Abstract
Current smoking cessation pharmacotherapies have modest efficacy, and most smokers relapse within the first few days after a quit attempt. Nicotine withdrawal-induced craving and cognitive impairments predict smoking relapse during abstinence and suggest that cognitive-enhancing drugs may prevent relapse. ABT-089 and ABT-107 are subtype-selective nAChR agonists that improve cognitive performance in laboratory animals. However, there are no studies examining the effects of ABT-089 and ABT-107 on nicotine self-administration and the reinstatement of nicotine-seeking behavior, an animal model of relapse in human smokers. The goal of the present study was to determine the effects of the α4β2*/α6β2* nAChR agonist ABT-089 and the α7 nAChR agonist ABT-107 on nicotine taking and seeking in rats. The effects of acute ABT-089 and ABT-107 pretreatment on nicotine self-administration and reinstatement were tested in male Sprague Dawley rats. Parallel studies of ABT-089 and ABT-107 on sucrose self-administration and reinstatement were tested in separate groups of rats to determine if the effects of these drug treatments generalized to other reinforced behaviors. Nicotine and sucrose self-administration behaviors were not altered following acute administration of ABT-089 (0, 0.12, 1.2 and 12.0 mg/kg) or ABT-107 (0, 0.03 and 0.3 mg/kg). In contrast, both ABT-089 and ABT-107 pretreatment dose-dependently attenuated nicotine reinstatement. These effects were reinforcer-specific as no effects of ABT-089 or ABT-107 pretreatment on sucrose seeking were noted. Taken together, these findings suggest that ABT-089 and ABT-107 do not affect nicotine consumption, but may reduce the likelihood that a smoking lapse will lead to relapse.
Keywords: smoking, relapse, addiction, self-administration
1. Introduction
Tobacco smoking remains the leading cause of preventable death worldwide. Even though smoking cessation at any age produces almost immediate health benefits that continue to improve as prolonged abstinence is maintained, only 3% of smokers are able to quit smoking successfully on their own [1]. Current FDA-approved smoking cessation medications only promote long-term smoking abstinence in 30% of patients [2]. Therefore, there is a critical need to develop novel, efficacious pharmacotherapies for smoking cessation.
Nicotine is the principal psychoactive chemical in tobacco that mediates tobacco’s reinforcing effects [3]. Nicotine is a non-selective nicotinic acetylcholine receptor (nAChR) agonist that binds to and activates neuronal nAChRs. There is clear evidence that neuronal nAChRs play critical roles in nicotine reinforcement and withdrawal [4-7], which supports the development of novel smoking cessation pharmacotherapies targeting these receptors. Varenicline is currently the best in class treatment for smoking cessation and was developed as such based on its action as a partial agonist at α4β2 nAChRs [8]. However, protracted smoking abstinence is only achieved in 23% of patients treated with varenicline [9, 10]. This limited therapeutic benefit may be due, in part, to increased expression of β2-containing nAChRs in the brain [11]. Similar to the effects of nicotine, chronic varenicline exposure is associated with up-regulation of neuronal α4β2 nAChRs, which is thought to promote nicotine craving and relapse during smoking abstinence [12-15]. Based on these findings, it has been proposed that subtype-selective nAChR agonists that do not increase nAChR expression in the brain may be more effective than varenicline and nicotine replacement therapies at maintaining long-term smoking abstinence [16].
A growing body of evidence indicates that α4β2* nAChRs and α7-containing nAChRs mediate the reinforcing effects of nicotine. In addition to regulating nicotine-taking and - seeking behaviors in rodents [17-23], both α4β2* nAChRs and α7-containing nAChRs have been shown to play important roles in cognitive function [24, 25]. Although many factors influence chronic smoking behavior, cognitive deficits are a core withdrawal phenotype that peak during the first few days of abstinence and predict smoking relapse [26, 27]. These findings indicate that cognitive-enhancing medications may prevent drug craving and relapse, in part, by reversing or normalizing nicotine withdrawal-induced cognitive impairments [28-30]. Therefore, novel subtype-selective α4β2* and α7 nAChR agonists that attenuate nicotine taking and seeking as well as improve nicotine withdrawal-induced cognitive deficits may represent efficacious smoking cessation pharmacotherapies [31].
ABT-089 is a partial agonist at neuronal α4β2* and α6β2* nAChRs that has been shown to increase cognitive performance in non-human primates and rodents [32-35]. Consistent with these findings, recent clinical studies have shown that ABT-089 improves cognition and is well tolerated in humans [36, 37]. ABT-107 is a high affinity agonist at neuronal α7 nAChRs that has also been shown to increase cognitive performance in laboratory animals [38-40]. ABT-107 is safe and well tolerated in humans in a Phase 1 clinical study [41]. Moreover, repeated administration of ABT-089 or ABT-107 during nicotine withdrawal does not increase expression of β2-containing nAChRs in the brain, which suggests that these compounds may promote smoking abstinence [42]. While these findings imply that ABT-089 and/or ABT-107 may reverse or normalize nicotine withdrawal-induced cognitive deficits and prevent relapse, no studies to date have examined the effects of these subtype-selective nAChR agonists on nicotine taking and seeking in rats.
