Abstract
Microglia are considered the brain’s resident immune cell involved in immune defense, immunocompetence, and phagocytosis. They maintain tissue homeostasis within the brain and spinal cord under normal condition and serves as its initial host defense system. However, when the central nervous system (CNS) faces injury, microglia respond through signaling molecules expressed or released by neighboring cells. Microglial responses are dual in nature. They induce a nonspecific immune response that may exacerbate CNS injury, especially in the acute stages, but are also essential to CNS recovery and repair.
The full range of microglial mechanisms have yet to be clarified, but there is accumulating knowledge about microglial activation in acute CNS injury. Microglial responses require hours to days to fully develop, and may present a therapeutic target for intervention with a much longer window of opportunity compare to other neurological treatments. The challenge will be to find ways to selectively suppress the deleterious effects of microglial activation without compromising its beneficial functions. This review aims to provide an overview of the recent progress relating on the deleterious and beneficial effect of microglia in the setting of acute CNS injury and the potential therapeutic intervention against microglial activation to CNS injury.
Keywords: Microglia, inflammation, brain, spinal cord, stroke, trauma
2. Introduction
Microglia represent anywhere from 5–20 % of the total glial population and are key modulators of the immune response in the brain (Kreutzberg 1996). Under normal physiological conditions, these highly dynamic and motile cells are spread throughout the brain and spinal cord and constantly survey their microenvironment for noxious agents and injurious processes (Nimmerjahn et al. 2005). They respond to extracellular signals and are responsible for clearing debris and toxic substances by phagocytosis, thereby maintaining normal cellular homeostasis in the central nervous system (CNS) (Hanisch and Kettenmann 2007). Therefore, under non-pathological conditions there is continuous low-level microglial activity in the CNS which is primarily involved in activity-dependent synaptic pruning and repair (El Khoury et al. 1998).
However, in the event of infection, inflammation, trauma, ischemia, and neurodegeneration, microglia quickly respond and can undergo morphologic transformation from a resting state referred to as “ramified” to an active “amoeboid” state, where they become virtually indistinguishable from circulating macrophages (Kreutzberg 1996; Thomas 1992). Therefore, activated microglia are often called “resident brain macrophages”. This is also stems from the fact that microglia are of mesodermal origin as are macrophages. The origin of brain microglia and whether microglia are renewed in situ or are replenished by precursors originating outside of the CNS is the subject of controversy (Lawson et al. 1992; Priller et al. 2001; Simard et al. 2006; Ajami et al. 2007; Mildner et al. 2007). The current thinking is that embryonic hemoatopoietic waves of microglial recruitment and differentiation occur in the CNS, and that maintenance and local expansion of microglia are solely dependent on the self-renewal of CNS resident cells (Ajami et al. 2007; Ginhoux et al. 2010), rather than continuous replenishment from the periphery.
Microglial normally display a ramified appearance, but when activated, microglia become amoeboid and it are indistinguishable from macrophages and circulating monocytes, not only morphologically, but also with regard to surface markers and function (Saijo and Glass 2011; Appel et al. 2011). Despite the many similarities between microglia and macrophages, there are a few reports regarding cell type specific gene transcription mechanisms in these tow cell types (Durafourt et al. 2012; Lee et al. 2014). There are also several reports using bone marrow chimera model obtained by transplantation of lethally irradiated recipients to distinguish the microglia to macrophages (Tang et al. 2012b; Evans et al. 2014). However, due to technical issues, such as BBB disruption by irradiation permitting influx of circulating myeloid cells, limit the interpretation of data generated by this approach (Ajami et al. 2007; Ginhoux et al. 2010).
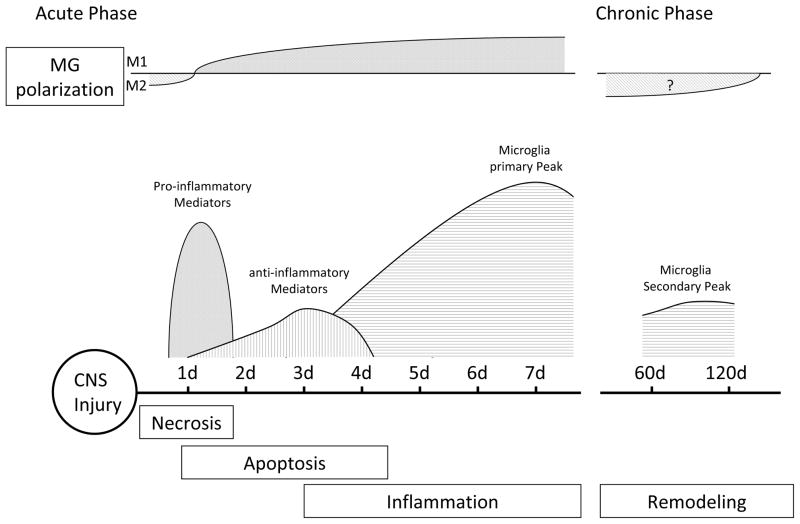
Accumulating evidence now indicates that activated microglial responses can be detrimental as well as beneficial after CNS injury (Figure 1). Once activated, microglia are thought to release a variety of inflammatory and cytotoxic mediators contributing to cell damage and cell death leading to exacerbated damage (Wood 1995; Lai and Todd 2006). Deleterious effects seem to be predominated especially in the acute stages of CNS injury, while beneficial activities characterize later stages. Recent studies demonstrated that microglia and macrophages can be activated into two major phenotypes: classically activated (M1) and alternatively activated (M2) (Chawla 2010; Gordon and Taylor 2005; Geissmann et al. 2010). Lipopolysaccharide (LPS) and the pro-inflammatory cytokine IFNγ promote M1 phenotype, which produces high levels of pro-inflammatory cytokines and oxidative metabolites such as IL-12, TNF-α, IL-6, IL-1β, and nitric oxide (NO), factors previously shown to cause additional damage. In contrast, M2 cells are activated in response to IL-4 or IL-13(Nair et al. 2006; Nguyen et al. 2011), which are thought to suppress inflammation, tissue repair, and promote wound healing (Colton 2009). Hu et al. and Wang et al. recently reported that local microglia and recruited macrophages assume a M2 phenotype at an early stage, peaking at around 5 days from injury, gradually transforming into a M1 phenotype at the sites of injury in ischemic stroke and traumatic brain injury. The M1 phenotype seems to be primed by ischemic neurons and leads to exacerbated neuronal damage, whereas M2 protects against it (Hu et al. 2012; Wang et al. 2013). This raises the importance of the understanding of different phenotype and their function of microglia (Mantovani et al. 2004; Mosser and Edwards 2008).
