortical neurons that overexpress TDP-43. This same PERK inhibitor dramatically ameliorated disease in scrapie mice (Moreno et al., 2013). These apparently conflicting results demonstrate that inhibiting the PERK pathway may have a beneficial effect in disorders involving protein misfolding. We suspect that the timing at which one perturbs PERK is important in its effect. Enhancing the effect of PERK may be beneficial early in a disease associated with misfolded proteins, but inhibiting PERK can ameliorate disease once the UPR is overwhelmed by the misfolded proteins and faces sustained and excessive ER stress. In addition, the extensive repression in translation caused by PERK may have unexpected and deleterious effects on genes that could accelerate disease. A rescue from the repression of synaptic proteins caused by the UPR has been offered as a possible explanation for the beneficial effect of the PERK inhibitor on scrapie. For this reason, it is valuable to proceed cautiously with clinical trials of drugs aimed at bolstering the UPR and ISR (Moreno et al., 2013).
Highlights.
Guanabenz enhances the UPR PERK pathway
uanabenz ameliorates mutant SOD1-induced disease in ALS mice
Guanabenz, an approved oral antihypertensive, is a candidate drug for the treatment of ALS
Acknowledgments
This work was supported by grants from the Dana Foundation, Target ALS (RPR and BP), and the NIH (NS078142-01 to RPR and NS34939 to BP). We thank Dr. Yulia Dzhashiashvili for reviewing the manuscript.
Addendum
This work was presented at the Society for Neuroscience in Nov. 2013. When this manuscript was in the final stages of submission, other investigators made available an ePub describing the effect of guanabenz in G93A mtSOD1 mice (Jiang et al., 2014). The ePub has similar findings to the present study with respect to the effect of guanabenz on onset and survival; however, the numbers of mice in the treated (n=9) and untreated group (n=12) are small and require confirmation with larger numbers of mice, as in the present study. The authors of the ePub used alternate day IP injections with 4 mg/kg of guanabenz (rather than the 8mg/kg of the present study) starting at 40 days (rather than 60 days in the present study). As noted in the body of this report, the known half life of guanabenz is 6 hours suggesting that more frequent dosing would have yielded a more ameliorative effect on mtSOD1-induced disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkin JD, et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase. J Biol Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzi SA, et al. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- Grad LI, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2014;111:3620–3625. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzman A, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva EV, et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111–3123. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- Jiang HQ, et al. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.03.047. ePub. [DOI] [PubMed] [Google Scholar]
- Kabashi E, et al. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann Neurol. 2007;62:553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- Kang SH, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H, et al. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci U S A. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus S, et al. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23:239–252. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Meacham RH, et al. Relationship of guanabenz concentrations in brain and plasma to antihypertensive effect in the spontaneously hypertensive rat. J Pharmacol Exp Ther. 1980;214:594–598. [PubMed] [Google Scholar]
- Moreno JA, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- Nassif M, et al. Amyotrophic lateral sclerosis pathogenesis: a journey through the secretory pathway. Antioxid Redox Signal. 2010;13:1955–1989. doi: 10.1089/ars.2009.2991. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrishevsky E, et al. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, et al. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, et al. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Tribouillard-Tanvier D, et al. Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions. PLoS One. 2008;3:e1981. doi: 10.1371/journal.pone.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, et al. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- Turner BJ, et al. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–117. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, et al. The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS. Faseb J. 2008;22:2476–2487. doi: 10.1096/fj.07-092783. [DOI] [PubMed] [Google Scholar]
- Urushitani M, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- Vaccaro A, et al. Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo. Neurobiol Dis. 2013;55:64–75. doi: 10.1016/j.nbd.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Walker AK, Atkin JD. Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis. IUBMB Life. 2011;63:754–763. doi: 10.1002/iub.520. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Mutant SOD1 knockdown in all cell types ameliorates disease in G85R SOD1 mice with a limited additional effect over knockdown restricted to motor neurons. J Neurochem. 2010;113:166–174. doi: 10.1111/j.1471-4159.2010.06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. The unfolded response in familial amyotrophic lateral sclerosis. Hum Mol Genet. 2011;20:1008–1015. doi: 10.1093/hmg/ddq546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum Mol Genet. 2014;23:2629–2638. doi: 10.1093/hmg/ddt658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol Dis. 2009;35:234–240. doi: 10.1016/j.nbd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wate R, et al. Expression of an endoplasmic reticulum-resident chaperone, glucose-regulated stress protein 78, in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol. 2005;110:557–562. doi: 10.1007/s00401-005-1080-y. [DOI] [PubMed] [Google Scholar]
- Wooley CM, et al. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]


