Highlights
-
•
Early measles vaccine and neonatal vitamin A supplementation may become policy in low-income countries.
-
•
Vaccines and vitamin A may interact.
-
•
In children who received early measles vaccine, neonatal vitamin A was associated with 5-fold higher overall mortality.
-
•
Implementation of both policies at the same time may lead to increased child mortality.
-
•
Co-packaging of child health interventions should be investigated for the effect on overall mortality.
Keywords: Measles vaccine, Vitamin A, Children, Mortality, Low-income countries
Abstract
Background
In Guinea-Bissau we conducted three trials of neonatal vitamin A supplementation (NVAS) from 2002 to 2008. None of the trials found a beneficial effect on mortality. From 2003 to 2007, an early measles vaccine (MV) trial was ongoing, randomizing children 1:2 to early MV at 4.5 months or no early MV, in addition to the usual MV at 9 months. We have previously found interactions between vitamin A and vaccines.
Objective
We investigated whether there were interactions between NVAS and early MV.
Design
We compared the mortality of NVAS and placebo recipients: first, from 4.5 to 8 months for children randomized to early MV or no early MV; and second, from 9 to 17 months in children who had received two MV or one MV. Mortality rates (MR) were compared in Cox models producing mortality rate ratios (MRR).
Results
A total of 5141 children were randomized to NVAS (N = 3015) or placebo (N = 2126) and were later randomized to early MV (N = 1700) or no early MV (N = 3441). Between 4.5 and 8 months, NVAS compared with placebo was associated with higher mortality in early MV recipients (MR = 30 versus MR = 0, p = 0.01), but not in children who did not receive early MV (p for interaction between NVAS and early MV = 0.03). From 9 to 17 months NVAS was not associated with mortality. Overall, from 4.5 to 17 months NVAS was associated with increased mortality in early MV recipients (Mortality rate ratio = 5.39 (95% confidence interval: 1.62, 17.99)).
Conclusions
These observations indicate that NVAS may interact with vaccines given several months later. This may have implications for the planning of future child intervention programs.
1. Introduction
Neonatal vitamin A supplementation (NVAS) is currently under investigation as a public health intervention to combat vitamin A deficiency and mortality in areas afflicted by vitamin A deficiency. We have studied the effect of NVAS on infant mortality in three randomized trials in Guinea-Bissau. One trial randomized normal birth weight neonates (≥2500 g) 1:1 to 50,000 IU vitamin A or placebo (VITA I, 2002–2004) [1]. A second trial randomized low birth weight neonates (<2500 g) 1:1 to 25,000 IU vitamin A or placebo (VITA II, 2005–2008) [2]. A third trial randomized normal birth weight neonates 1:1:1 to 50,000 IU vitamin A, 25,000 IU vitamin A or placebo (VITA III, 2004–2007) [3].
We observed that NVAS interacted with subsequent routine vaccinations in a sex-differential manner; the effect of NVAS tended to be negative in females once they started receiving the diphtheria–tetanus–pertussis vaccine (DTP) recommended at 6 weeks of age [2,4].
From 2003 to 2007 a trial randomizing children to early measles vaccine (MV) at 4.5 months of age or no early MV in addition to the usual MV at 9 months of age was also conducted [5,6]. When analyzing the data from the early MV trial it became clear that there were strong interactions between early MV and NVAS. Early MV had no effect on overall mortality in children who had received NVAS, whereas a strong beneficial effect was seen among children who had not received NVAS, either because they had been randomized to placebo or because they had not participated in the NVAS trials [5].
Though neither NVAS nor early MV is currently recommended, the situation may change. Three new NVAS trials are ongoing [7] and NVAS may become policy if these new trials show a beneficial effect. The early MV trial showed a remarkably strong beneficial effect of early MV in children who had not received NVAS. The trial is currently being repeated in several West African countries which do not use NVAS. If results are replicable early MV may also become policy. It is therefore important to assess whether there is interaction between NVAS and early MV.
In the present paper we analyzed the potential interaction between NVAS and early MV in 5141 children who participated in both an NVAS trial and the early MV trial. We compared the mortality of NVAS and placebo recipients: first, in the time window from 4.5 to 8 months for children randomized to early MV or no early MV, and second, from 9 to 17 months in children who had received two MV or one MV, respectively.
2. Methods
The study was a reanalysis of previously conducted randomized trials of NVAS and early MV, respectively. The trials were conducted to study the effect of NVAS and MV, respectively, and the idea to study the potential interactions between the two interventions only occurred after the completion of the trials. Hence, the size of the present study was not based on a prespecified hypothesis and corresponding sample size calculations, but defined by the number of children who had participated in both a NVAS trial and the early MV trial. Information on exposure (randomization to NVAS and early MV) and outcome (overall mortality) was available from the trial databases.
