Abstract
Borrelia burgdorferi s.s., the bacterium that causes Lyme disease in North America, circulates among a suite of vertebrate hosts and their tick vector. The bacterium can be differentiated at the outer surface protein C (ospC) locus into 25 genotypes. Wildlife hosts can be infected with a suite of ospC types but knowledge on the transmission efficiencies of these naturally infected hosts to ticks is still lacking. To evaluate the occupancy and detection of ospC types in wildlife hosts, we adapted a likelihood-based species patch occupancy model to test for the occurrence probabilities (ψ – “occupancy”) and transmission efficiencies (ε – “detection”) of each ospC type. We detected differences in ospC occurrence and transmission efficiencies from the null models with HIS (human invasive strains) types A and K having the highest occurrence estimates, but both HIS and non-HIS types having high transmission efficiencies. We also examined ospC frequency patterns with respect to strains known to be invasive in humans across the host species and phylogenetic groups. We found that shrews and to a lesser extent, birds, were important host groups supporting relatively greater frequencies of HIS to non-HIS types. This novel method of simultaneously assessing occurrence and transmission of ospC types provides a powerful tool in assessing disease risk at the genotypic level in naturally infected wildlife hosts and offers the opportunity to examine disease risk at the community level.
Keywords: Lyme disease, ospC, Occurrence probability, Transmission efficiency, Likelihood model, Species occupancy model
1. Introduction
Lyme disease in North America is caused by infection with the spirochete bacterium, Borrelia burgdorferi s.s. (Burgdorfer et al., 1982). This bacterium circulates within vertebrate host species, vectored primarily by the black-legged tick, Ixodes scapularis. Within local tick populations, the B. burgdorferi population is genetically heterogeneous, consisting of a group of distinct genotypes (Wang et al., 1999; Qiu et al., 2002; Gatewood et al., 2009; Barbour and Travinsky, 2010; Hamer et al., 2011; Brisson et al., 2012; Margos et al., 2012). These genotypes are differentiated by genetic differences at the highly variable antigenic site of the outer surface protein C (ospC) locus (Ohnishi et al., 2001; Liang et al., 2004). B. burgdorferi s.s. exhibits 25 alleles (types), of which 17 are known to occur in the northeastern United States (Qiu et al., 2002; Barbour and Travinsky, 2010).
Previous studies had detected differential infection frequencies of vertebrate hosts by particular ospC types (Brisson and Dykhuizen, 2004; Hanincova et al., 2006). Although B. burgdorferi s.s. varies in its reservoir-competence levels over a large suite of host species (Battaly and Fish, 1993; Rand et al., 1998; Giardina et al., 2000; Richter et al., 2000; LoGiudice et al., 2003; Ginsberg et al., 2005; Brisson et al., 2008; Taragel’ova et al., 2008; Keesing et al., 2009), the role of host species in supporting genotypic variation of the bacterium is not well understood. Here, we utilize the ospC locus as a marker for genetic diversity (Brisson et al., 2011) to determine the presence and frequencies of ospC genotypes in the vertebrate hosts.
Recent studies of associations between hosts and B. burgdorferi genotypes are limited by their focus on subsets of the hosts occurring at any single site, e.g., mammals or birds, but not both (Brisson and Dykhuizen, 2004; Alghaferi et al., 2005; Anderson and Norris, 2006; Hanincova et al., 2006; Ogden et al., 2008; but see MacQueen et al. (2012) for an exception). Although there is some information on the transmission rates of B. burgdorferi from host to tick, based on needle and tick infections of mouse models in experimental inoculation studies, (Hofmeister et al., 1999; Hanincova et al., 2008; Baum et al., 2012), there is less information on transmission rates of strains from naturally infected wildlife host individuals to ticks. Understanding transmission and occurrence patterns is important since all ospC genotypes can infect humans, but the probability of the bacterium invading humans following a tick bite varies by genotype (Seinost et al., 1999; Dykhuizen et al., 2008; Wormser et al., 2008). Additionally, ospC genotypes appear to vary in their Lyme disease severity (Strle et al., 2011; Hanincova et al., 2013). In humans, most diagnosed cases involve only five of the seventeen ospC types, specifically A, B, I, K, and N (Seinost et al., 1999; Dykhuizen et al., 2008). For this study, we label these five types human invasive strains (HIS). Hence, understanding the relative occurrence and differential transmission efficiencies of B. burgdorferi genotypes can offer important insights to Lyme disease risk at the finer, genotypic scale.
In this study, we addressed the following two questions here: (1) What are the probabilities of occurrence of ospC types in hosts and the transmission efficiencies of the various ospC types from infected hosts to ticks? (2) How do the relative frequencies of HIS types and non-HIS types differ among phylogenetically distinct but frequently co-occurring host groups (e.g. shrews vs. rodents vs. birds)? Due to different ecological, behavioral and physiological traits among the groups, these traits could influence host–tick interactions, infection probability, and the potential to spread the bacterium at different geographic scales. Thus, examining ospC variation among these basic groups provides a good foundation for future investigations on ospC genotypic variation at the host community level.