Here, we examined the ability of acute systemic administration of ABT-089 and ABT-107 to attenuate nicotine self-administration and the reinstatement of nicotine seeking-behavior, an animal model of relapse in abstinent human smokers. In addition to examining the effects of ABT-089 and ABT-107 on nicotine taking and seeking, we also assessed the role of these subtype-selective nAChR agonists in modulating sucrose self-administration and reinstatement in order to examine the specificity of these drug treatments in appetitive/reinforced behaviors.
2. Materials and Methods
2.1. Animals and housing
Male Sprague Dawley rats (Rattus norvegicus) approximately 3 months of age were obtained from Taconic Laboratories (Germantown, NY, USA). All subjects were mildly food restricted (20-25 g chow daily) to 85-90% of their free-feeding body weight following recovery from surgery for the duration of these experiments. Water was continuously available in the home cage. Rats were housed in a colony maintained on a 12-h/12-h reverse light/dark cycle, with lights off at 7:00 a.m. All experimental protocols were in accordance with the guidelines set forth by the National Institutes of Health and were approved by the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
2.2. Materials
All self-administration experiments were conducted in ventilated, sound attenuating operant chambers purchased from Med-Associates Inc. (East Fairfield, VT, USA). Each operant chamber was equipped with both active and inactive response levers, a sucrose pellet dispenser, cue lights, tone generator, as well as an automated injection pump for administering drug or vehicle solutions intravenously.
2.3. Surgery
Rats were handled daily and allowed one week to acclimate to their home cages upon arrival. Prior to surgery, the rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma Aldrich/RBI, St. Louis, MO, USA). For rats self-administering nicotine, an indwelling silicone catheter (CamCaths; Cambridge, UK) was inserted into the right, external jugular vein and sutured securely in place. The catheter was attached to a mesh backmount that was implanted subcutaneously above the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.3 ml of a solution of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA, USA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH, USA). When not in use, catheters were sealed with plastic obturators.
2.4. Nicotine self-administration and reinstatement of nicotine seeking
After surgery, rats were allowed 7 days to recover before behavioral testing commenced. Nicotine self-administration was preformed as described previously [43, 44]. Initially, rats were placed in operant chambers and allowed to lever-press for intravenous infusions of nicotine (0.03 mg/kg nicotine/59 μl saline, infused over a 5 s period) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each nicotine infusion was paired with a light/tone cue. Stable responding on the FR schedules of reinforcement was defined as less than 20% variation in response rates over three consecutive self-administration days. After stable responding was achieved, the schedule of reinforcement was increased to fixed-ratio 3 (FR3) for 3-5 days and then finally increased to a fixed-ratio-5 (FR5) schedule. A 20 s time-out period followed each nicotine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the operant sessions.
The effects of acute ABT-089 and ABT-107 were examined in separate cohorts of rats that acquired stable nicotine self-administration on a FR5 schedule of reinforcement. A between-session, within-subjects design was used to test the effects of ABT-089 and ABT-107 on nicotine taking (Figure 1A). Each test day was separate by at least 2 days of nicotine self-administration to ensure that nicotine taking had stabilized between test sessions. Subjects were pretreated with ABT-089 (0, 0.12, 1.2 and 12.0 mg/kg, i.p.) 10 min prior to the beginning of the operant session. ABT-107 (0, 0.03 and 0.3 mg/kg, i.p.) was administered 30 min prior to self-administration sessions. Following pretreatment with ABT-089 or ABT-107, rats were placed into the operant chambers and allowed to self-administer intravenous nicotine. All doses of ABT-089 and ABT-107 were counterbalanced and tested in each rat in order to avoid potential rank order effects of drug pretreatment. Doses of ABT-089 and ABT-107 and pretreatment times were selected based on previously published reports demonstrating cognitive-enhancing properties of these compounds in rodents [33, 34, 39, 45].
Figure 1. Timeline for the nicotine and sucrose self-administration and reinstatement studies.
A between-sessions and within-subjects design was used to study the effects of ABT-089 (vehicle, 0.12, 1.2 and 12.0 mg/kg) and ABT-107 (vehicle, 0.03 and 0.3 mg/kg) pretreatments on nicotine and sucrose self-administration behaviors (A) as well as the reinstatement of nicotine- and sucrose-seeking behaviors (B) in separate cohorts of rats. See Methods for more detail.