Fig. 1.
Schematic of the time course of microglial activation after CNS injury. Temporal evolution of M1 and M2 microglial polarization ratios is indicated.
Microglia require longer time windows to fully develop as described later, and thus presents a good target for therapeutic intervention with a much longer window of opportunity compare to other neurological treatment. The challenge will be to find ways to selectively suppress the deleterious effects of microglial activation after CNS injury without compromising repair and remodeling.
In this review, the authors will focus on the deleterious and beneficial effect of microglia in the setting of acute CNS injury and the potential therapeutic intervention against microglial activation to the CNS injury.
3. Mechanisms of microglial cytotoxicity and cellular protection
Microglia, like macrophages, respond to invading pathogens by facilitating rapid sequestration and killing of microorganisms and limit the effects of damage and cell necrosis (Ransohoff and Perry 2009). These responses include rapid migration, proliferation, and release of superoxide, nitric oxide (NO), proteases, cytokines, and phagocytosis of the damaged cells. However, some of these reactions may lead to deleterious effects to the CNS.
Superoxide and Nitric oxide
Superoxide, produced by the partial reduction of molecular oxygen, is a reactive species which interacts with other molecules to produce more highly reactive oxygen species, such as peroxynitrite, hypochlorous acid, carbonyl radical, and hydroxyl radical, all of which are directly cytotoxic to neurons and other cells. Superoxide and other reactive species are also pro-inflammatory signaling molecules which promote microglial activation in a feed-forward manner (Mander et al. 2006; Kauppinen et al. 2008). The production of superoxide in microglia occurs primarily by NADPH oxidase (NOX), of which several isoforms have been characterized (Groemping and Rittinger 2005; Lambeth 2004). The major isoform found in immune cells including microglia is NOX2, or professional NOX. Activation of NOX through has been demonstrated in brain ischemia and related disorders, and its inhibition or deficiency has been shown to be protective (Tang et al. 2012a). While NOX2 is present in both microglia and circulating immune cells, one study using a bone marrow chimera model suggested that the detrimental effects of NOX-generated superoxide was due to NOX present in brain cells (Walder et al. 1997). However, work from our own group using a similar approach indicated that while superoxide generated by NOX in both microglia and circulating immune cells contributed to ischemic brain injury (Tang et al. 2011; Yenari et al. 2006), NOX in circulating immune cells contributed more to injury.
Nitric oxide (NO) is another major reactive species produced by immune cells. Activated microglia produce NO through inducible nitric oxide synthase (iNOS). The cytotoxicity of NO is thought to be due primarily to its reactive metabolite, peroxynitrite, which is formed by reaction with superoxide (Beckman and Koppenol 1996). However, like superoxide, NO is also a powerful signaling molecule, and also promotes pro-inflammatory responses in a feed forward manner.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are proteases that can break down extracellular proteins, such as collagen, and are involved in extracellular matrix remodeling. MMPs are normally found in the microglial cytosol as an inactivated state, they are cleaved by proteases such as plasmin or other MMPs to their active state (Rosenberg 2002). Some MMPs, notably MMP-2 also have direct cytotoxic effects and can disrupt the blood-brain barrier (Candelario-Jalil et al. 2009). Microglia are the major source of MMP, especially MMP-3 and -9(Rosenberg et al. 2001; del Zoppo et al. 2007). Recent work has indicated that fibronectin and vitronectin, substances typically found in the plasma, can activate microglial cells to generate pro-MMP-9 (del Zoppo et al. 2012). Bone marrow chimera models, where bone marrow of host animals is transplanted with marrow from host marrow, have shown that MMPs derived from immune cells contribute to worsened ischemic injury, although the contribution from leukocytes appears more significant that that contributed by microglia (Gidday et al. 2005; Wang et al. 2009).
Chemokines, cytokines, and trophic factors
Resting microglia release a variety of chemokines and cytokines, and pattern of this release is dramatically altered after CNS injury (Lucas et al. 2006). These factors function primarily as intercellular signaling molecules, and many have feed-forward effects in driving the inflammatory response. Some, such as tumor necrosis factor-α (TNFα), can also have direct cytotoxic effects and promote disruption of the blood-brain barrier (Shohami et al. 1999; Vexler et al. 2006). On the other hand, microglia also release a number of neurotrophic factors, such as TGFβ1, BDNF, and GDNF, and these are thought to be important in maintaining neuronal integrity after CNS injury (Lehrmann et al. 1998; Suzuki et al. 1999; Lee et al. 2002; Batchelor et al. 1999).