2. Methods
2.1. Maximum likelihood model
To obtain probabilities of occurrence and transmission efficiencies from infected hosts to ticks, we used a likelihood-based occupancy approach (MacKenzie et al., 2002), which utilizes field data on naturally occurring B. burgdorferi infection in various hosts and the ticks that feed upon them. The principle of this approach is based on well-known ecological species occupancy models, which seeks to estimate the occurrence of species in habitats that may be difficult to survey, and in which detection is uncertain (MacKenzie et al., 2002; McCallum, 2013). Our model is a corollary to such models; here the aim is to detect ospC types (“species”) within target hosts (“habitat”) through the use of multiple larval ticks feeding on those host species (with the ticks serving as the “detection” method). The method requires multiple attempts at “detection” (ticks feeding) per “habitat” (host), and uses the numbers of true and false negatives and positives (transmission to one or more of the ticks feeding on a given host) to provide maximum likelihood estimates of both the occurrence rate of any particular ospC genotype in a given host species and the probability of transmission (“detection”) of that ospC genotype from that host to the ticks feeding on that host.
Because transmission efficiencies of ospC types from hosts to tick are assumed to be less than 1 (Brisson and Dykhuizen, 2004; Hanincova et al., 2006), “detection” probabilities are also (routinely) less than 1. Thus, absence of an ospC type from a given tick could be the result either of the absence of that type from that host or from failure of transmission of the ospC type from the host to the tick being sampled. This method provides simultaneous maximum likelihood estimates of both occupancy (ψ rates) and probabilities of successful transmission (ε rates) of those ospC types to ticks from different vertebrate host species. The approach is robust for even small numbers of replicate ticks per host, as long as the detection probabilities of the ospC types in the host are greater than approximately 0.3 (MacKenzie et al., 2002). The important features of the method are that it accounts for variation in both host and tick sample sizes, allows for sampling variation associated with both hosts and ticks (e.g., genetic, feeding success, intra-specific variation, etc.), and that both parameters (ψ and ε) are estimated from information on ospC types from the sampled ticks. The novel use of a patch-occupancy model for estimation of infection and transmission rates of ospC types drawn from different vertebrate hosts should provide a powerful approach for the elucidation of disease risk associated with B. burgdorferi, and can be extended to other vector-borne zoonotic diseases.
For each of the ospC genotypes, we compared two alternative models: (a) a null model that ignored the identity of the vertebrate host and estimated a separate probability of occurrence (ψ) for each ospC type, averaged over all host species, and an average transmission efficiency (ε) of that ospC type from the vertebrate hosts to ticks; and (b) a contrasting species-specific model of separate (ψ) rates for each host species and average (ε) for each ospC type. Only host species with at least one positive tick for a particular ospC type were included in the model, as the method cannot infer both ψ and ε from an absence of bacteria. We did not analyze ospC type J, because we only detected this genotype in a single host species, so that cross-species comparisons were not possible. Hence, only 16 of 17 recovered types were analyzed for the competing models.
Maximum likelihood estimates and two-unit support intervals (likelihood analogues of 95% confidence intervals) for the parameters of each model were obtained after 2500 iterations, using global optimization methods in the likelihood 1.5 package (Murphy, 2012) in R version 2.15.1 (R, 2012). The two models (null vs. species- & strain-specific) were compared using AIC, corrected for small sample size (AICc) (Burnham and Anderson, 2002). The comparison provides an explicit evaluation of the hypothesis that ψ and ε values for a given ospC type differ among species of vertebrate hosts, with the null hypothesis of no differences rejected if the more elaborate model had a lower AICc (ΔAICc > 2). The magnitude of the difference in AICc between the two models provides a measure of the strength of evidence for the best model, after controlling for the different numbers of parameters in the two models (Burnham and Anderson, 2002).
Lastly, we examined for relative differences in HIS and non-HIS types among different species and their phylogenetic groups using contingency table analysis. All analyses were conducted using R version 2.15.1 (R, 2012).
2.2. Field methods
To assess the association of ospC types with reservoir hosts, animals carrying ticks were captured during the summers of 2008–2010 at the Cary Institute of Ecosystem Studies in Millbrook, NY, an area of endemic Lyme disease and high incidence rates (NYSDOH, 2013). Many of the species examined were captured as part of a larger study that examined host reservoir competency for various tick-borne pathogens (Keesing et al., 2009, 2012; Hersh et al., 2012). Mammals were live-trapped and birds were mist netted (IACUC #06-01 and 09-01) in mid to late summer each year, coinciding with the peak activity for larval ticks at our sites (Ostfeld et al., 1996). All animals were moved temporarily (<1 week) to the laboratory, where they were held in appropriately sized cages with wire mesh floors and pans of water or moistened paper towels beneath the floors. We collected fully fed larvae that dropped from the hosts and allowed them to molt into nymphs before flash-freezing them for DNA extraction and B. burgdorferi characterization (protocol following Keesing et al., 2009). As transovarial transmission of B. burgdorferi is very rare, questing larval ticks are essentially uninfected. Therefore, any B. burgdorferi infections present in the fully fed larval ticks are due to the acquisition of the bacteria from the hosts they fed upon. We did not collect vertebrate tissue samples to test for ospC types, because the absence of an ospC type from host tissue might reflect either an absence of that type from the host or failure to detect the ospC type, similarly to testing the larval ticks. We also did not test feeding nymphal ticks from the hosts, because that would require destructive sampling of the ticks, while they were still feeding, thus precluding later estimation of their transmission efficiency to the hosts. Moreover, feeding nymphal ticks might have been infected previously by the hosts they fed upon as larvae, so ospC types in feeding nymphal ticks might result from either previous or current feeds.