The effects of ABT-089 and ABT-107 on the reinstatement of nicotine-seeking behavior were tested in separate cohorts of rats that were not previously tested with these compounds during the self-administration phase of the experiment. Following 28 daily nicotine self-administration sessions, drug-taking behavior was extinguished by replacing the nicotine with 0.9% saline such that every 5 active lever responses resulted in a saline infusion. Light/tone cues that were paired with nicotine infusions during the self-administration phase were turned off during extinction. Daily extinction sessions continued until responding on the active lever was less than 20% of the response rate maintained by nicotine self-administration under the FR5 schedule of reinforcement. Typically, it took 3-7 days for rats to meet this criterion. Once nicotine self-administration was extinguished, the ability of an acute priming injection of nicotine (0.2 mg/kg, s.c.) to reinstate nicotine seeking was assessed. During each reinstatement test session, subjects were allowed to respond for light/tone cues that were previously paired with nicotine infusions. Therefore, satisfaction of the response requirement (i.e. every 5 active lever responses) during reinstatement test sessions resulted in an infusion of saline along with contingent presentations of the light/tone cues. On subsequent reinstatement test days, ABT-089 (0, 0.12, 1.2 and 12.0 mg/kg, i.p.) and ABT-107 (0, 0.03 and 0.3 mg/kg, i.p.) was administered 10 and 30 min, respectively, prior to a priming injection of nicotine. Animals were placed immediately into the operant chambers following administration of a priming injection of nicotine and a 2-hour reinstatement session began. Using a between-sessions reinstatement paradigm, each reinstatement test session was followed by extinction sessions until responding was again less than 20% of the response rate maintained by nicotine self-administration (Figure 1B). Generally, 1-2 days of extinction were necessary to reach extinction criterion between reinstatement test sessions. This reinstatement procedure combines nicotine priming- and cue-induced reinstatement, which has been used in several previous studies [46-48], that elicits more robust drug seeking than when only nicotine or only the cues are presented. The combination of nicotine and cues may be a more relevant model of smoking relapse in humans than cues alone or nicotine alone.
2.5. Sucrose self-administration and reinstatement of sucrose seeking
Reinforcer-specificity and potential nonspecific rate-suppressing effects of ABT-089 and ABT-107 were evaluated by assessing the influence of these compounds on sucrose taking and seeking in separate cohorts of rats. Rats were trained initially to self-administer 45 mg sucrose pellets (Research Diets, New Brunswick, NJ) on a FR1 schedule of reinforcement. Once animals achieved stable responding for sucrose (defined as less than 20% variation in responding over three consecutive days) on the FR1 schedule of reinforcement, the response requirement was increased to an FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets within each 1-hour daily operant session. Each successful completion of the response requirement resulted in delivery of a sucrose pellet as well as contingent presentations of light/tone cues. Subjects were mildly food restricted in order to maintain consistency with the nicotine self-administration experiments (i.e. to ensure similar motivational states). A between-session, within-subjects design was used to test the effects of ABT-089 and ABT-107 in rats stably self-administering sucrose pellets (Figure 1A). Each test day was separate by at least 2 days of sucrose self-administration to ensure that sucrose taking had stabilized between test sessions. Subjects were pretreated with ABT-089 (0, 0.12, 1.2 and 12.0 mg/kg, i.p.) 10 min prior to the beginning of the operant session. ABT-107 (0, 0.03 and 0.3 mg/kg, i.p.) was administered 30 min prior to self-administration sessions. All doses of ABT-089 and ABT-107 were counterbalanced and tested in each rat.
The effects of ABT-089 and ABT-107 on sucrose seeking were tested in separate cohorts of rats. After 14 days of sucrose-maintained responding on a FR5 schedule of reinforcement, rats underwent an extinction phase where lever pressing no longer resulted in sucrose delivery and presentation of light/tone cues. Once lever responding decreased to less than 20% of the maximum number of responses completed during sucrose self-administration, animals proceeded to reinstatement testing. During the reinstatement test sessions, rats were allowed to respond for light/tone cues that were previously paired with sucrose pellet delivery during the self-administration phase. Doses of ABT-089 and ABT-107 that attenuated nicotine priming-induced reinstatement of drug seeking were administered 10 min or 30 min, respectively, prior to the beginning of the reinstatement session. Using a within-subjects design, each animal served as its own control. The experimenter remotely administered one sucrose pellet every 2 min for the first 10 min of the reinstatement session. Moreover, non-contingent presentation of light/tone cues was delivered for 5 seconds at the beginning of each reinstatement test session and repeated once every 2 minutes for the first 10 minutes of the reinstatement session. A between-session paradigm was used so that each daily reinstatement test session was followed by an extinction session the following day until responding was again less than 20% of the response rate maintained by sucrose (Figure 1B). Doses of ABT-089 and ABT-107 were counterbalanced across reinstatement test sessions. Rats were tested for sucrose seeking in the absence of a systemic drug infusion at the end of the experiment to ensure that reinstatement of sucrose seeking had not extinguished.