2.1. Setting
The Bandim Health Project (BHP) maintains a demographic surveillance system in six suburban districts of the capital of Guinea-Bissau and covers approximately 102,000 inhabitants. There are three health centers in the study area, one has a maternity ward. The national hospital where many women from the study area give birth is a few kilometers away. BHP assistants are placed at the health centers and the hospital to register all study area children. All houses in the study area are visited monthly to register new pregnancies and births. All children below 3 years of age are followed through home visits every third month.
UNICEF classifies Guinea-Bissau as having vitamin A deficiency as a public health problem [8]. The country has implemented VAS campaigns for children between 6 months and 5 years of age. During the study period there were vitamin A campaigns in November 2003, December 2004, December 2005, May 2006, November 2006, July 2007, December 2007, July 2008, and January 2009.
2.2. Vitamin A trials
From 2002 to 2008, we conducted three trials of NVAS. VITA I randomized normal birth weight neonates (≥2500 g) 1:1 to 50,000 IU vitamin A or placebo (2002–2004) [1]. VITA II randomized low birth weight neonates (<2500 g) 1:1 to 25,000 IU vitamin A or placebo (2005–2008) [2]. VITA III randomized normal birth weight neonates 1:1:1 to 50,000 IU vitamin A, 25,000 IU vitamin A or placebo (2004–2007) [3]. The trials are presented in more detail in Table 1.
Table 1.
The three neonatal vitamin A trials in Guinea-Bissau.
| VITA I | VITA II | VITA III | |
|---|---|---|---|
| Enrollment period | 11/2002–11/2004 | 05/2005–01/2008 | 11/2004–05/2007 |
| Recruitment area | BHP study area | BHP study area and Bissau City | BHP study area |
| Participants | Normal birth weight neonates | Low birth weight neonates | Normal birth weight neonates |
| Number of participants | 4345 | 1717 | 6048 |
| Infant mortality rate | 47/1000 | 106/1000 | 39/1000 |
| Dose(s) of vitamin A | 50,000 IU (N = 2145) | 25,000 IU (N = 854) | 50,000 IU (N = 2015) 25,000 IU (N = 2011) |
| Placebo | Yes (N = 2200) | Yes (N = 863) | Yes (N = 2022) |
| Proportion of children participating in early MV trial | 2185 (50%) | 130 (8%) | 2826 (47%)a |
| Overall results of the trials | |||
| Mortality rate ratio (95% CI) NVAS versus placebo |
1.07 (0.79–1.44) | 1.08 (0.79–1.47) | 1.28 (0.91–1.81) |
| Effect in males | 0.84 (0.55–1.27) | 0.74 (0.45–1.22) | 1.35 (0.84–2.16) |
| Effect in females | 1.39 (0.90–2.14) | 1.42 (0.94–2.15) | 1.21 (0.73–2.01) |
| Results when censoring children at the date they received early measles vaccine | |||
| Mortality rate ratio (95% CI) NVAS versus placebo |
1.02 (0.76–1.38) | 1.08 (0.79–1.46) | 1.18 (0.84–1.68) |
| Effect in males | 0.81 (0.53–1.23) | 0.74 (0.46–1.20) | 1.22 (0.76–1.97) |
| Effect in females | 1.32 (0.85–2.04) | 1.43 (0.94–2.16) | 1.14 (0.68–1.90) |
One more than reported in VITA III; this child was excluded from VITA III because the weight was below 2.5 kg.
2.3. Early MV trial
The Early MV trial enrolled 4.5 months old children from August 2003 to April 2007 as described in detail elsewhere [5]. Children were randomized 1:1:1 to three treatment groups: a standard dose of Edmonston-Zagreb (EZ) MV at 4.5 months of age and at 9 months of age (group A); no vaccine at 4.5 months and EZ MV at 9 months of age (group B); no vaccine at 4.5 months and Schwarz MV at 9 months of age (group C). All children were enrolled and randomized at 4.5 months of age. It was a condition for entering the trial that the children had received the third dose of DTP (DTP3) at least four weeks before enrollment; hence, children in groups B and C had DTP3 as their most recent vaccination between 4.5 and 8 months of age. Children in groups B and C who received MV at 9 months of age were randomized to an additional MV or no additional MV at 18 months of age. We found no differences between groups B and C, and hence the two groups have been combined [5].
2.4. Outcome
The vitamin A trials had mortality by 12 months of age as main outcome; the early MV trial had mortality by 3 years of age as main outcome. In the present reanalysis we studied the effect of NVAS versus placebo between 4.5 and 8 months of age, when the children had early MV or DTP3 as their most recent vaccine, and from 9 to 17 months, when the children according to the protocol had two doses of MV or one dose of MV as their most recent vaccine. Follow-up was censored at age 18 months when children in the one-dose MV group were randomized to a booster dose of MV or no booster and many children received booster DTP.
2.5. Ethical issues
The trials were registered at clinicaltrials.gov (VITA I: NCT00168597; VITA II and III: NCT00168610; Early MV trial: NCT00168558). All trials were approved by the Research Coordination and Ethical Committee of the Ministry of Health in Guinea-Bissau and the Danish Central Ethical Committee gave its consultative approval.