Phagocytosis
Microglia and macrophages phagocytose injured cells, thereby clearing necrotic debris and setting the stage for recovery. A few signals that lead to phagocytosis have been recently identified. One model proposes that necrotic cells release nucleic acid remnants into the extracellular space where they can bind appropriate receptors on phagocytes. Some of these phagocytosis initiating signals have been referred to as DAMPs, or danger associated molecular patterns (An et al. 2013). Those identified in stroke and related injury models include purines such as UTP, ADP and ATP, and signal through purinergic receptor systems to lead to phagocytosis (Koizumi et al. 2007). However, phagocytosis through these signaling systems, while leading to the clearance of injured cells, may also worsen cell death either by causing microglia to phagocytose viable cells or generate neurotoxic substances (Emmrich et al. 2013; Neher et al. 2013). Our laboratory has recently begun to study a recently characterized microglial receptor, triggering receptor expressed on myeloid cells (TREM2). TREM2 was originally characterized by its ability to bind pathogens such as bacteria and initiate phagocytosis (N’Diaye et al. 2009). It has been described on activated macrophages and microglia (Sessa et al. 2004; Takahashi et al. 2005; Daws et al. 2001), and binds to one or more ill-defined ligands on eukaryotic cells including neurons and astrocytes (Hsieh et al. 2009; Stefano et al. 2009; Daws et al. 2003). TREM2 has been shown to mediate phagocytosis of apoptotic neurons without stimulating a typical inflammatory response (Hsieh et al. 2009). Conversely, loss of TREM2 impairs phagocytosis and promotes inflammation (Takahashi et al. 2005). In a model of neuroprotection by therapeutic hypothermia, our group found that while therapeutic cooling led to decreased activated microglia and other pro-immune responses, TREM2 was actually increased on microglia of brains protected from ischemia, suggesting that TREM2 was correlated to improved stroke outcome (Kawabori et al. 2013). Thus, some phagocytic pathways of microglia may potentiate damage, while other pathways may ameliorate it. Preliminary work in our lab suggests that preventing phagocytosis by deleting TREM2 may exacerbate functional neurological recovery (Kawabori et al, International Stroke Conference 2014 abstract).
Phagocytic activities of microglia are also shown to regulate the numbers of synapses and affect the structural plasticity of the CNS. Microglia are engaged in the regulation and remodeling of synapses both in perinatal and postnatal periods (Ji et al. 2013; Paolicelli et al. 2011). These observations highlight the fact that microglial activities are crucial not only under pathological condition, but also under physiological condition.
4. Microglial activation in Stroke
Stroke is a leading cause of death and disability in the industrialized world (Onwuekwe and Ezeala-Adikaibe 2012; Towfighi and Saver 2011). Stroke is a heterogeneous condition consisting of several subtypes. Regardless, stroke can be broadly categorized into ischemic and hemorrhagic types. Microglia have been documented to play an important role in both, contributing to inflammatory responses both negatively and positively.
Microglia in ischemic stroke
Ischemic stroke constitutes 87% of all strokes and is caused by the occlusion of a blood vessel due to either embolism or thrombus. As a result, brain tissue is deprived of blood glucose and oxygen. This leads to neuronal death, release of reactive oxygen species and other substances. Many of these molecules are shown to activate microglia and causes secondary damage to the injured and alive cells that escaped the damage (Yenari and Han 2012; Taylor and Sansing 2013). Accumulating data shows that TNF-α, glutamate, heat shock protein (HSP), Adenosine triphosphate (ATP), CD14 receptors, followed by toll-like receptor 4 (TRL4) have been documented in activated microglia in the infarct brain (Saito et al. 2000; Beschorner et al. 2002b; Lehnardt et al. 2003).
Activated microglia can be detected as early as 2 hours after ischemia, whereas blood-derived macrophages do not enter the brain before 10 hours. By 22–46 hours after the insult, activated microglia and macrophages are distributed throughout the entire lesion and are detectable up to 1 week after the insult (Zhang et al. 1995; Nilupul Perera et al. 2006; Stoll et al. 1998; Dirnagl et al. 1999; Clausen et al. 2008).
Direct evidence supporting a damaging role of microglia/macrophages was demonstrated when their direct application potentiated neuron cell death (Giulian et al. 1993; Lehnardt et al. 2003; Zhang et al. 1997; Huang et al. 2010), and microglia were also shown to express and release various kind of inflammatory mediators as described above, most of which are cytotoxic. Recent work has shown that microglia can potentiate injury to blood-brain barrier constituents (astrocytes and endothelial cells) via NOX-mediated superoxide in cell culture models of ischemia (Yenari et al. 2006). Several groups also show that mice deficient in the gp91 subunit of NOX2 have smaller infarcts than do wild-type mice (Kahles et al. 2007; Walder et al. 1997; Chen et al. 2009), and that outcomes from experimental cerebral ischemia reperfusion are improved with early administration of the pharmacological NOX inhibitor, apocynin (Chen et al. 2009; Tang et al. 2007; Tang et al. 2005; Tang et al. 2008). Pharmacological inhibition of iNOS reduces infarct volume (Iadecola et al. 1995), and iNOS null mice have smaller infarcts and better neurological outcomes than wild-type control animals (Zhao et al. 2000). Therapeutic hypothermia after ischemia likewise reduces microglial iNOS expression and NO production (Han et al. 2002). Inhibition of MMP at the acute stage of ischemia are also shown to reduce infarct size, brain edema, and recombinant tissue plasminogen activator-induced hemorrhage (Pfefferkorn and Rosenberg 2003), and mice deficient in MMP-9 or MMP-3 have reduced ischemic injury compare to wild-type (Asahi et al. 2000; Walker and Rosenberg 2009). However, because of the neurovascular remodeling function of the protease, prolonged inhibition of MMPs after ischemia may have deleterious effects on function recovery (Zhao et al. 2006). Of translational relevance, the widely used antiplatelet agent, clopidogrel, is also an antagonist of the P2Y12 purinergic receptor. P2Y12 is known to mediate microglial chemotaxis under conditions of injury. P2Y12 deletion or its inhibition by clopidogrel led to reduced microglial migration to areas of injury, and also protected the brain from global cerebral ischemia (Webster et al. 2013).
In addition to the pro-inflammatory aspects of microglia, microglia have also been shown to have anti-inflammatory properties, which are neuroprotective. Microglia produce the growth factor TGF-β1(Watanabe et al. 2000; Lai and Todd 2006). When microglial proliferation was inhibited in transgenic mice, infarct size was increased following ischemia, and suggests that proliferating microglia cells exert a beneficial role (Lalancette-Hebert et al. 2007). There are some possible mechanisms underlying these observations. First, microglia produce neurotrophic factors which stimulate neurogenesis and plasticity. Secondly, phagocytosis of neutrophils by activated microglia may prevent the release of toxic mediators (Weston et al. 2007; Frank-Cannon et al. 2009). Finally, resident macrophages scavenge and remove necrotic debris and other potentially harmful substances (Frank-Cannon et al. 2009).