2.6. Drugs
(−)Nicotine hydrogen tartrate salt (Sigma Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline (pH was adjusted to 7.4±0.5 with sodium hydroxide). Nicotine doses are reported as free base concentrations. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride] and ABT-107 [5-(6-[(3R)-1-azabicyclo[2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole] were synthesized by AbbVie (North Chicago, IL, USA) and dissolved in sterile 0.9% saline [33, 39]. Doses of ABT-089 and ABT-107 used in these studies represent behaviorally relevant doses that have been shown previously to produce cognitive-enhancing effects in rodents [33, 34, 39, 45].
2.7. Statistical analyses
Data are presented as means and standard errors of the mean (S.E.M.) Total active and inactive lever responses during nicotine and sucrose self-administration and reinstatement experiments were analyzed with two-way mixed-factors analyses of variance (ANOVAs). Pairwise comparisons were made with Bonferroni post hoc test following two-way ANOVAs (P<0.05).
3. Results
3.1. Acute ABT-089 administration has no effect on nicotine and sucrose self-administration in rats
Total lever responses for animals self-administering nicotine on a FR5 schedule of reinforcement are shown in Figure 2A (n = 16). Prior to the start of the operant session, rats were pretreated with 0, 0.12, 1.2 or 12 mg/kg ABT-089. These data were analyzed with a two-way ANOVA, which revealed a significant main effect of lever [F(1,120)=391.6, p<0.0001] but no effect of treatment [F(3,120)=0.48, p=0.70]. Total lever responses for animals self-administering sucrose pellets on a FR5 schedule of reinforcement are shown in Figure 2B. Prior to the start of the self-administration session, animals were pretreated with 0, 0.12, 1.2, or 12 mg/kg ABT-089 (n = 10). These data were analyzed with a two-way ANOVA, which revealed a significant main effect of lever [F(1,72)=5587, p<0.0001] but no effect of treatment [F(3,72)=0.51, p=0.67]. These findings indicate that acute ABT-089 administration does not influence nicotine or sucrose self-administration in rats.
Figure 2. Acute, systemic administration of ABT-089 did not affect nicotine and sucrose self-administration.
(A) ABT-089 pretreatment did not alter nicotine-taking behavior in rats self-administering nicotine on a FR5 schedule of reinforcement. No significant differences in responding (mean±SEM) on the active and inactive levers were found in rats pretreated with vehicle, 0.12, 1.2 and 12.0 mg/kg ABT-089 (n = 16/treatment) prior to nicotine self-administration test sessions (p>0.05). (B) Total number of active lever responses (mean±SEM) in subjects self-administering sucrose pellets on a FR5 schedule of reinforcement (n = 10/treatment). No significant differences in responding were noted between subjects pretreated with vehicle, 0.12, 1.2 and 12.0 mg/kg ABT-089 (p>0.05).
3.2. Acute ABT-107 administration has no effect on nicotine and sucrose self-administration in rats
Total active and inactive lever responses in animals pretreated with 0, 0.03 and 0.3 mg/kg ABT-107 are shown in Figure 3A (n = 7). These data were analyzed with a two-way ANOVA, which revealed a significant main effect of lever [F(1,36)=91.44, p<0.0001] but no effect of treatment [F(2,36)=0.05, p=0.95]. The effects of ABT-107 pretreatment on sucrose self-administration are shown in Figure 3B (n = 9). There was no effect of drug pretreatment on lever responding [F(2,48)=0.07, p=0.93] suggesting that acute ABT-107 administration has no effect on nicotine or sucrose taking in rats.
Figure 3. Nicotine and sucrose self-administration were not altered in rats pretreated with ABT-107.
(A) No significant differences in responding (mean±SEM) on the active and inactive levers were found in rats pretreated with vehicle, 0.03 and 0.3 mg/kg ABT-107 (n = 7/treatment) prior to nicotine self-administration test sessions (p>0.05). (B) Acute, systemic ABT-107 administration did not alter sucrose-taking behavior in rats (n = 9/treatment). No significant differences in responding were noted between subjects pretreated with vehicle, 0.03 and 0.3 mg/kg ABT-107 (p>0.05).