2.6. Statistical analysis
All analyses were done using Stata 12.1 (StataCorp, College Station, TX).
Characteristics at enrollment into the early MV trial were compared using chi-square test (categorical variables), t-test (normally distributed continuous variables), and Kruskall–Wallis test (non-normally distributed continuous variables).
We compared mortality rates (MR) between NVAS and placebo recipients within strata of early and no early MV in Cox proportional hazards models with age as the underlying time variable. Hence, age was inherently adjusted for. All analyses were furthermore adjusted for NVAS trial (VITA I–III).
In the analysis between 4.5 and 8 months of age the children entered at the date of randomization to MV or no early MV and were censored at the date of the 9-month-MV; in the analysis from 9 to 17 months the children entered at the date of the 9-month MV and were censored at age 18 months. Children who were lost to follow-up were censored at the date when they were last seen alive. As NVAS may interact with subsequent VAS [9] we conducted an analysis in which we censored children at the time of the first VAS opportunity after they reached 6 months of age.
Finally we calculated a combined estimate of the three NVAS trials with censoring of children at the time of early MV.
The analyses were post hoc analyses in the sense that the original trials were not designed to test the potential interaction, but prespecified in the sense that we conceived the idea to study the interaction, based on observations from other studies, prior to conducting the analyses.
All the analyses are interaction analyses, since we evaluated NVAS effects in strata of the NVAS trial participants, namely those who did and those who did not receive early MV. The interaction analyses were stratified by sex, as both the NVAS and the early MV trial found sex-differences. They were also stratified by the two age windows (4.5–8 months and 9–17 months) which were inherent in the design of the early MV trial. Hence, the potential interaction between NVAS and early MV was assessed overall and in 4 subgroups defined by sex and age. We did not perform other interaction analyses than those described. With this limited number of subgroup analyses we did not find it indicated to adjust for multiple testing.
3. Results
A total of 5141 children participated both in NVAS trials and in the early MV trial; 2185 (42.5%) participated in VITA I, 130 (2.5%) in VITA II, and 2826 (55.0%) in VITA III.
The random allocation seemed conserved at age 4.5 months as the baseline characteristics at enrollment was evenly distributed between NVAS and placebo groups except that slightly more NVAS recipients in VITA I were allocated to early MV, and NVAS recipients compared with placebo recipients in the no early MV group had very slightly higher mid-upper-arm circumference (MUAC) (Table 2). Ninety-six percent of the children were breastfed at enrollment; 22% of these were exclusively breastfed. By 9 months of age, 92% were still breastfed, the proportions at both time points were similar in males and females (data not shown).
Table 2.
Characteristics at enrollment into the early measles vaccine trial among children randomized to vitamin A and placebo at birth and measles vaccine or no measles vaccine at 4.5 months of age.a
| Early measles vaccine (N = 1700) |
No early measles vaccine (N = 3441) |
|||
|---|---|---|---|---|
| Vitamin A (N = 1022) | Placebo (N = 678) | Vitamin A (N = 1993) | Placebo (N = 1448) | |
| Male sex | 51% (526) | 52% (350) | 51% (1021) | 51% (733) |
| Median age (25–75% range) at enrollment (months) | 4.8 (4.7, 5.1) | 4.8 (4.7, 5.2) | 4.8 (4.7, 5.2) | 4.8 (4.6, 5.1) |
| Enrollment in rainy season | 52% (529) | 47% (321) | 51% (1009) | 50% (729) |
| Bandim suburb | 45% (456) | 47% (317) | 44% (871) | 46% (672) |
| Breastfed at enrollment | 96% (981) | 97% (655) | 97% (1921) | 96% (1388) |
| Exclusively breastfed at enrollment (among breastfed) | 21% (201) | 19% (125) | 23% (429) | 22% (300) |
| Hospitalized before enrollment | 3% (26) | 4% (27) | 3% (50) | 2% (28) |
| Pigs in household | 17% (171) | 16% (104) | 19% (368) | 17% (250) |
| Median no of people/sleeping room (25–75% range) |
4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Toilet inside house | 18% (179) | 15% (102) | 15% (293) | 15% (217) |
| Functioning electricity | 25 (252) | 28 (187) | 27 (537) | 26 (370) |
| Mean weight in kg (SD) | 7.2 (0.96) | 7.2 (0.96) | 7.2 (0.99) | 7.2 (1.00) |
| Mean weight-for-age z-score (SD)d | −0.08 (1.11) | −0.02 (1.10) | −0.07 (1.11) | −0.09 (1.16) |
| Mean length in cm (SD) | 64.3 (2.6) | 64.3 (2.7) | 64.2 (2.8) | 64.1 (2.6) |
| Mean length-for-age z-score (SD)d | −0.28 (1.13) | −0.32 (1.18) | −0.31 (1.21) | −0.34 (1.19) |
| Mean weight-for-length z-score (SD)d | 0.22 (1.24) | 0.32 (1.29) | 0.26 (1.30) | 0.25 (1.34) |
| Mean MUAC in mm (SD) | 142.2 (11.7) | 142.6 (11.6) | 142.6 (12.0)b | 141.6 (12.2)b |
| Mean maternal MUAC in mm (SD) | 277.7 (35.1) | 274.4 (33.4) | 275.6 (34.2) | 274.8 (34.2) |
| NBW VITA I | 377 (52%)c | 342 (48%)c | 703 (48%)c | 763 (52%)c |
| LBW VITA II | 19 (50%) | 19 (50%) | 46 (50%) | 46 (50%) |
| NBW VITA III | 626 (66%) | 317 (34%) | 1244 (66%) | 639 (34%) |
Compared using chi-square test (categorical variables), t-test (normally distributed continuous variables), and Kruskall–Wallis test (non-normally distributed continuous variables, only age at enrollment and people/sleeping room).