Minocycline, a tetracycline family antibiotic, was shown to provide significant protection against brain ischemia by inhibiting microglial activation and proliferation (Yrjanheikki et al. 1998; Yrjanheikki et al. 1999), and minocycline has shown to protect against permanent cerebral ischemia in wild-type but not in MMP-9 deficient mice (Koistinaho et al. 2005). Edaravone, a novel free radical scavenger, significantly reduced infarct volume and improved neurological deficit scores for ischemic mice by reducing microglial activation (Zhang et al. 2005). In spontaneously hypertensive rats with permanent MCAO, repetitive hyperbaric oxygen (HBO) treatment reduced infarct volume by suppressing microglia activation (Gunther et al. 2005). Protection by hypothermia has been shown to be related, in part, to inhibiting microglial activation and reducing elaboration of many pro-inflammatory immune molecules (Yenari and Han 2012; Han et al. 2002). However, as mentioned above, hypothermic neuroprotection has also been associated with the upregulation of the pro-phagocytic molecule, TREM2 (Kawabori et al. 2013).
Recombinant human tissue plasminogen activator (rt-PA) is the only approved thrombolytic treatment of ischemic stroke at the current medical practice. Beside from its thrombolytic aspect, recent evidence shows that t-PA and its substrate plasmin enhances microglial cell activation and recruitment to the injured site through several chemokines. Activated microglia are shown to exacerbate the neurological damage after ischemic stroke (Sheehan et al. 2007; Lenglet et al. 2014).
Microglia in intracerebral hemorrhage (ICH)
Intracerebral hemorrhage occurs when a blood vessels in the brain parenchyma ruptures, most commonly due to hypertension and accounts for 10–15% of all strokes (Manno 2012). ICH has a high mortality rate: 30–50% of patients die within the first 30 days (Qureshi et al. 2009). Despite the recent advances in the intracerebral hemorrhage research, no specific treatment for this currently exists (Morgenstern et al. 2010). The introduction of blood components, including thrombin, heme, and leukocytes and platelets, into the brain creates the basis for a secondary injury due to microglial activation and neuroinflammation resulting in the recruitment of leukocytes into a normally immune privileged site (Keep et al. 2012).
Microglial activation may also have a dual role after ICH. While some microglial activities may be beneficial, microglia have also been shown to play a role in the secondary injury that occurs after ICH (Keep et al. 2012). A major role of microglial cells after ICH is to phagocytose the debris and red blood cells left in the brain after hemorrhage. They have been shown to endocytose heme and hemoglobin. These processes are mediated through scavenger receptors, such as CD36(Aronowski and Zhao 2011). As in the case of ischemic stroke, microglia also produce proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, in models of ICH (Wang 2010; Aronowski and Zhao 2011; Wang and Dore 2007b).
The activation of microglia/macrophage occurs early following ICH. Activated microglia have been observed within the peri-hematomal region as early as 1 hour following in the collagenase model ICH (where collagenase disrupts extracellular matrix proteins and causes primary brain hemorrhage) and within 4 hours in the ICH model where whole blood is directly injected into the brain (Wang and Dore 2007a; Xue and Del Bigio 2000). Microglial production of IL-1β in rats can be seen as early as 6 hours and can persist up to 24 hours (Wasserman et al. 2007). The numbers of the activated microglia/macrophages reaches peak at around 72 hours in the peri-hematomal region, and returns to basal levels between three and four weeks (Wang 2010; Yabluchanskiy et al. 2010).
Mediators of microglial activation after ICH have also been studied and some candidates have been found to activate the microglia (Donovan et al. 1997). Thrombin, a serine protease necessary for coagulation, has been shown to play a pivotal role. Direct injection of thrombin into the striatum led to upregulation of CD11b in microglia, and microglia changed from the resting, ramified to activated morphology to an amoeboid shape within 4 hours. Activated microglia were also immunopositive for iNOS by 24 hours and the number of microglia/macrophages were increased by 72 hours (Fujimoto et al. 2007). The effect of thrombin on microglial proinflammatory cytokine and MMPs has also been described. Microglia express thrombin receptors and produce IL-1β, TNF-α, and MMP-9 when stimulated with thrombin (Wu et al. 2008; Xue et al. 2006). Product of red blood cell lysis, including heme and iron, are also active initiators of microglial activation and neuroinflammation (Wu et al. 2008).
Microglia in subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH), especially aneurysmal SAH, is often a catastrophic condition of CNS. Although accounting for only 5% of all strokes, SAH imposes a significant burden on society and economy, as it affects mainly middle-aged patients, leading to high mortality and disability rates (Venti 2012). Early and delayed brain injury after SAH have been well documented, but the underlying mechanisms especially the effect of neuroinflammation have not been well elucidated. Recent findings have highlighted a strong contribution of neuroinflammation to the early brain injury, the vasospasm after SAH (Fassbender et al. 2001; Pradilla et al. 2010). A total of 30 to 40% of aneurysmal SAH patients will have delayed cerebral ischemia from vasospasm, anywhere from 4 to 14 days after the insult, resulting in increased morbidity and mortality. A recent report from Hanafy showed that microglia and the TLR4 signaling pathway play important roles in the development of vasospasm and also in the acute phase of neuronal apoptosis after SAH (Hanafy 2013). Deficiency of TLR4 downstream adaptor molecules MyD88 and TRIF reduced vasospasm as well as neuronal apoptosis. Conversely, another group showed that administration of the TLR4 agonist, lipopolysaccharide (LPS) worsened vasospasm (Smithason et al. 2012).