Our dataset includes the following ten host species (and the number of positive individuals): white-footed mouse (Peromyscus leucopus) (12), eastern chipmunk (Tamius striatus) (10), short-tailed shrew (Blarina brevicauda) (10), masked shrew (Sorex cinereus) (3), eastern gray squirrel (Sciurus carolinensis) (4), red squirrel (Tamiasciurus hudsonicus) (7), striped skunk (Mephitis mephitis) (1), American robin (Turdus migratorius) (13), Veery (Catharus fuscescens) (16), and Wood Thrush (Hylocichla mustelina) (4). We removed the single skunk from the analyses, in view of the small sample size, and because we detected only ospC type B from the ticks feeding on that skunk, precluding meaningful analysis.
2.3. Laboratory method
We amplified the ospC gene from positively infected tick samples using newly developed primers OC−368F/OC693R (5′-ATAAACGCCAATTTCTCTAATTCTTC-3′/5′-GACTTTATTTTTCCAGTTACTTTTTT-3′) and nested primers OC4+F/OC643 (5′-GAAAAAGAATACATTAAGTG-3′/5-TAATTAAGGTTTTTTTGGA-3′) (Devevey et al. ***unpublished results). All samples were subjected to 1% gel electrophoresis to determine the presence of the ospC gene, before being tested with the reverse line blot technique (RLB) to determine specific ospC types infecting the vertebrate host species (Qiu et al., 2002; Brisson and Dykhuizen, 2004). We tested a minimum of three PCR positive ticks per host individual and up to seven positive randomly selected ticks, if there were more than seven positive ticks per individual.
3. Results
3.1. Occurrence probabilities and transmission efficiencies of ospC types
For most ospC types, the addition of host species as a covariate did not improve the model, relative to the null models (Table 1). Only types C and T yielded lower AICc values for the species-specific models, and the difference in AICc between the alternate models was <1 for type C. Overall, we detected only weak support for the more elaborate model. Maximum likelihood estimates of occurrence probabilities were high (ψ > 0.5) for types A and K, low (ψ < 0.2) for types H, L and U, and intermediate (~0.3 < ψ < ~0.4) for the remaining types. Although the support intervals for these estimates were relatively wide, the lack of overlap between the support intervals for types with high and low occurrence probabilities indicates disparate occurrence probabilities among strains, averaged across the host community.
Table 1.
Maximum likelihood estimates and AICc values from the null and species-specific models, and delta AICc differences between the two models. Overall, the null model had lower AICc values, except for ospC C and T, compared to the species-specific model (bolded).
| HIS/non- HIS |
ospC type | Null model | Species-specific model | |Null AICc–species AICc| | ||||
|---|---|---|---|---|---|---|---|---|
| No. parameters | ψ (low–high) (occurrence) | ε (low–high) (transmission) | AICc | No. parameters | AICc | Δ AICc | ||
| HIS | A | 2 | 0.56 (0.45–0.67) | 0.55 (0.49–0.61) | 463.82 | 9 | 479.22 | 15.40 |