p = 0.02 for same MUAC among NVAS and placebo recipients in the no early MV group (t-test).
p = 0.05 for equal distribution of NVAS and placebo in the early MV and the no early MV group in VITA I (chi-square test).
Computed using the WHO Child Growth Standards (Software available at http://www.who.int/nutgrowthdb/software/en/).
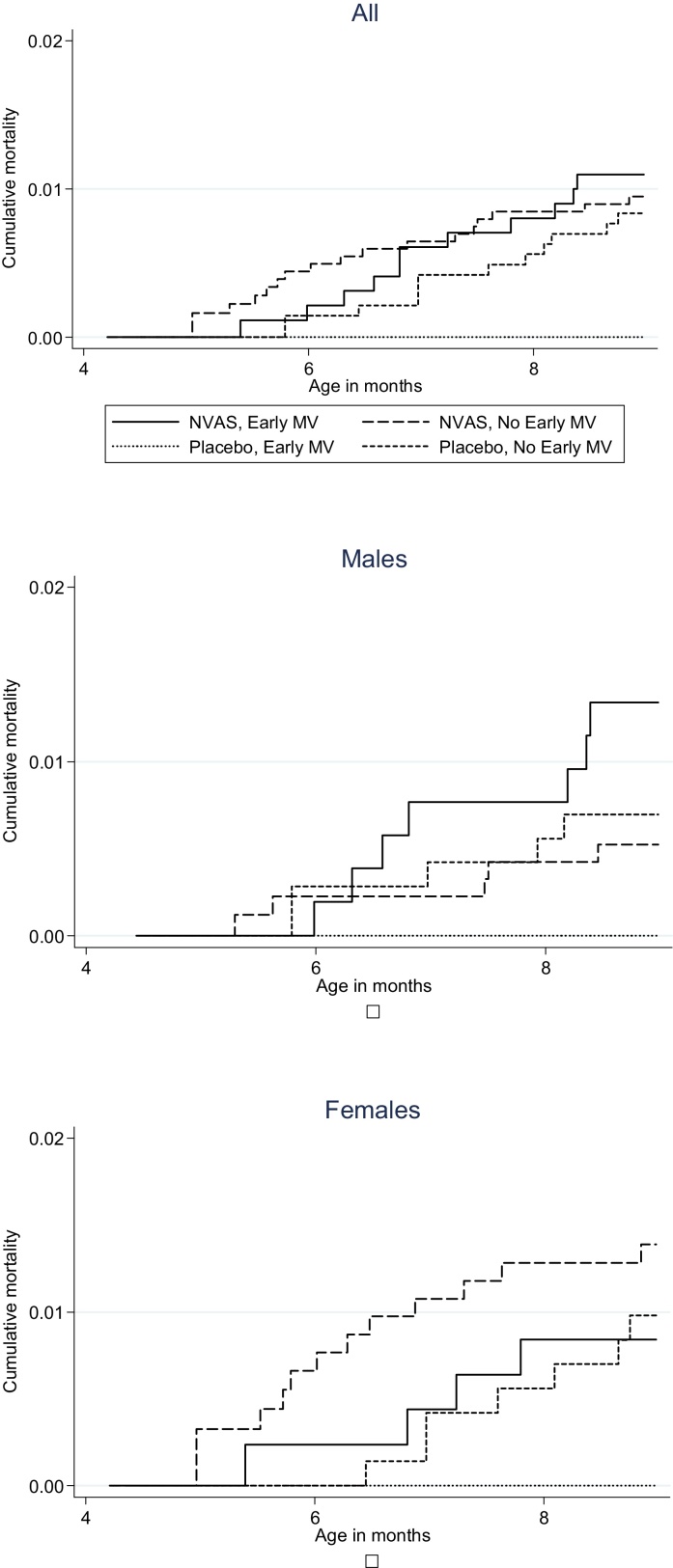
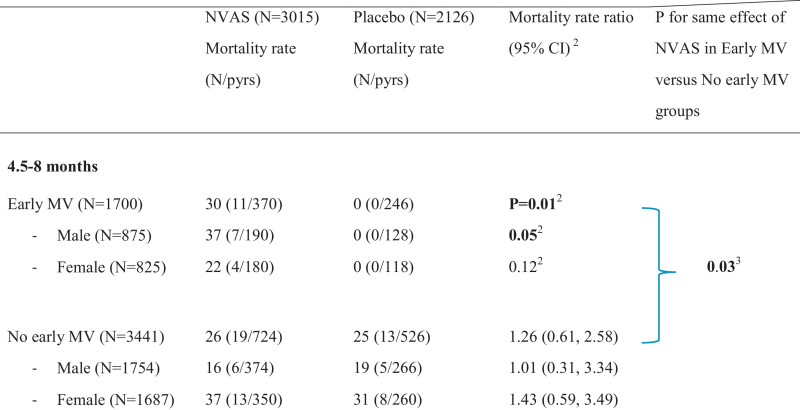
Between enrollment into the early MV trial and 9 months of age, at the time of the usual MV, 43 deaths occurred in 1865 pyrs corresponding to a mortality rate (MR) of 23/1000 pyrs. However, the MR varied between the different groups (Fig. 1). In the early MV group having received NVAS was associated with significantly higher mortality compared with placebo (MR = 30 versus MR = 0, p = 0.01, Table 3). The effect was significant in males (p = 0.05) but not in females (p = 0.12). When comparing the effect of NVAS in the early MV group with the effect of NVAS in the no early MV group, the effect of NVAS was more marked in the early MV group than in the no early MV group (MR = 26 versus MR = 25), resulting in a significant interaction between NVAS and early MV (p = 0.03, Table 3).