Microglial activation in trauma
Traumatic brain injury (TBI) and spinal cord injury (SCI) are the leading cause of morbidity and mortality in the younger generations, and have substantial direct, and indirect cost to society (Potts et al. 2006). TBI and SCI are a highly complex disorders caused by both primary and secondary injury mechanisms (Kumar and Loane 2012). Primary injury mechanisms result from the mechanical damage that occurs at the time of trauma to neurons, axons, glia and blood vessels as a result of shearing, tearing, or stretching, consequently these damages induce secondary injury mechanisms that evolve over minutes to days after the initial traumatic insult and result from delayed neuronal damage (McIntosh et al. 1996). The secondary injury includes delayed events, such as ischemia, lipid degradation, free radical formation, excitotoxity, and protease release (Bao et al. 2005; Hausmann 2003), leading to demyelination, axonal degeneration, neuronal death, cavitation, and glial scarring surrounding the area of initial damage (Fitch et al. 1999; Dusart and Schwab 1994; Koshinaga and Whittemore 1995). Inflammation by microglia is thought to play an important role in these expanded secondary damage (Potts et al. 2006; Hausmann 2003), as they not only release proinflammatory cytokines, but rapidly recruit other immune cells and exacerbate injury (Dusart and Schwab 1994; Kigerl et al. 2009). However, as described before, these microglial responses are thought to have not only harmful effects, but beneficial effects as shown in stroke models.
Similar to the response in stroke, microglia have been shown to react within a few hours with a migratory response toward the lesion site following CNS trauma. In fact, in vivo two-photon microscopy imaging studies of microglia following laser-induced injury documented rapid proliferation and movement of ramified microglial cells to the site of injury in response to extracellular ATP released by the injured tissue (Davalos et al. 2005; Haynes et al. 2006). Microglial processes then coalesce to form an area of containment between healthy and injured tissues, suggesting that microglia may represent the first line of defense following injury (Davalos et al. 2005). In human TBI, microglial activation has been reported as early as 72 hours after injury (Engel et al. 2000), and can remain elevated for months after injury as well as in the rodent model of TBI (Beschorner et al. 2002a; Gentleman et al. 2004; Csuka et al. 2000; Maeda et al. 2007).
Previous studies have shown that microglia and blood-derived macrophages release potentially neurotoxic agents after spinal cord injury (reactive oxygen species; NO and peroxynitrite, cytokines; TNF-α and IL-1β)(Satake et al. 2000; Bao et al. 2004). Activation of microglia/macrophages through Toll-like receptors (TLRs) induces neuronal cell death and neurite degeneration (Fitch et al. 1999; Lehnardt et al. 2002; Popovich et al. 2002). White matter is also quite sensitive to these immune molecules, and treatments aimed at reducing the microglial/macrophage response and subsequent neurotoxicity are often protective (Blight 1994; Popovich et al. 1999; Park et al. 2004; Byrnes et al. 2009). These interventions may be expected to prevent the second late phase of axonal dieback (Stirling et al. 2004; Horn et al. 2008). IL-1β, and TNF-α levels are also elevated in both the serum and CSF of patients with severe TBI (Ross et al. 1994; Goodman et al. 1990). TNF-a expression after experimental TBI is detectable after 1 hours, peaks between 3 and 8 hours, and returns to normal level at 24 hours after injury (Stover et al. 2000; Shohami et al. 1994; Fan et al. 1996).
Also in the chronic stage of the injury, activated microglia surround the lesion and remain chronically activated for weeks and months after the initial brain trauma (Maeda et al. 2007). Persistent long-term microglial activation was observed in the traumatized cortex 3 months after experimental brain injury and was associated with increased expression of proinflammatory cytokines, IL-1β and TNF-α (Holmin and Mathiesen 1999). In humans, long-term microglial activation and chronic inflammation after injury may persist for many years in brain injury survivors (Gentleman et al. 2004). These long-term persistent inflammatory changes may cause post-traumatic neurodegeneration, which could form the basis of the cognitive decline that is often observed in long-term survivors of TBI.
Anti-inflammatory cytokine levels are also modulated by TBI. In humans, IL-10 and TFG-b levels are elevated acutely after injury (Morganti-Kossmann et al. 1999; Csuka et al. 1999), and experimental studies have shown that IL-10 has beneficial effects following trauma (Knoblach and Faden 1998). Injection of the anti-inflammatory cytokine TGF-β after injury in rodents reduces damaged lesion size, improves function, and reduces iNOS expression (Tyor et al. 2002; Hamada et al. 1996). Intravenous administration of IL-10 after experimental TBI in rats improved neurological recovery and significantly reduced TNF-α and IL1β expression in the traumatized cortex and hippocampus. These neuroprotective effects may be the result of suppressed microglial activation, in that IL-10 treatment has been shown to decrease production of proinflammatory cytokines (Kremlev and Palmer 2005).