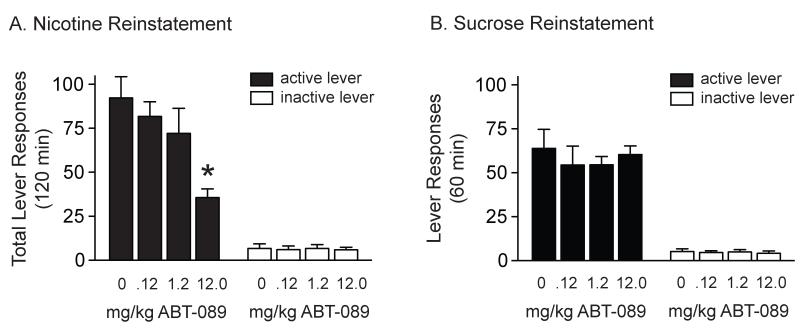
3.3. Acute ABT-089 administration attenuated nicotine, but not sucrose, reinstatement in rats
Total active and inactive lever responses for rats pretreated with 0, 0.12, 1.2 and 12.0 mg/kg ABT-089 prior to a systemic priming injection of nicotine are shown in Figure 4A (n = 11). Total lever responses were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(3,80)=4.00, p<0.05] and lever [F(1,80)=121.5, p<0.0001] as well as a significant treatment × lever interaction [F(3,80)=3.83, p<0.05]. Subsequent pairwise analyses showed that the total active lever responses were significantly different between rats pretreated with vehicle and 12.0 mg/kg ABT-089 (Bonferroni, p<0.05). Total lever responses for animals pretreated with 0 (n = 10), 0.12, 1.2 and 12.0 mg/kg ABT-089 (n = 9) prior to sucrose reinstatement tests are shown in Figure 4B. These data were analyzed with a two-way ANOVA, revealed a significant main effect of lever [F(1,66)=152.7, p<0.0001] but no effect of treatment [F(3,66)=0.37, p=0.81]. Taken together, these data indicate that ABT-089 administration attenuates the reinstatement of nicotine-seeking behavior and that these effects are reinforcer-specific.
Figure 4. Acute, systemic administration of ABT-089 attenuated nicotine, but not sucrose, seeking.
(A) Total number of responses (mean±SEM) on the active and inactive levers during the reinstatement test session following a priming injection of nicotine (0.2 mg/kg, s.c.) in rats pretreated with vehicle, 0.12, 1.2 and 12.0 mg/kg ABT-089 (n=11/treatment). ABT-089 dose-dependently attenuated drug seeking induced by a priming injection of nicotine and cues previously associated with nicotine self-administration. The asterisk represents a significant decrease in responding on the active lever in animals treated with 12.0 mg/kg ABT-089 when compared with saline-treated controls (Bonferroni, p<0.05). (B) No differences in total active lever responses (mean±SEM) during sucrose reinstatement test sessions were noted in animals pretreated with vehicle (n=10), 0.12 (n=9), 1.2 (n=9) or 12.0 (n=9) mg/kg ABT-089 (p>0.05).
3.4. Acute ABT-107 administration attenuated nicotine, but not sucrose, reinstatement in rats
Total lever responses in rats pretreated with 0, 0.03 and 0.3 mg/kg ABT-107 (n = 8) prior to a systemic priming injection of nicotine are shown in Figure 5A. These data were analyzed with a two-way ANOVA, which revealed significant main effects of treatment [F(2,42)=8.07, p<0.01] and lever [F(1,42)=124.2, p<0.0001] as well as a significant treatment × lever interaction [F(2,42)=6.90, p<0.01]. Subsequent pairwise analyses showed that the total active lever responses were significantly different between rats pretreated with vehicle and 0.3 mg/kg ABT-107 (Bonferroni, p<0.05). The effects of ABT-107 pretreatment on sucrose seeking were tested in a separate cohort of rats (n = 8). Total lever responses during the sucrose reinstatement test sessions are shown in Figure 5B and were analyzed with a two-way ANOVA. No effects of ABT-107 pretreatment on sucrose seeking were observed [F(2,42)=2.24, p=0.12]. These results indicate that ABT-107 administration selectively attenuates nicotine-seeking behavior in rats.
Figure 5. Acute, systemic administration of ABT-107 dose-dependently attenuated nicotine, but not sucrose, seeking.
(A) Total number of responses (mean±SEM) on the active and inactive levers during the reinstatement test session following a priming injection of nicotine (0.2 mg/kg, s.c.) in rats pretreated with vehicle, 0.03 or 0.3 ABT-107 (n = 8/treatment). Total active lever responses were significantly decreased in rats pretreated with 0.3 mg/kg ABT-107 when compared to saline-treated controls (Bonferroni, *p<0.05). (B) No differences in total active lever responses (mean±SEM) during sucrose reinstatement test sessions were observed in rats pretreated with vehicle, 0.03 and 0.3 mg/kg ABT-107 (n = 8/treatment)(p>0.05).
