Abstract
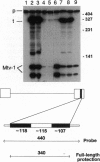
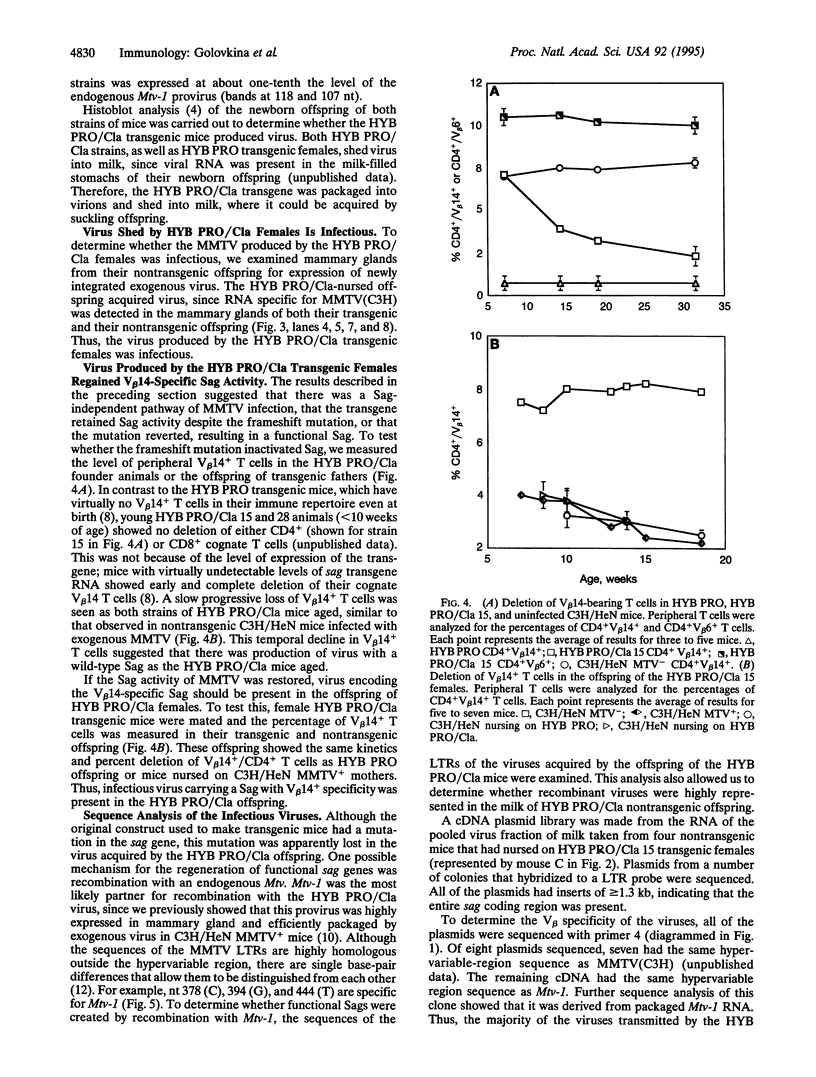
Mouse mammary tumor virus (MMTV) encodes a superantigen that is important for viral infectivity in vivo. To determine whether superantigen function was required for infection by milk-borne MMTV, we created HYB PRO/Cla transgenic mice. These mice produced a full-length, packaged viral RNA with a frameshift mutation that caused premature termination of the superantigen protein. Young HYB PRO/Cla mice showed no deletion of their cognate V beta 14+ T cells, although they shed virus in their milk. The nontransgenic offspring of the HYB PRO/Cla mice were infected with this virus, since transgene-specific viral transcripts were detected in their mammary glands. Surprisingly, these offspring demonstrated the progressive deletion of V beta 14+ T cells characteristic of exogenous MMTV (C3H) infection. Sequence analysis demonstrated that these newly acquired viruses had reconstituted superantigen open reading frames resulting from recombination between the HYB PRO/Cla and endogenous Mtv-1 proviral RNAs. Thus, there is selection during the infection process for MMTVs with functional superantigen genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Beutner U., Kraus E., Kitamura D., Rajewsky K., Huber B. T. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994 May 1;179(5):1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S., Wheeler D. Phylogenetic and structural analyses of MMTV LTR ORF sequences of exogenous and endogenous origins. Virology. 1993 Mar;193(1):171–185. doi: 10.1006/viro.1993.1113. [DOI] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Gallahan D., D'Hoostelaere L., Potter M. Novel class of mouse mammary tumor virus-related DNA sequences found in all species of Mus, including mice lacking the virus proviral genome. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4113–4117. doi: 10.1073/pnas.79.13.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Hoggan M. D., Willey R. L., Strebel K., Martin M. A., Repaske R. Genetic recombination of human immunodeficiency virus. J Virol. 1989 Mar;63(3):1455–1459. doi: 10.1128/jvi.63.3.1455-1459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Prescott J. A., Janeway C. A., Jr, Ross S. R. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J Exp Med. 1994 Feb 1;179(2):439–446. doi: 10.1084/jem.179.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Jaffe A. B., Ross S. R. Coexpression of exogenous and endogenous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J Virol. 1994 Aug;68(8):5019–5026. doi: 10.1128/jvi.68.8.5019-5026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Tikhonenko A. T., Vassetzky N. S., 3rd, Sheftel B. I., Gudkov A. V. Distribution of mouse mammary tumor virus-related sequences does not correlate with the taxonomic position of their hosts. Virus Genes. 1990 Jun;4(1):85–92. doi: 10.1007/BF00308568. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Shakhov A. N., Waanders G., Scarpellino L., Luethy R., Kraehenbuhl J. P., MacDonald H. R., Acha-Orbea H. An exogenous mouse mammary tumor virus with properties of Mls-1a (Mtv-7). J Exp Med. 1992 Jun 1;175(6):1623–1633. doi: 10.1084/jem.175.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Acha-Orbea H., MacDonald H. R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993 Aug 13;74(3):529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- Henrard D., Ross S. R. Endogenous mouse mammary tumor virus is expressed in several organs in addition to the lactating mammary gland. J Virol. 1988 Aug;62(8):3046–3049. doi: 10.1128/jvi.62.8.3046-3049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Maltzman J., Raulet D. H. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989 Jul 1;170(1):135–143. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9655–9659. doi: 10.1073/pnas.85.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Wang H., Acha-Orbea H., Pauley R. J., Wei W. Z. A new infectious mammary tumor virus in the milk of mice implanted with C4 hyperplastic alveolar nodules. Eur J Immunol. 1993 Nov;23(11):2765–2769. doi: 10.1002/eji.1830231107. [DOI] [PubMed] [Google Scholar]
- Stuhlmann H., Berg P. Homologous recombination of copackaged retrovirus RNAs during reverse transcription. J Virol. 1992 Apr;66(4):2378–2388. doi: 10.1128/jvi.66.4.2378-2388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonari K., Fairchild S., Rosenwasser O. A. Influence of viral superantigens on V beta- and V alpha-specific positive and negative selection. Immunol Rev. 1993 Feb;131:131–168. doi: 10.1111/j.1600-065x.1993.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Nagase H., Nakano H., Matsuzawa A., Nariuchi H. A V beta 8.2-specific superantigen from exogenous mouse mammary tumor virus carried by FM mice. Eur J Immunol. 1994 Jul;24(7):1612–1619. doi: 10.1002/eji.1830240724. [DOI] [PubMed] [Google Scholar]