Fig. 1.
Cumulative mortality according to randomization to neonatal vitamin A supplementation (NVAS) and early measles vaccine (MV), from 4 to 8 months.
Table 3.
Mortality rate ratios in different age groups according to randomization to neonatal vitamin A supplementation (NVAS) or placebo and early or no early measles vaccine (MV). Note: all children received MV at 9 months of age.a
 |
 |
 |
Bold text marks significant differences at the 5% level.
pyrs = person-years of risk.
Mortality rate ratio estimates derived from Cox proportional hazards models adjusted for Trials I–III.
b Note that it was not possible to calculate mortality rate ratio for the early MV group from 4.5 to 8 months; instead we calculated a p-value for equal mortality rates using log-rank test.
cp-value from exact Poisson regression using command expoisson in Stata.
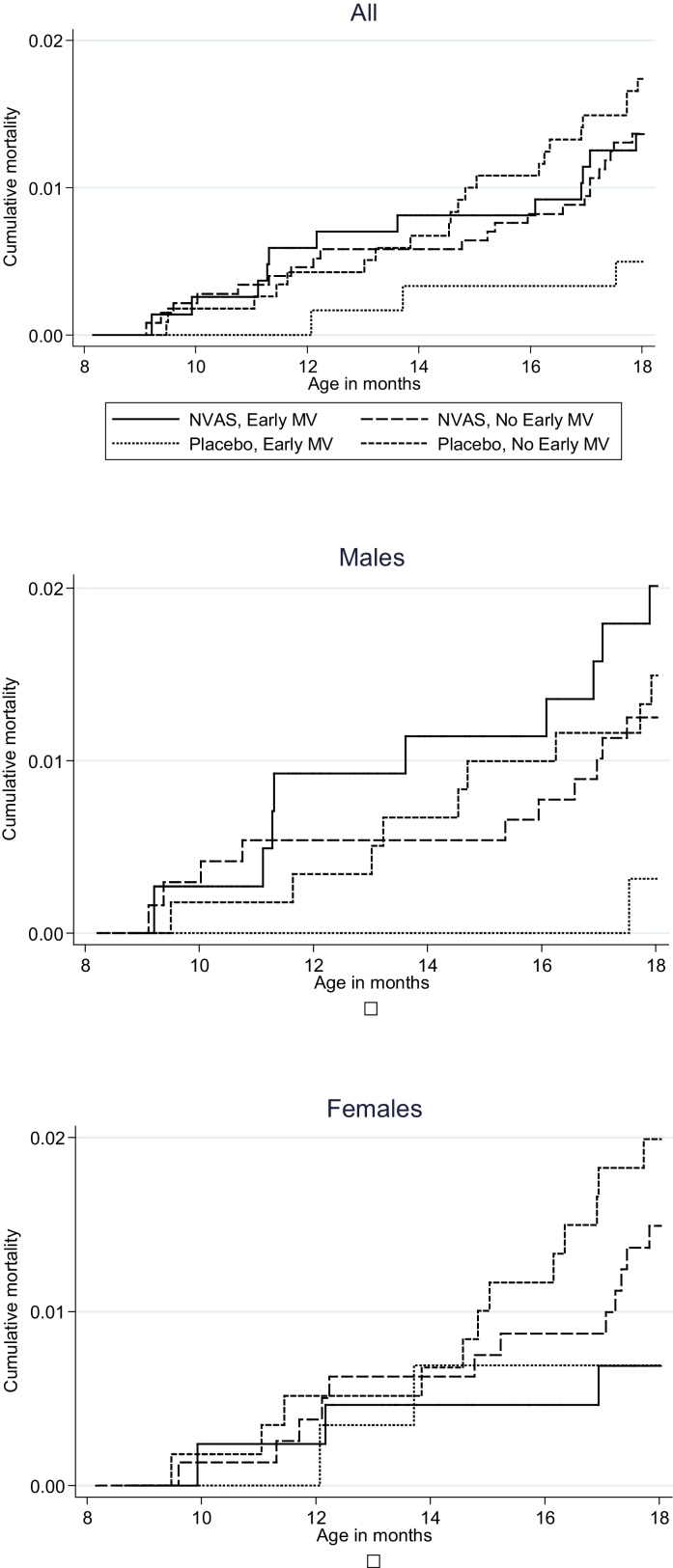
As expected the MR was lower between 9 and 17 months of age (58 deaths/3231 pyrs, MR = 18/1000). There was no longer any significant negative effect of receiving NVAS compared with placebo in the early MV group (Table 3, Fig. 2)
Fig. 2.