4. Discussion
This is the first study to examine the effects of the α4β2* and α6β2* nAChR partial agonist ABT-089 and the α7 nAChR agonist ABT-107 on nicotine self-administration and the reinstatement of nicotine-seeking behavior. Acute administration of ABT-089 and ABT-107 did not influence nicotine or sucrose self-administration in rats. In contrast, acute administration of ABT-089 and ABT-107 dose-dependently attenuated the reinstatement of nicotine-seeking behavior in rats. Moreover, ABT-089 and ABT-107 administration did not influence sucrose seeking indicating that these effects are reinforcer specific and not due to general motor-suppressant effects of drug treatment. Taken together, these results indicate that α4β2*, α6β2* and α7nAChRs play a critical role in the reinstatement of nicotine-seeking behavior and that subtype-selective agonists targeting these nAChRs may have efficacy in preventing smoking relapse during abstinence.
The lack of effect of acute ABT-089 administration on nicotine self-administration was surprising given the role for α4-, α6- and β2-containing nAChR subtypes in mediating the reinforcing effects of nicotine. Partial agonists of α4β2* nAChRs and antagonists of α4β2* nAChRs decrease nicotine self-administration in rats [17-20, 49]. Consistent with these findings, mutant mice lacking β2* nAChRs self-administer less nicotine than wildtype controls, deficits that are reversed following viral-mediated re-expression of β2 nAChR subunits in the ventral tegmental area [50-52]. Moreover, the reinforcing properties of nicotine are also mediated by α4* nAChRs. Nicotine self-administration is reduced in mutant mice lacking α4* nAChRs compared to wildtype controls [52] [however see, 53]. Transgenic mice expressing hypersensitive α4* nAChRs have increased sensitivity to nicotine reward providing further support for the role of α4* nAChRs in nicotine reinforcement [53, 54]. α6β2* nAChRs also play an important role in nicotine self-administration. Pharmacological inhibition of α6β2* nAChRs decrease nicotine taking in rats [55, 56] and mutant mice lacking α6* nAChRs do not acquire nicotine self-administration [52]. Taken together, these findings support a key role for α4-, α6- and β2-containing nAChR subtypes in regulating nicotine self-administration. However, despite considerable evidence from behavioral pharmacology and genetic studies that decreased α4β2* and α6β2* nAChR function reduces nicotine taking in rodents [6], we found no effect of the α4β2* and α6β2* nAChR partial agonist ABT-089 on nicotine self-administration. The doses of ABT-089 used in this study were based on doses that improve cognitive performance in rats [33, 34]. It is possible that higher acute doses of ABT-089 may be required to attenuate nicotine self-administration in rats. Moreover, chronic dosing of smoking cessation medications is required to prevent nicotine craving and relapse in human smokers. Therefore, it is possible that sustained elevated plasma levels of ABT-089, not achieved through our acute dosing regimen, are necessary to attenuate nicotine self-administration in rats. While future studies are needed to examine the effects of repeated ABT-089 on nicotine self-administration, the present findings indicate that acute ABT-089 does not affect nicotine consumption at the doses tested.
The present findings also demonstrate that acute administration of the α7 nAChR agonist ABT-107 has no effect on stable nicotine self-administration in rats. These results are not surprising given the mixed evidence supporting a role for α7 nAChRs in mediating the reinforcing properties of nicotine. Pharmacological inhibition of α7 nAChRs and decreased expression of α7 nAChR subunits does not alter nicotine taking in rodents [52, 57]. In contrast, one study demonstrated reduced nicotine taking in rats pretreated with an α7 nAChR antagonist [21]. While the precise contribution of α7 nAChRs to nicotine reinforcement remains to be determined, our findings suggest that α7 nAChRs do not play a role in nicotine taking. However, there is some evidence that α7 nAChRs regulate nicotine consumption in a temporally dependent manner. Mice with constitutive knockdown of neuronal α7 nAChR expression display reduced consumption of nicotine compared to controls after prolonged, but not acute, nicotine exposure [58]. Therefore, the present study examining the effects of ABT-107 on nicotine taking may be limited by the short duration (i.e. 28 days) of nicotine self-administration. It is possible that the effects of ABT-107 on drug taking may not be realized until rats have self-administered nicotine for a minimum of 60 days.
Both the locomotor-stimulating effects and the reinforcing properties of nicotine are mediated, in part, by increased dopamine signaling in the ventral striatum [59-61]. When compared to nicotine, ABT-089 is 30% less efficacious and 25-fold less potent in stimulating dopamine release from rat striatal slices [62]. While these findings suggest that the lack of effect of ABT-089 on nicotine self-administration may be due, in part, to its reduced potency to stimulate dopamine release, future studies are required to determine the exact neurochemical effects of ABT-089 in rat self-administering nicotine. With regard to ABT-107, a recent study demonstrated that ABT-107 administration does not produce locomotor sensitization or cross-sensitize to nicotine when administered to nicotine-experienced rats [39]. In contrast to the behavioral-activating effects of nicotine, these results suggest that ABT-107 does not stimulate striatal dopamine release to promote hyperlocomotion. Thus, it is possible that rats continue to self-administer nicotine at baseline levels following pretreatment with ABT-089 and ABT-107 due to the relatively weak influence of these agonists on nucleus accumbens dopamine transmission.