| B | 2 | 0.39 (0.27–0.53) | 0.61 (0.52–0.69) | 250.08 | 6 | 259.14 | 9.06 | |
| I | 2 | 0.32 (0.21–0.46) | 0.33 (0.24–0.43) | 232.21 | 7 | 240.21 | 8.00 | |
| K | 2 | 0.52 (0.40–0.65) | 0.38 (0.31–0.44) | 396.25 | 8 | 399.70 | 3.45 | |
| N | 2 | 0.34 (0.23–0.47) | 0.38 (0.29–0.46) | 279.92 | 8 | 289.98 | 10.06 | |
| Non-HIS | C | 2 | 0.29 (0.18–0.42) | 0.44 (0.34–0.54) | 216.16 | 6 | 215.27 | 0.89 |
| D | 2 | 0.29 (0.19–0.40) | 0.46 (0.37–0.55) | 256.90 | 8 | 269.34 | 12.44 | |
| E | 2 | 0.32 (0.22–0.44) | 0.42 (0.34–0.51) | 302.95 | 9 | 312.85 | 9.90 | |
| F | 2 | 0.40 (0.28–0.54) | 0.39 (0.31–0.48) | 288.73 | 8 | 298.77 | 10.04 | |
| G | 2 | 0.44 (0.33–0.55) | 0.54 (0.47–0.61) | 387.69 | 9 | 396.02 | 8.33 | |
| H | 2 | 0.15 (0.07–0.25) | 0.61 (0.47–0.73) | 127.27 | 7 | 133.17 | 5.90 | |
| L | 2 | 0.11 (0.04–0.24) | 0.40 (0.20–0.61) | 62.29 | 4 | 66.11 | 3.82 | |
| M | 2 | 0.33 (0.23–0.45) | 0.44 (0.35–0.52) | 281.25 | 8 | 283.62 | 2.37 | |
| O | 2 | 0.29 (0.15–0.48) | 0.38 (0.26–0.53) | 117.72 | 4 | 121.20 | 3.48 | |
| T | 2 | 0.32 (0.21–0.47) | 0.69 (0.59–0.77) | 189.20 | 6 | 181.56 | 7.64 | |
| U | 2 | 0.17 (0.09–0.28) | 0.67 (0.54–0.78) | 135.04 | 7 | 138.99 | 3.95 | |
The estimates for transmission efficiency (ε) from an infected host to a tick also varied, but generally had tighter support intervals than did the occurrence (ψ) estimates (Table 1). Types T and U had high transmission efficiencies (ε ≈ 0.7); types A, B, G, and H had medium efficiencies (0.55 < ε < 0.6), and the remaining types had the lowest efficiencies (0.35 < ε < 0.45). All transmission efficiency estimates were greater than 0.3, although the lower support interval did fall below 0.3 for several ospC types. (See Appendix A for species-specific model parameter estimates). The support intervals are again wide, but the non-overlapping values of high and low transmission efficiencies indicate that large variation exists in how effectively strains transmit from their vertebrate hosts to ticks, provided the type is actually present in the host.
3.2. HIS and non-HIS proportions among hosts
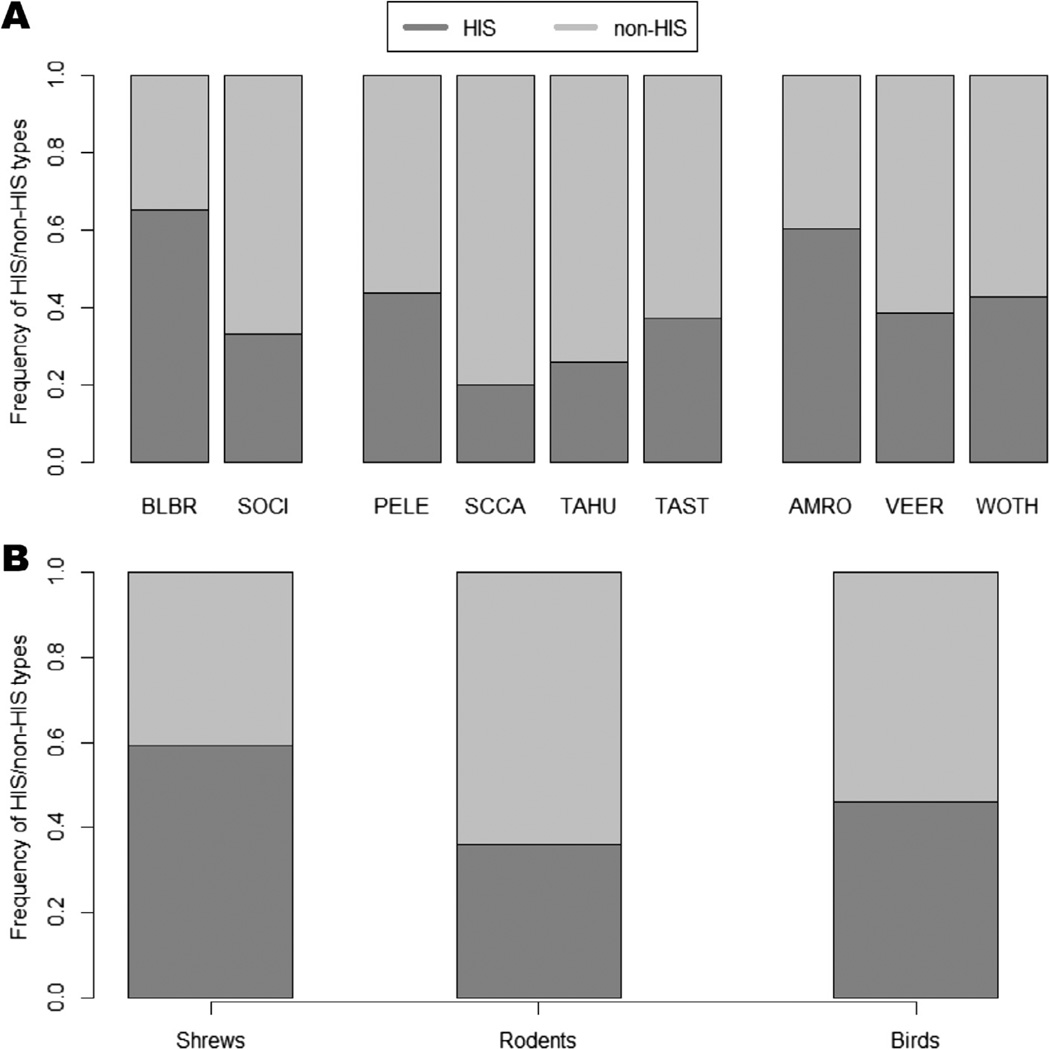
Vertebrate host individuals contained an average of 4.05 (±2.29 sd) ospC types, while individual ticks contained an average of 2.07 (±1.24 sd) types. The frequency of HIS versus non-HIS (Fig. 1A) types detected was significantly different among the nine host species (χ2 = 18.557, df = 8, p = 0.0167), with HIS types less frequent in gray and red squirrels (~0.20 < fr(HIS) < 0.25), while short-tailed shrews and American robins had the highest frequency of HIS types (fr(HIS) > 0.60). Intermediate levels of HIS infection (0.30 < fr(HIS) < 0.45) occurred for all other host species. We also detected a significant difference in HIS types among phylogenetic groups (χ2 = 6.734, df = 2, p = 0.0345), with rodents and birds having lower HIS frequencies than did shrews (Fig. 1B). (See Appendix B for strain-specific positive infections per host species.)