Cumulative mortality according to randomization to neonatal vitamin A supplementation (NVAS) and early measles vaccine (MV), from 9 to 17 months.
Between 4.5 and 17 months of age, due to the strong negative effect observed up to 9 months of age, NVAS compared with placebo was associated with significantly increased mortality in early MV recipients, overall (5.39 (1.62, 17.99)) and in males (11.31 (1.50, 85.47)), again resulting in a significant interaction between NVAS and early MV (p = 0.008, Table 3).
When we censored follow-up at the time of the first vitamin A opportunity occurring after the children had reached 6 months of age, the results remained largely unchanged (from 4.5 to 17 months of age the estimate for NVAS versus placebo was 4.28 (1.25–14.62); 8.16 (1.05–63.42) in males).
3.1. Effect of neonatal VAS in all previous trials when censoring for early MV
We conducted a reanalysis of VITA I–III to assess the effect of neonatal VAS on infant mortality if we censored children when they received early MV. The estimates for the three trials are shown in Table 1. The combined estimate for the three trials was 1.08 (0.90, 1.30); 0.89 (0.69, 1.16) in males and 1.31 (1.01, 1.70) in females (p for same effect in males and females = 0.04).
4. Discussion
In this analysis we combined information on children who had participated first in an NVAS trial, and subsequently in an early MV trial, and found significant interactions between the two immune-modulatory interventions. Having received NVAS as compared with placebo was associated with a strong negative effect on overall mortality after receiving early MV. The negative effect was pronounced from 4.5 to 8 months of age. It was significant in its own right among males.
None of the three NVAS trials from Guinea-Bissau found a beneficial effect [1–3]. Some children from all three trials also participated in the early MV trial, and a negative interaction between NVAS and early MV could have led to an underestimation of the benefits of NVAS for children, who follow the currently recommended vaccination schedule. However, a reanalysis of the three trials with censoring at the time of early MV still showed no beneficial effect of NVAS and a significant negative effect in females. Hence, early MV does not explain the lack of beneficial effect of NVAS in Guinea-Bissau.
4.1. Strengths and weaknesses
Though this analysis should not be interpreted as a 2-by-2 factorial trial it still has some of the strengths of a trial, as the children were randomized to both treatments. However, it is a relatively small study, it was not sized to study interactions, and it may be subject to random fluctuations in mortality among subgroups.
4.2. Interpretation
More than 10 years ago, our group proposed the hypothesis that VAS amplifies the non-specific effects of vaccines [10]. This hypothesis was based on two main observations: first, the routine childhood vaccinations have non-specific effects, the live BCG and MV reduce mortality more than can be explained by prevention of the target diseases [11,12], whereas the inactivated DTP vaccine is associated with increased mortality in areas with herd immunity to pertussis [13,14]; second, the mortality benefit pattern after VAS resembles that of vaccines, with a beneficial effect in the time windows dominated by BCG (at birth) and MV (after 6 months of age) but no beneficial effect between 1 and 5 months of age, in the time window of DTP [10]. The hypothesis implied that VAS would probably be beneficial when provided with the live BCG and MV, but harmful when provided with DTP vaccine. We have subsequently tested the hypothesis in observational studies [15,16], randomized trials [1–3,17] and by reanalyzing old trials [18] and we have been able to show repeatedly that VAS and vaccines interact.