Conclusions
Inflammation following acute CNS injury is increasingly recognized as a key element in its progression. In this review we have focused on several elements which are involved in inflammatory responses following ischemic stroke, intracerebral hemorrhage, SAH, and traumatic injury. Although, early inflammatory responses may potentiate ischemic injury, late responses may be important in recovery and repair. The precise mechanisms of the inflammatory responses are still to be elucidated. And future work and better understanding against this field will shed light on new therapeutic methods to these injuries.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS40516, to MY), the Veteran’s Merit Award (MY), the Uehara Foundation (2013 Research Fellowship, to MK). Grants to MY were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10 (12):1538–1543. doi: 10.1038/nn2014. nn2014 [pii] [DOI] [PubMed] [Google Scholar]
- An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, Leak RK, Gao Y, Sun BL, Zheng P, Chen J. Molecular dialogs between the ischemic brain and the peripheral immune system: Dualistic roles in injury and repair. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011;30 (1):4–8. [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42 (6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. STROKEAHA.110.596718 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20 (12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88(6):1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. 2240 [pii] [DOI] [PubMed] [Google Scholar]
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94 (5):1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. JNC3280 [pii] [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19 (5):1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271 (5 Pt 1):C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002a;103 (6):541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002b;126(1–2):107–115. doi: 10.1016/s0165-5728(02)00046-2. S0165572802000462 [pii] [DOI] [PubMed] [Google Scholar]
- Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60(1):263–273. doi: 10.1016/0306-4522(94)90220-8. 0306-4522(94)90220-8 [pii] [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica B, Riccio A, Pajoohesh-Ganji A, Loane DJ, Faden AI. Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann Neurol. 2009;66 (1):63–74. doi: 10.1002/ana.21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158 (3):983–994. doi: 10.1016/j.neuroscience.2008.06.025. S0306-4522(08)00895-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106 (10):1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. 106/10/1559 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29 (7):1262–1272. doi: 10.1038/jcbfm.2009.47. jcbfm200947 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. 1742-2094-5-46 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4 (4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csuka E, Hans VH, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC. Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport. 2000;11 (11):2587–2590. doi: 10.1097/00001756-200008030-00047. [DOI] [PubMed] [Google Scholar]
- Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101(2):211–221. doi: 10.1016/s0165-5728(99)00148-4. S0165572899001484 [pii] [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8 (6):752–758. doi: 10.1038/nn1472. nn1472 [pii] [DOI] [PubMed] [Google Scholar]
- Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol. 2001;31 (3):783–791. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. doi:10.1002/1521-4141(200103)31:3<783::AID-IMMU783>3.0.CO;2-U [pii] 10.1002/1521-4141(200103)31:3<783::AID-IMMU783>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171 (2):594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, Wang X, Hosomi N, Mabuchi T, Koziol JA. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32 (5):919–932. doi: 10.1038/jcbfm.2012.11. jcbfm201211 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38 (2 Suppl):646–651. doi: 10.1161/01.STR.0000254477.34231.cb. 38/2/646 [pii] [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. S0166-2236(99)01401-0 [pii] [DOI] [PubMed] [Google Scholar]
- Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17 (14):5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60 (5):717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6 (5):712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19 (1 Suppl):S81–84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Emmrich JV, Hornik TC, Neher JJ, Brown GC. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013;280 (20):5030–5038. doi: 10.1111/febs.12401. [DOI] [PubMed] [Google Scholar]
- Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner HD, Meyermann R. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000;100 (3):313–322. doi: 10.1007/s004019900172. [DOI] [PubMed] [Google Scholar]
- Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 2014;254C:109–120. doi: 10.1016/j.expneurol.2014.01.013. S0014-4886(14)00026-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain Res Mol Brain Res. 1996;36(2):287–291. doi: 10.1016/0169-328x(95)00274-v. 0169328X9500274V [pii] [DOI] [PubMed] [Google Scholar]
- Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, Fritzinger M, Horn P, Vajkoczy P, Kreisel S, Brunner J, Schmiedek P, Hennerici M. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70 (4):534–537. doi: 10.1136/jnnp.70.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19 (19):8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. 1750-1326-4-47 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144 (2):694–701. doi: 10.1016/j.neuroscience.2006.09.049. S0306-4522(06)01311-X [pii] [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10 (6):453–460. doi: 10.1038/nri2784. nri2784 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, Nicoll JA. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146 (2–3):97–104. doi: 10.1016/j.forsciint.2004.06.027. S0379-0738(04)00398-6 [pii] [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289 (2):H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330 (6005):841–845. doi: 10.1126/science.1194637. science.1194637 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36 (6):681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30 (2–3):213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5 (12):953–964. doi: 10.1038/nri1733. nri1733 [pii] [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386 (Pt 3):401–416. doi: 10.1042/BJ20041835. BJ20041835 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther A, Kuppers-Tiedt L, Schneider PM, Kunert I, Berrouschot J, Schneider D, Rossner S. Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. Eur J Neurosci. 2005;21 (11):3189–3194. doi: 10.1111/j.1460-9568.2005.04151.x. EJN4151 [pii] [DOI] [PubMed] [Google Scholar]
- Hamada Y, Ikata T, Katoh S, Katoh K, Niwa M, Tsutsumishita Y, Fukuzawa K. Effects of exogenous transforming growth factor-beta 1 on spinal cord injury in rats. Neurosci Lett. 1996;203(2):97–100. doi: 10.1016/0304-3940(95)12271-0. 0304-3940(95)12271-0 [pii] [DOI] [PubMed] [Google Scholar]
- Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22(10):3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. 20026332 22/10/3921 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10:83. doi: 10.1186/1742-2094-10-83. 1742-2094-10-83 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10 (11):1387–1394. doi: 10.1038/nn1997. nn1997 [pii] [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–378. doi: 10.1038/sj.sc.3101483. 3101483 [pii] [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9 (12):1512–1519. doi: 10.1038/nn1805. nn1805 [pii] [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport. 1999;10 (9):1889–1891. doi: 10.1097/00001756-199906230-00017. [DOI] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28 (38):9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. 28/38/9330 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109 (4):1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. JNC6042 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43 (11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. STROKEAHA.112.659656 [pii] [DOI] [PubMed] [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol. 2010;43 (4):325–331. doi: 10.5115/acb.2010.43.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995;268 (1 Pt 2):R286–292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8(2):e56293. doi: 10.1371/journal.pone.0056293. PONE-D-12-37806 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38 (11):3000–3006. doi: 10.1161/STROKEAHA.107.489765. STROKEAHA.107.489765 [pii] [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28 (22):5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. 28/22/5827 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M, Hokari M, Zheng Z, Kim JY, Calosing C, Hsieh CL, Nakamura MC, Yenari MA. Triggering Receptor Expressed on Myeloid Cells-2 Correlates to Hypothermic Neuroprotection in Ischemic Stroke. Ther Hypothermia Temp Manag. 2013;3(4):189–198. doi: 10.1089/ther.2013.0020. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11 (8):720–731. doi: 10.1016/S1474-4422(12)70104-7. S1474-4422(12)70104-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29 (43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. 29/43/13435 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153 (1):143–151. doi: 10.1006/exnr.1998.6877. S0014-4886(98)96877-7 [pii] [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25 (4):460–467. doi: 10.1038/sj.jcbfm.9600040. 9600040 [pii] [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446 (7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;12 (2):209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Palmer C. Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol. 2005;162 (1–2):71–80. doi: 10.1016/j.jneuroim.2005.01.010. S0165-5728(05)00016-0 [pii] [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. 0166-2236(96)10049-7 [pii] [DOI] [PubMed] [Google Scholar]
- Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26 (8):1191–1201. doi: 10.1016/j.bbi.2012.06.008. S0889-1591(12)00150-X [pii] [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84 (1):49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27 (10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. 27/10/2596 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. nri1312 [pii] [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–415. doi: 10.1016/0306-4522(92)90500-2. 0306-4522(92)90500-2 [pii] [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim SK, Kim SH, Kim K, Lim CH, Park J, Roh TY, Kim N, Chai YG. Transcription-related element gene expression pattern differs between microglia and macrophages during inflammation. Inflamm Res. 2014 doi: 10.1007/s00011-014-0711-y. [DOI] [PubMed] [Google Scholar]
- Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Expression disparity of brain-derived neurotrophic factor immunoreactivity and mRNA in ischemic hippocampal neurons. Neuroreport. 2002;13 (17):2271–2275. doi: 10.1097/01.wnr.0000043410.7262. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22(7):2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. 20026268 22/7/2478 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. 1432609100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24(4):437–448. doi: 10.1002/(SICI)1098-1136(199812)24:4<437::AID-GLIA9>3.0.CO;2-X. [pii] [DOI] [PubMed] [Google Scholar]
- Lenglet S, Montecucco F, Denes A, Coutts G, Pinteaux E, Mach F, Schaller K, Gasche Y, Copin JC. Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.9. jcbfm20149 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–240. doi: 10.1038/sj.bjp.0706400. 0706400 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang MR, Suzuki K, Suhara T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res. 2007;1157:100–111. doi: 10.1016/j.brainres.2007.04.054. S0006-8993(07)00918-3 [pii] [DOI] [PubMed] [Google Scholar]
- Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176(2):1046–1052. doi: 10.4049/jimmunol.176.2.1046. 176/2/1046 [pii] [DOI] [PubMed] [Google Scholar]
- Manno EM. Update on intracerebral hemorrhage. Continuum (Minneap Minn) 2012;18(3):598–610. doi: 10.1212/01.CON.0000415430.99394.3e. 00132979-201206000-00011 [pii] [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25 (12):677–686. doi: 10.1016/j.it.2004.09.015. S1471-4906(04)00295-9 [pii] [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74 (2):315–342. [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10 (12):1544–1553. doi: 10.1038/nn2015. nn2015 [pii] [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma. 1999;16 (7):617–628. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41 (9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. STR.0b013e3181ec611b [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8 (12):958–969. doi: 10.1038/nri2448. nri2448 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184 (2):215–223. doi: 10.1083/jcb.200808080. jcb.200808080 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177(3):1393–1399. doi: 10.4049/jimmunol.177.3.1393. 177/3/1393 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A. 2013;110 (43):E4098–4107. doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480 (7375):104–108. doi: 10.1038/nature10653. nature10653 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA. Inflammation following stroke. J Clin Neurosci. 2006;13 (1):1–8. doi: 10.1016/j.jocn.2005.07.005. S0967-5868(05)00341-3 [pii] [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308 (5726):1314–1318. doi: 10.1126/science.1110647. 1110647 [pii] [DOI] [PubMed] [Google Scholar]
- Onwuekwe I, Ezeala-Adikaibe B. Ischemic stroke and neuroprotection. Ann Med Health Sci Res. 2012;2(2):186–190. doi: 10.4103/2141-9248.105669. AMHSR-2-186 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333 (6048):1456–1458. doi: 10.1126/science.1202529. science.1202529 [pii] [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21 (6):754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. 01.STR.0000083051.93319.28 [pii] [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61 (7):623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158(2):351–365. doi: 10.1006/exnr.1999.7118. S0014-4886(99)97118-2 [pii] [DOI] [PubMed] [Google Scholar]
- Potts MB, Koh SE, Whetstone WD, Walker BA, Yoneyama T, Claus CP, Manvelyan HM, Noble-Haeusslein LJ. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx. 2006;3 (2):143–153. doi: 10.1016/j.nurx.2006.01.006. S1545-5343(06)00018-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradilla G, Chaichana KL, Hoang S, Huang J, Tamargo RJ. Inflammation and cerebral vasospasm after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21 (2):365–379. doi: 10.1016/j.nec.2009.10.008. S1042-3680(09)00117-X [pii] [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7(12):1356–1361. doi: 10.1038/nm1201-1356. nm1201-1356 [pii] [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373 (9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. S0140-6736(09)60371-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39 (3):279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893(1–2):104–112. doi: 10.1016/s0006-8993(00)03294-7. S0006-8993(00)03294-7 [pii] [DOI] [PubMed] [Google Scholar]
- Ross SA, Halliday MI, Campbell GC, Byrnes DP, Rowlands BJ. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg. 1994;8 (4):419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11 (11):775–787. doi: 10.1038/nri3086. nri3086 [pii] [DOI] [PubMed] [Google Scholar]