Fig. 1.
Frequency of each HIS to non-HIS types within the host species (A) and among phylogenetic groups (B). Species codes: BLBR = short-tailed shrew, SOCI = masked shrew, PELE = white-footed mouse, SCCA = eastern gray squirrel, TAHU = red squirrel, TAST = eastern chipmunk, AMRO = American Robin, VEER = Veery, WOTH = Wood Thrush.
4. Discussion
4.1. Occurrence (ψ) and transmission efficiency (ε) estimates
Our likelihood models provide a novel approach for examining occurrence and transmission rates of ospC types of B. burgdorferi from a suite of host species to their larval tick vectors. The resulting parameter estimates provided important information on potential disease risk at the genotypic level, as different pathogen genotypes can have different disease outcomes (Strle et al., 2011; Hanincova et al., 2013). In general, our data supported the null models, rather than the more elaborate host species-specific models (Table 1), with the exception of ospC C (which provided inconclusive evidence of a difference) and ospC T (where the data clearly supported the species-specific model). This latter support for the species-specific model reflects the relatively high frequency of ospC T in chipmunks and, to a lesser extent, red squirrels (Table 2), as previously shown by Brisson and Dykhuizen (2004).
The varying occurrence and transmission efficiency rates among ospC types, averaged across the host community, are indicative of variation in bacterial strain circulation among vertebrate hosts and ticks. All models had transmission efficiency (“detection”) values greater than 0.3, with some types (A, G, H, T, and U) having much higher transmission efficiencies. However, of these commonly detected types, several of them (H, T, and U) tended to have low occurrence probabilities (Table 1). This pattern of low occurrence coupled with high transmission efficiency offers the opportunity for types to continually circulate among hosts and ticks, potentially helping to maintain the high ospC diversity observed in wild populations. Alternatively, factors such as strain facilitation (Andersson et al., 2013), competition (Balmer and Tanner, 2011), bacteremia levels (Barbour et al., 2009), or even timing of infection (Ogden et al., 2007) could also affect the overall circulation of strains in the wildlife community, and hence the overall ospC diversity in both hosts and ticks. Our study was not designed to test the mechanisms that influence occupancy or transmission efficiencies, but rather to estimate these probabilities given the infections we detected from fed larval ticks collected from the hosts.
Our models provided a reasonable representation of Lyme disease risk to be expected from the wildlife hosts and tick vectors. Relatively large estimates of occurrence and transmission efficiencies for several HIS types suggest that Lyme disease risk present in this host community may be high (Table 1). The coupling of greater HIS circulation with approximately 30% infection prevalence of B. burgdorferi in questing nymphal ticks southeastern New York (Horobik et al., 2006) implies high Lyme disease risk in this area. While our sampling was primarily carried out on the Cary Institute property, other areas in the northeast have similar percentages of nymphal infection prevalence (Daniels et al., 1998; Levin et al., 1999; Tsao et al., 2004) and relative ospC frequencies (e.g., high A and K) in questing nymphal ticks (Brisson et al., 2011) as our study area. However, different biotic and abiotic factors could have important influences on the realized circulation of ospC types in other areas (e.g. different assemblages of vertebrate hosts). Regardless, we propose that parameter estimates from models such as those presented here have general value with regard to providing information on genotype circulation and potential Lyme disease risk.