In contrast to a growing literature examining the role of nAChR subtypes in nicotine reinforcement, there is a paucity of studies investigating nAChR subtypes in the reinstatement of nicotine-seeking behavior. Administration of varenicline, an α4β2 nAChR partial agonist and α7 nAChR agonist, attenuates nicotine seeking in rats [17, 23] [however see, 63]. Furthermore, chronic nicotine exposure increases α7 nAChR/NMDA receptor complex formation in the brain and disrupting this interaction attenuates cue-induced nicotine seeking [22]. Our results extend these findings and indicate that administration of the α4β2* and α6β2* nAChR partial agonist ABT-089 and the α7 nAChR agonist ABT-107 is sufficient to attenuate the reinstatement of nicotine seeking elicited by a priming injection of nicotine and cues previously paired with nicotine taking. A recent study, however, found that administration of the α7 nAChR antagonist MLA attenuated cue-induced nicotine seeking in rats [64]. The reason for these discrepant findings is not clear but may involve altered brain permeability of MLA following chronic nicotine exposure and/or non-specific effects of MLA at other nAChR subtypes [65, 66]. The ability of ABT-089 and ABT-107 to attenuate nicotine, but not sucrose, seeking indicates that these effects do not generalized to other reinforced behaviors or are due to general motor impairments following drug administration. Collectively, these data highlight the need to further identify the role of nAChRs in the reinstatement of nicotine-seeking behavior and investigate the efficacy of subtype-selective nAChR agonists to attenuate nicotine seeking. One limitation of the current study is that it does not examine the effects of ABT-089 and ABT-107 on drug priming-versus cue-induced reinstatement of nicotine seeking. Future studies are required to identify the effects of these nAChR agonists on regulating these two aspects of nicotine seeking.
Chronic dosing of smoking cessation medications is required to prevent nicotine craving and relapse in human smokers. Therefore, it will be necessary to study the effects of repeated dosing of ABT-089 and ABT-107 during nicotine withdrawal on subsequent drug seeking. It is possible that ABT-089 and ABT-107 treatment alone may have limited efficacy in human smokers. Whether ABT-089 and/or ABT-107 represent first-line pharmacotherapies or adjunctive therapies for smoking cessation remains to be determined. Interestingly, a recent study found that acute administration of ABT-089 or ABT-107 reversed/normalized anxiety-like behaviors during nicotine withdrawal in mice [42]. Together with the present findings, these results indicate that the nAChR partial agonists ABT-089 and ABT-107 may alleviate nicotine withdrawal-induced anxiety and attenuate nicotine relapse in human smokers.
The present results indicate that acute administration of ABT-089 and ABT-107 is sufficient to attenuate the reinstatement of nicotine-seeking behavior in rats. These results suggest that subtype-selective nAChR agonists that target neuronal α4β2*, α6β2* and α7 nAChRs may prevent nicotine craving and relapse in abstinent human smokers. Future clinical trials are required to determine the efficacy of ABT-089 and ABT-107 as smoking cessation pharmacotherapies in treatment-seeking smokers.
Highlights.
Acute administration of ABT-089 or ABT-107 attenuated the reinstatement of nicotine seeking.
ABT-089 or ABT-107 administration did not affect sucrose-seeking behavior.
Nicotine and sucrose self-administration were not affected by ABT-089 or ABT-107 pretreatment.
Acknowledgments
This work was supported, in part, by a K01 training grant (K01 DA030445) from the National Institutes of Health (NIH) and a pilot grant from the Center for Interdisciplinary Research on Nicotine Addiction (CIRNA), supported by the National Cancer Institute (P50 CA 143187), at UPenn. The authors would like to thank AbbVie for generously providing ABT-089 and ABT-107 for these studies. We also thank Kelsey Ige for her technical assistance, Dr. Lynne Rueter (Associate Director II, Neuroscience Discovery; AbbVie) for her helpful discussions, and Dr. Caryn Lerman for her critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no potential conflict of interest relating to this study.