- Saito S, Matsuura M, Tominaga K, Kirikae T, Nakano M. Important role of membrane-associated CD14 in the induction of IFN-beta and subsequent nitric oxide production by murine macrophages in response to bacterial lipopolysaccharide. Eur J Biochem. 2000;267(1):37–45. doi: 10.1046/j.1432-1327.2000.00956.x. ejb956 [pii] [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000;85(1–2):114–122. doi: 10.1016/s0169-328x(00)00253-9. S0169328X00002539 [pii] [DOI] [PubMed] [Google Scholar]
- Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur J Neurosci. 2004;20 (10):2617–2628. doi: 10.1111/j.1460-9568.2004.03729.x. EJN3729 [pii] [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Zhou C, Gravanis I, Rogove AD, Wu YP, Bogenhagen DF, Tsirka SE. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27 (7):1738–1745. doi: 10.1523/JNEUROSCI.4987-06.2007. 27/7/1738 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10(2):119–130. doi: 10.1016/s1359-6101(99)00008-8. S1359610199000088 [pii] [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14 (4):615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49 (4):489–502. doi: 10.1016/j.neuron.2006.01.022. S0896-6273(06)00075-4 [pii] [DOI] [PubMed] [Google Scholar]
- Smithason S, Moore SK, Provencio JJ. Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarachnoid hemorrhage through a myeloid cell-dependent mechanism. Neurocrit Care. 2012;16 (2):327–334. doi: 10.1007/s12028-011-9651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem. 2009;110 (1):284–294. doi: 10.1111/j.1471-4159.2009.06130.x. JNC6130 [pii] [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24(9):2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. 24/9/2182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56(2):149–171. doi: 10.1016/s0301-0082(98)00034-3. S0301-0082(98)00034-3 [pii] [DOI] [PubMed] [Google Scholar]
- Stover JF, Schoning B, Beyer TF, Woiciechowsky C, Unterberg AW. Temporal profile of cerebrospinal fluid glutamate, interleukin-6, and tumor necrosis factor-alpha in relation to brain edema and contusion following controlled cortical impact injury in rats. Neurosci Lett. 2000;288(1):25–28. doi: 10.1016/s0304-3940(00)01187-3. S0304-3940(00)01187-3 [pii] [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, Fukuuchi Y. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19 (11):1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201 (4):647–657. doi: 10.1084/jem.20041611. jem.20041611 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, Zhang JH. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94 (5):1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. JNC3292 [pii] [DOI] [PubMed] [Google Scholar]
- Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35 (4):517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154 (2):556–562. doi: 10.1016/j.neuroscience.2008.03.090. S0306-4522(08)00523-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012a;34 (4):338–345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol. 2011;70 (4):606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Zheng Z, Yenari MA. Bone marrow chimeras in the study of experimental stroke. Transl Stroke Res. 2012b;3 (3):341–347. doi: 10.1007/s12975-012-0169-6. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17 (1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42 (8):2351–2355. doi: 10.1161/STROKEAHA.111.621904. STROKEAHA.111.621904 [pii] [DOI] [PubMed] [Google Scholar]
- Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL. Treatment of spinal cord impact injury in the rat with transforming growth factor-beta. J Neurol Sci. 2002;200(1–2):33–41. doi: 10.1016/s0022-510x(02)00113-2. S0022510X02001132 [pii] [DOI] [PubMed] [Google Scholar]
- Venti M. Subarachnoid and intraventricular hemorrhage. Front Neurol Neurosci. 2012;30:149–153. doi: 10.1159/000333625. 000333625 [pii] [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke. Clin Neurosci Res. 2006;6 (5):293–313. doi: 10.1016/j.cnr.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28 (11):2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Walker EJ, Rosenberg GA. TIMP-3 and MMP-3 contribute to delayed inflammation and hippocampal neuronal death following global ischemia. Exp Neurol. 2009;216 (1):122–131. doi: 10.1016/j.expneurol.2008.11.022. S0014-4886(08)00444-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Guo Q, Hossain M, Fazio V, Zeynalov E, Janigro D, Mayberg MR, Namura S. Bone marrow-derived cells are the major source of MMP-9 contributing to blood-brain barrier dysfunction and infarct formation after ischemic stroke in mice. Brain Res. 2009;1294:183–192. doi: 10.1016/j.brainres.2009.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33 (12):1864–1874. doi: 10.1038/jcbfm.2013.146. jcbfm2013146 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92 (4):463–477. doi: 10.1016/j.pneurobio.2010.08.001. S0301-0082(10)00144-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007a;130 (Pt 6):1643–1652. doi: 10.1093/brain/awm095. 130/6/1643 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007b;27 (5):894–908. doi: 10.1038/sj.jcbfm.9600403. 9600403 [pii] [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. S0006-8993(07)01954-3 [pii] [DOI] [PubMed] [Google Scholar]
- Watanabe H, Abe H, Takeuchi S, Tanaka R. Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neurosci Lett. 2000;289(1):53–56. doi: 10.1016/s0304-3940(00)01252-0. S0304-3940(00)01252-0 [pii] [DOI] [PubMed] [Google Scholar]
- Webster CM, Hokari M, McManus A, Tang XN, Ma H, Kacimi R, Yenari MA. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8 (8):e70927. doi: 10.1371/journal.pone.0070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston RM, Jones NM, Jarrott B, Callaway JK. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab. 2007;27 (1):100–114. doi: 10.1038/sj.jcbfm.9600324. 9600324 [pii] [DOI] [PubMed] [Google Scholar]
- Wood PL. Microglia as a unique cellular target in the treatment of stroke: potential neurotoxic mediators produced by activated microglia. Neurol Res. 1995;17 (4):242–248. doi: 10.1080/01616412.1995.11740321. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y. Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:59–65. doi: 10.1007/978-3-211-09469-3_13. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283(3):230–232. doi: 10.1016/s0304-3940(00)00971-x. S030439400000971X [pii] [DOI] [PubMed] [Google Scholar]
- Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci. 2006;26 (40):10281–10291. doi: 10.1523/JNEUROSCI.2806-06.2006. 26/40/10281 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Sawle P, Homer-Vanniasinkam S, Green CJ, Motterlini R. Relationship between leukocyte kinetics and behavioral tests changes in the inflammatory process of hemorrhagic stroke recovery. Int J Neurosci. 2010;120 (12):765–773. doi: 10.3109/00207454.2010.523129. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13 (4):267–278. doi: 10.1038/nrn3174. nrn3174 [pii] [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37 (4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. 01.STR.0000206281.77178.ac [pii] [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95 (26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96 (23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36 (10):2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. 01.STR.0000182241.07096.06 [pii] [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682 (1–2):182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Powers C. Temporal profile of microglial response following transient (2 h) middle cerebral artery occlusion. Brain Res. 1997;744 (2):189–198. doi: 10.1016/S0006-8993(96)01085-2. S0006-8993(96)01085-2 [pii] [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12 (4):441–445. doi: 10.1038/nm1387. nm1387 [pii] [DOI] [PubMed] [Google Scholar]
- Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872(1–2):215–218. doi: 10.1016/s0006-8993(00)02459-8. S0006-8993(00)02459-8 [pii] [DOI] [PubMed] [Google Scholar]