Although we have detected patterns in the occurrence and transmission efficiencies of ospC types in our study, we advise caution on the interpretation of the data for several reasons. First, even though we trapped over three summers, the numbers of infected animals that had sufficient ticks to test were rather low, especially for red squirrels and masked shrews, which are difficult to capture. If there are rare strains that occur with host species we have not been able to successfully trap, we may miss these genotypes in our tick samples. Second, frequencies of ospC types detected in the animals may change from year to year, resulting in annual changes in frequencies of strains resident in questing nymphs (Qiu et al., 1997). Hence, there could be more variation in ospC occurrence and transmission efficiencies that could be accounted for with a longer term study. Third, factors such as host and tick infectivity variation, endemicity, and host community composition may influence the relative frequencies of ospC types circulating in the community. And last, although all transmission efficiency estimates were above 0.3, the lower support intervals for several ospC types fell below the 0.3 detection probability. Hence, the level of information from the presence/absence of genotypes detected from the tick is small, and makes it difficult for the model to determine a true absence or low detection of the genotype (MacKenzie et al., 2002).
While there is a need for increased sampling and additional host taxa to capture a greater representation of the wildlife host community, and to provide tighter probability estimates of occurrence and transmission efficiencies, what is clear is that the addition of this patch occupancy model to our analytical toolkit will provide more detail on these parameters for B. burgdorferi genotypes, as well as other vector-borne zoonotic pathogens. Another consideration for future research may include testing host tissue samples or feeding nymphal ticks to compare the utility of the model to PCR based methods. Nonetheless, the utilization of this model for host communities in other areas of the US, or even Europe, offers additional insights into the risk of Lyme disease at the genotypic level, which is important, as these genotypes vary in their disease severity.
4.2. ospC distribution patterns
We expected differences in the ospC frequency distribution among the phylogenetic host groups, due to differences in their ecological and physiological traits. Specifically, the contribution of hosts circulating HIS types could be either species- or group-specific. For example, shrews supported greater frequencies of HIS types and lower frequencies of non-HIS types than did either rodents or birds, except for the robins (Fig. 1B). Although we had only four B. burgdorferi positive masked shrew individuals with sufficient numbers of ticks to assay, which limits our statistical power, the results are nevertheless intriguing, as only one HIS type (B) was detected in this species, as opposed to two or more HIS types in other host species. There is little information on ospC types detected in shrews (Brisson and Dykhuizen, 2004), but the high proportions of type B acquired from both shrew species and their relatively high reservoir competencies (LoGiudice et al., 2003; Brisson et al., 2008) lends credence to the importance of these inconspicuous hosts in Lyme disease risk in this endemic area of New York State.
Among birds, American robins supported relatively higher proportions of HIS types than did the other species. Their commonness in human-dominated landscapes (Whittaker and Marzluff, 2009) and high HIS proportions make robins a particularly important avian host for increased transmission to humans, as previously suggested (Battaly and Fish, 1993; Ginsberg et al., 2005; Ogden et al., 2008; Scott et al., 2010, 2012; Brinkerhoff et al., 2011; Mathers et al., 2011). However, factors such as grooming, foraging behaviors, and habitat where the birds occur, in addition to density, may influence host–tick contact rates and the competency of robins to be effective reservoirs (Richter et al., 2000). Although robins may contribute relatively more to the potential transmission of HIS types, their smaller population size in the northeastern forests (LoGiudice et al., 2003) limits their capability as important hosts contributing to Lyme disease risk. But due to their partial migration patterns, robins may play a role in expanding the range of the B. burgdorferi and the ospC types they support (Ogden et al., 2008, 2011; Brinkerhoff et al., 2011).
Among rodents, squirrels had relatively low HIS frequencies (Fig. 1A) and appear to be poor reservoirs for B. burgdorferi (Keesing et al., 2009), suggesting that squirrels contribute little to Lyme disease risk. On the other hand, white-footed mice and chipmunks supported relatively high HIS frequencies, and are known to be competent reservoirs (Keesing et al., 2009), which reinforces their role as important hosts for B. burgdorferi (LoGiudice et al., 2003). In light of the array of HIS frequencies within this greater host community, as well as past studies on shrew contribution to Lyme disease risk (Brisson et al., 2008), our results argue for including the short-tailed shrew, and to a lesser extent American robins, as important hosts influencing potential HIS types circulating in the wildlife and tick populations.
Although the host community we sampled was primarily located at a single site, the pattern of ospC separation among phylogenetic groups may potentially be generalized, inasmuch as the frequencies of ospC types we detected in our host species were similar to those from other studies in the northeastern US and Canada (Brisson and Dykhuizen, 2004; Hanincova et al., 2006; Ogden et al., 2008, 2011; Barbour and Travinsky, 2010; MacQueen et al., 2012). Further investigation is warranted to determine whether the frequency patterns of HIS and non-HIS hold true for a larger number of host species, host individuals, and fed larval ticks in other areas where Lyme disease is endemic or expanding. Regardless, the present study provides us with better and broader ospC occurrence and transmission efficiency information that can be incorporated with host community composition and diversity (LoGiudice et al., 2003, 2008) parameters to broaden our understanding of Lyme disease risk at the community level.