References
- [1].Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311:193–4. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- [3].Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–81. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- [4].Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- [5].Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nature reviews Neuroscience. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- [6].Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochemical pharmacology. 2011;82:984–95. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annual review of neuroscience. 2011;34:105–30. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- [9].Hays JT, Ebbert JO, Sood A. Efficacy and safety of varenicline for smoking cessation. Am J Med. 2008;121:S32–42. doi: 10.1016/j.amjmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- [10].Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- [11].Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13:41–6. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–6. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- [13].Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. The Journal of pharmacology and experimental therapeutics. 1983;226:817–25. [PubMed] [Google Scholar]
- [14].Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buccafusco JJ, Beach JW, Terry AV., Jr. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. The Journal of pharmacology and experimental therapeutics. 2009;328:364–70. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, et al. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. The Journal of pharmacology and experimental therapeutics. 2012 doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208:365–76. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- [18].Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- [19].Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–51. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- [20].Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306:407–20. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- [21].Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–73. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- [22].Li S, Li Z, Pei L, Le AD, Liu F. The alpha7nACh-NMDA receptor complex is involved in cue-induced reinstatement of nicotine seeking. J Exp Med. 2012;209:2141–7. doi: 10.1084/jem.20121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2012;15:1265–74. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Levin ED. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharmacol. 2013;86:1145–52. doi: 10.1016/j.bcp.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- [26].Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- [27].Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106:61–4. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacology, biochemistry, and behavior. 2011;99:285–94. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–63. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert opinion on drug discovery. 2014;9:579–94. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol. 2009;78:795–802. doi: 10.1016/j.bcp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin NH, Gunn DE, Ryther KB, Garvey DS, Donnelly-Roberts DL, Decker MW, et al. Structure-activity studies on 2-methyl-3-(2(S)-pyrrolidinylmethoxy) pyridine (ABT-089): an orally bioavailable 3-pyridyl ether nicotinic acetylcholine receptor ligand with cognition-enhancing properties. J Med Chem. 1997;40:385–90. doi: 10.1021/jm960233u. [DOI] [PubMed] [Google Scholar]
- [34].Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–58. [PubMed] [Google Scholar]
- [35].Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, et al. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10:167–82. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, et al. Efficacy and safety of the novel alpha(4)beta(2) neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl) 2012;219:715–25. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- [37].Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–70. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- [38].Malysz J, Anderson DJ, Gronlien JH, Ji J, Bunnelle WH, Hakerud M, et al. In vitro pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J Pharmacol Exp Ther. 2010;334:863–74. doi: 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- [39].Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther. 2010;334:875–86. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- [40].Radek RJ, Robb HM, Stevens KE, Gopalakrishnan M, Bitner RS. Effects of the novel alpha7 nicotinic acetylcholine receptor agonist ABT-107 on sensory gating in DBA/2 mice: pharmacodynamic characterization. J Pharmacol Exp Ther. 2012;343:736–45. doi: 10.1124/jpet.112.197970. [DOI] [PubMed] [Google Scholar]
- [41].Othman AA, Lenz RA, Zhang J, Li J, Awni WM, Dutta S. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol. 2011;51:512–26. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- [42].Yohn NL, Turner JR, Blendy JA. Activation of alpha4beta2*/alpha6beta2* Nicotinic Receptors Alleviates Anxiety during Nicotine Withdrawal Without Upregulating Nicotinic Receptors. J Pharmacol Exp Ther. 2014;349:348–54. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an Acetylcholinesterase Inhibitor and Positive Allosteric Modulator of Nicotinic Acetylcholine Receptors, Attenuates Nicotine Taking and Seeking in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD. Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addiction Biology. 2012 doi: 10.1111/adb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kohlhaas KL, Bitner RS, Gopalakrishnan M, Rueter LE. Effects of alpha7 nicotinic acetylcholine receptor agonists on antipsychotic efficacy in a preclinical mouse model of psychosis. Psychopharmacology (Berl) 2012;220:823–33. doi: 10.1007/s00213-011-2535-6. [DOI] [PubMed] [Google Scholar]
- [46].LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–13. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [47].Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:685–96. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–41. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–24. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- [50].Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- [51].Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, et al. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006;187:189–99. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- [52].Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cahir E, Pillidge K, Drago J, Lawrence AJ. The necessity of alpha4* nicotinic receptors in nicotine-driven behaviors: dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology. 2011;36:1505–17. doi: 10.1038/npp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- [55].Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–73. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, et al. bPiDI: a novel selective alpha6beta2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol. 2011;163:346–57. doi: 10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, et al. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–9. [PubMed] [Google Scholar]
- [58].Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–13. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- [60].Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–41. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC, et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–46. [PubMed] [Google Scholar]
- [63].Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216:267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu X. Effects of blockade of alpha4beta2 and alpha7 nicotinic acetylcholine receptors on cue-induced reinstatement of nicotine-seeking behaviour in rats. Int J Neuropsychopharmacol. 2014;17:105–16. doi: 10.1017/S1461145713000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lockman PR, Van der Schyf CJ, Abbruscato TJ, Allen DD. Chronic nicotine exposure alters blood-brain barrier permeability and diminishes brain uptake of methyllycaconitine. J Neurochem. 2005;94:37–44. doi: 10.1111/j.1471-4159.2005.03162.x. [DOI] [PubMed] [Google Scholar]
- [66].Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]