We have also learned in the process. Initially, we did not emphasize sex as an important covariate. However, in most [1,2,4,17,18], though not all studies [3,15,16], we have found that VAS provided close to DTP had a negative effect for females, but not for males. Furthermore, we had not envisaged that VAS could interact with vaccines given months after. We first became aware of this possibility when analyzing the first NVAS trial, observing an increase in mortality in female NVAS recipients, which occurred when the children started receiving DTP several months after NVAS [4]. The present analysis suggests that NVAS may interact with vaccines given as much as 4–5 months later. If true, this is surprising, not only because it occurred so many months after NVAS, but also because the interaction between NVAS and early MV was negative. If anything we would have expected the opposite. The explanation may be the three intermediate DTP vaccinations. In the early MV trial, all children were visited at the ages of DTP1, DTP2, and DTP3 and their mothers were encouraged to bring them for vaccination. Hence, all participants had received three DTP vaccines with short intervals, and they were enrolled in the early MV trial 4 weeks later. The cocktail of first NVAS, then three DTP and then early MV may have been too much. In a trial of BCG revaccination we found a negative effect of receiving BCG at 19 months of age followed by DTP and then VAS in a campaign [19].
4.3. Biological mechanisms
We have discovered interactions between NVAS and the following vaccines: DTP (negative for females) [2,4], and early MV (negative for males). Furthermore, we have found that NVAS primes a beneficial response to a subsequent dose of VAS provided after 12 months of age, particularly in females [9,16]. Hence, NVAS seems to have profound effects on the immune system's response to subsequent stimulations with both vaccines and micronutrients – and these effects may differ in males and females.
A single high dose of vitamin A will quickly be distributed into the tissues and only released under homeostatic control. It may help prevent vitamin A deficiency, but it seems unlikely that this would have so profound long-term effects on the response to vaccines. A recent review has addressed vitamin A's potential epigenetic effects and emphasized vitamin A's powerful effects on stem cell differentiation [20]. From our perspective the most plausible explanations for the observed long-term effects of NVAS is that NVAS has epigenetic effects, resulting in fundamental priming effects on the neonatal immune system which determine the response to subsequent challenges. The result may be a reduction in mortality after the child receives MV at 9 months of age or after a subsequent high dose of vitamin A – but the present study indicated that it primes for a detrimental response to an early MV given shortly after three doses of DTP.
4.4. Implications
Though the existing four NVAS trials in Africa have all shown negative trends [1–3,21,22], three new NVAS trials are ongoing [7]. NVAS may become policy if these new trials show a beneficial effect. This could potentially happen if the trials are carried out in areas with high neonatal mortality but low subsequent mortality, or in areas with combined BCG and DTP vaccination – in such areas a negative interaction between NVAS and DTP in females would not be seen. If introduced, it will be very important to ensure that NVAS does not interact negatively with DTP in females, and to be alert about potential interactions with other health interventions. MV is currently being recommended from age 6 months of age in areas with a high incidence of both HIV infection and measles [23]. Hence, if NVAS is being introduced it is possible that it may have negative long-term effects on overall mortality in such settings. The early MV trial is being repeated in two African countries of which none uses NVAS, and if the results are replicable early MV may become a common policy. If there are indeed negative interaction between NVAS and early MV it will be important that the two policies are not both implemented.
Conclusion
The present study adds to the evidence that VAS interacts with vaccines. The interactions may sometimes be beneficial but sometimes negative, increasing mortality. The interactions between health interventions are not considered when global policies are designed and implemented. However, with the trend to co-package interventions, it should become increasingly important to consider interactions to optimize the beneficial effect of child intervention programs.
Names for PubMed indexing
Benn, Martins, Fisker, Diness, Garly, Balde, Rodrigues, Whittle, Aaby.
Acknowledgement
C.S.B. was the PI for the vitamin A trials, with assistance from A.F., B.R.D. and I.B. C.M., M.L.G., H.W. and P.A. were responsible for the early measles vaccine trial. A.R. supervised the routine registration system. C.B. and P.A. conducted the statistical analyses. C.B. wrote the first manuscript draft. All authors contributed to the data interpretation, commented upon the paper and approved the final version. C.B. is the guarantor.
Conflict of interest: None of the authors had any conflict of interest. Funding: The original NVAS trials were funded by the EU (ICA4-CT-2002-10053), the Danish Medical Research Council (22-03-0621), University of Copenhagen, March of Dimes (#6-FY04-51), and the Ville Heise Foundation. The early MV trial was funded by DANIDA and the Danish National Research Foundation. The trial also received support from Fonden til Lægevidenskabens Fremme and Novo Nordisk Foundation. C.S.B. holds an ERC Starting Grant (ERC-StG-243149). B.R.D. received a PhD grant from the Graduate School of International Health. P.A. holds a research professorship grant from Novo Nordisk Foundation. The Bandim Health Project receives support from DANIDA. CVIVA is funded by the Danish National Research Foundation (DNRF108). The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Footnotes
Clinical trial registration: The trials were registered at clinicaltrials.gov (VITA I: NCT00168597; VITA II and III: NCT00168610; Early MV trial: NCT00168558).