Supplementary Material
Acknowledgements
The authors would like to thank the field crews of 2008–2010, especially Shannon Duerr and Deanna Lynn Briehof, for their exceptional field and animal husbandry work. We would also like to thank Felicia Keesing for the tick samples; David Moriarty and Peter Thrall for previously reviewing the manuscript; and Julien Papaix for assistance with R graphics. Additional thanks to two anonymous reviewers who have helped improve the manuscript. Funding support included: Bevier Fellowship – Graduate School New Brunswick, Rutgers University, Sep 2011***–May 2012 (H.B.V.); NIAID AI 076342 and AI097137 (D.B.); USDA/NJAES-17111 (P.E.S.); NSF grant DEB 0949702 (R.S.O. and C.C.); and NSF grant EF 0813035 (R.S.O. and F. Keesing).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.meegid.2013.12.011.
Contributor Information
Holly B. Vuong, Email: Holly.vuong@csiro.au.
Charles D. Canham, Email: Canhamc@caryinstitute.org.
Dina M. Fonseca, Email: Dinafons@rci.rutgers.edu.
Dustin Brisson, Email: Dbrisson@sas.upenn.edu.
Peter J. Morin, Email: Pjmorin@rci.rutgers.edu.
Peter E. Smouse, Email: Smouse@aesop.rutgers.edu.
Richard S. Ostfeld, Email: Ostfeldr@caryinstitute.org.
References
- Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, Dumler JS. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J. Clin. Microbiol. 2005;43:1879–1884. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Norris DE. Genetic diversity of Borrelia burgdorferi sensu stricto, in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in southern Maryland. Appl. Environ. Microbiol. 2006;72:5331–5341. doi: 10.1128/AEM.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Scherman K, Raberg L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am. Nat. 2013;181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- Balmer O, Tanner M. Prevalence and implications of multiple-strain infections. Lancet Infect. Dis. 2011;11:868–878. doi: 10.1016/S1473-3099(11)70241-9. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Travinsky B. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. mBio. 2010;1 doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaly GR, Fish D. Relative importance of bird species as hosts for immature Ixodes dammini (Acari: Ixodidae) in a suburban residential landscape of southern New York state. J. Med. Entomol. 1993;30:740–747. doi: 10.1093/jmedent/30.4.740. [DOI] [PubMed] [Google Scholar]
- Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio. 2012;3 doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Folsom-O’Keefe CM, Tsao K, Diuk-Wasser MA. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. Front. Ecol. Environ. 2011;9:103–110. [Google Scholar]
- Brisson D, Baxamusa N, Schwartz I, Wormser GP. Biodiversity of Borrelia burgdorferi strains in tissues of Lyme disease patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE. OspC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, Ostfeld R. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. R. Soc. B: Biol. Sci. 2008;275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Vandermause MF, Meece JK, Reed KD, Dykhuizen D. Evolution of northeastern and midwestern Borrelia burgdorferi, United States. Emerg. Infect. Dis. 2012;16:911–917. doi: 10.3201/eid1606.090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease–A tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2002. [Google Scholar]
- Daniels TJ, Boccia TM, Varde S, Marcus J, Le J, Bucher DJ, Falco RC, Schwartz I. Geographic risk for Lyme disease and human granulocytic ehrlichiosis in southern New York State. Appl. Environ. Microbiol. 1998;64:4663–4669. doi: 10.1128/aem.64.12.4663-4669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, Schwartz I. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am. J. Trop. Med. Hyg. 2008;78:806–810. [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser MA. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl. Environ. Microbiol. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina AR, Schmidt KA, Schauber EM, Ostfeld RS. Modeling the role of songbirds and rodents in the ecology of Lyme disease. Can. J. Zool. 2000;78:2184–2197. [Google Scholar]
- Ginsberg HS, Buckley PA, Balmforth MG, Zhioua E, Mitra S, Buckley FG. Reservoir competence of native North American birds for the Lyme disease spirchete, Borrelia burgdorferi. J. Med. Entomol. 2005;42:445–449. doi: 10.1603/0022-2585(2005)042[0445:RCONNA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Rosen ME, Walker ED, Tsao JI. Diverse Borrelia burgdorferi strains in a bird-tick cryptic cycle. Appl. Environ. Microbiol. 2011;77:1999–2007. doi: 10.1128/AEM.02479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 2006;12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Mukherjee P, Ogden NH, Margos G, Wormser GP, Reed KD, Meece JK, Vandermause MF, Schwartz I. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One. 2013;8:e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Ogden NH, Diuk-Wasser M, Pappas CJ, Iyer R, Fish D, Schwartz I, Kurtenbach K. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 2008;74:153–157. doi: 10.1128/AEM.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 2012;18:1951–1957. doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister EK, Glass GE, Childs JE, Persing DH. Population dynamics of a naturally occurring heterogeneous mixture of Borrelia burgdorferi clones. Infect. Immun. 1999;67:5709–5716. doi: 10.1128/iai.67.11.5709-5716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horobik VC, Keesing F, Ostfeld RS. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest-field edges. Ecohealth. 2006;3:262–268. [Google Scholar]
- Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H, Ostfeld RS. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B-Biol. Sci. 2009;276:3911–3919. doi: 10.1098/rspb.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Hersh MH, Tibbetts M, McHenry DJ, Duerr S, Brunner J, Killilea M, LoGiudice K, Schmidt K, Ostfeld R. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 2012;18:2013–2016. doi: 10.3201/eid1812.120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg. Infect. Dis. 1999;5:204–208. doi: 10.3201/eid0502.990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 2004;72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K, Duerr STK, Newhouse MJ, Schmidt KA, Killilea ME, Ostfeld RS. Impact of host community composition on Lyme disease risk. Ecology. 2008;89:2841–2849. doi: 10.1890/07-1047.1. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie DI, Nichols JD, Lachman GB, Droege S, Andrew Royle J, Langtimm CA. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 2002;83:2248–2255. [Google Scholar]
- MacQueen DD, Lubelczyk C, Elias SP, Cahill BK, Mathers AJ, Lacombe EH, Rand PW, Smith RP., Jr Genotypic diversity of an emergent population of Borrelia burgdorferi at a costal Maine island recently colonized by Ixodes scapularis. Vector Borne Zoonotic Dis. 2012;12:456–461. doi: 10.1089/vbz.2011.0811. [DOI] [PubMed] [Google Scholar]
- Margos G, Tsao JI, Castillo-Ramirez S, Girard YA, Hamer SA, Hoen AG, Lane RS, Raper SL, Ogden NH. Two boundaries separate Borrelia burgdorferi populations in North America. Appl. Environ. Microbiol. 2012;78:6059–6067. doi: 10.1128/AEM.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers A, Smith RP, Cahill B, Lubelczyk C, Elias SP, Lacombe E, Morris SR, Vary CP, Parent CE, Rand PW. Strain diversity of Borrelia burgdorferi in ticks dispersed in North America by migratory birds. J. Vector Ecol. 2011;36:24–29. doi: 10.1111/j.1948-7134.2011.00137.x. [DOI] [PubMed] [Google Scholar]
- McCallum J. Changing use of camera traps in mammalian field research: habitats, taxa and study types. Mammal Rev. 2013;43:196–206. [Google Scholar]
- Murphy L. Likelihood: methods for maximum likelihood estimation. R package v 1.5. 2012 < http://CRAN.R-project.org/package=likelihood>. [Google Scholar]
- NYSDOH. New York State Department of Health: communicable diseases. 2013 < http://www.health.ny.gov/statistics/diseases/communicable/>.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Kurtenbach K, Lindsay LR, Charron DF. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134:209–227. doi: 10.1017/S0031182006001417. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Hanincova K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O’Callaghan CJ, Schwartz I, Thompson RA. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008;74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Margos G, Aanensen DM, Drebot MA, Feil EJ, Hanincová K, Schwartz I, Tyler S, Lindsay LR. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl. Environ. Microbiol. 2011;77:3244–3254. doi: 10.1128/AEM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Hazler KR, Cepeda OM. Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. J. Med. Entomol. 1996;33:90–95. doi: 10.1093/jmedent/33.1.90. [DOI] [PubMed] [Google Scholar]
- Qiu WG, Bosler EM, Campbell JR, Ugine GD, Wang IN, Luft BJ, Dykhuizen DE. A population genetic study of Borrelia burgdorferi sensu stricto from eastern Long Island, New York, suggested frequency-dependent selection, gene flow and host adaptation. Hereditas. 1997;127:203–216. doi: 10.1111/j.1601-5223.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. Version 2.15. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Rand PW, Lacombe EH, Smith RP, Ficker J. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. J. Med. Entomol. 1998;35:270–276. doi: 10.1093/jmedent/35.3.270. [DOI] [PubMed] [Google Scholar]
- Richter D, Spielman A, Komar N, Matuschka FR. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000;6:133–138. doi: 10.3201/eid0602.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012;98:49–59. doi: 10.1645/GE-2874.1. [DOI] [PubMed] [Google Scholar]
- Scott JD, Lee MK, Fernando K, Durden LA, Jorgensen DR, Mak S, Morshed MG. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J. Vector Ecol. 2010;35:124–139. doi: 10.1111/j.1948-7134.2010.00038.x. [DOI] [PubMed] [Google Scholar]
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC Type A) genotype is associated with greater inflammation and more severe Lyme disease. Am. J. Pathol. 2011;178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taragel’ova V, Koci J, Hanincova K, Kurtenbach K, Derdakova M, Ogden NH, Literak I, Kocianova E, Labuda M. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of Borreliosis in Central Europe. Appl. Environ. Microbiol. 2008;74:1289–1293. doi: 10.1128/AEM.01060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: Vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I, Dykhuizen DE, Qui W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker KA, Marzluff JM. Species-specific survival and relative habitat use in an urban landscape during the postfledging period. Auk. 2009;126:288–299. [Google Scholar]
- Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, Nadelman RB, Ludin S, Schwartz I. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 2008;198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.