References
- 1.Benn C.S., Diness B.R., Roth A., Nante E., Fisker A.B., Lisse I.M. Effect of 50 000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ. 2008;336:1416–1420. doi: 10.1136/bmj.39542.509444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benn C.S., Fisker A.B., Napirna B.M., Roth A., Diness B.R., Lausch K.R. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ. 2010;340:c1101. doi: 10.1136/bmj.c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benn C.S., Diness B.R., Balde I., Rodrigues A., Lausch K.R., Martins C. Two different doses of supplemental vitamin A did not affect mortality of normal-birth-weight neonates in Guinea-Bissau in a randomized controlled trial. J Nutr. July 2014 doi: 10.3945/jn.114.192674. pii: jn.114.192674 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Benn C.S., Rodrigues A., Yazdanbakhsh M., Fisker A.B., Ravn H., Whittle H. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine. 2009;27:2891–2898. doi: 10.1016/j.vaccine.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 5.Aaby P., Martins C.L., Garly M.L., Bale C., Andersen A., Rodrigues A. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins C.L., Garly M.L., Bale C., Rodrigues A., Ravn H., Whittle H.C. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. BMJ. 2008;337:a661–a670. doi: 10.1136/bmj.a661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neovita Study Author Group, Bahl R., Bhandari N., Dube B., Edmond K., Fawzi W. Efficacy of early neonatal vitamin A supplementation in reducing mortality during infancy in Ghana, India and Tanzania: study protocol for a randomized controlled trial. Trials. 2012;13:22. doi: 10.1186/1745-6215-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO Global Database on Vitamin A Deficiency; 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [Google Scholar]
- 9.Fisker A.B., Aaby P., Rodrigues A., Frydenberg M., Bibby B.M., Benn C.S. Vitamin A supplementation at birth might prime the response to subsequent vitamin A supplements in girls. Three year follow-up of a randomized trial. PLoS ONE. 2011;6:e5 2326. doi: 10.1371/journal.pone.0023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn C.S., Bale C., Sommerfelt H., Friis H., Aaby P. Hypothesis: vitamin A supplementation and childhood mortality: amplification of the non-specific effects of vaccines? Int J Epidemiol. 2003;32:822–828. doi: 10.1093/ije/dyg208. [DOI] [PubMed] [Google Scholar]
- 11.Roth A., Garly M.L., Jensen H., Nielsen J., Aaby P. Bacillus Calmette-Guerin vaccination and infant mortality. Expert Rev Vaccin. 2006;5:277–293. doi: 10.1586/14760584.5.2.277. [DOI] [PubMed] [Google Scholar]
- 12.Aaby P., Samb B., Simondon F., Seck A.M., Knudsen K., Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995;311:481–485. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaby P., Benn C.S., Nielsen J., Lisse I.M., Rodrigues A., Jensen H. DTP vaccination and child survival in observational studies with incomplete vaccination data. Trop Med Int Health. 2007;12:15–24. doi: 10.1111/j.1365-3156.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- 14.Aaby P., Benn C., Nielsen J., Lisse I.M., Rodrigues A., Ravn H. Testing the hypothesis that diphtheria–tetanus–pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn C.S., Martins C., Rodrigues A., Ravn H., Fisker A.B., Christoffersen D. The effect of vitamin A supplementation administered with missing vaccines during national immunization days in Guinea-Bissau. Int J Epidemiol. 2009;38:304–311. doi: 10.1093/ije/dyn195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisker A.B., Aaby P., Bale C., Balde I., Biering-Sorensen S., Agergaard J. Does the effect of vitamin A supplements depend on vaccination status? An observational study from Guinea-Bissau. BMJ Open. 2012;2:e480004. doi: 10.1136/bmjopen-2011-000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benn C.S., Martins C., Rodrigues A., Jensen H., Lisse I.M., Aaby P. Randomised study of effect of different doses of vitamin A on childhood morbidity and mortality. BMJ. 2005;331:1428–1432. doi: 10.1136/bmj.38670.639340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benn C.S., Aaby P., Nielsen J., Binka F.N., Ross D.A. Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana Vitamin A supplementation trial. Am J Clin Nutr. 2009;90:629–639. doi: 10.3945/ajcn.2009.27477. [DOI] [PubMed] [Google Scholar]
- 19.Roth A.E., Benn C.S., Ravn H., Rodrigues A., Lisse I.M., Yazdanbakhsh M. Effect of revaccination with BCG in early childhood on mortality: randomised trial in Guinea-Bissau. BMJ. 2010;340:c671. doi: 10.1136/bmj.c671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudas L.J., Wagner J.A. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malaba L.C., Iliff P.J., Nathoo K.J., Marinda E., Moulton L.H., Zijenah L.S. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr. 2005;81:454–460. doi: 10.1093/ajcn.81.2.454. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey J.H., Iliff P.J., Marinda E.T., Mutasa K., Moulton L.H., Chidawanyika H. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization Conference report WHO position on measles vaccines. Vaccine. 2009;27:7219–7221. doi: 10.1016/j.vaccine.2009.09.116. [DOI] [PubMed] [Google Scholar]