Abstract
Objective
To investigate the association between obesity and multiple sclerosis (MS) while accounting for established genetic and environmental risk factors.
Methods
Participants included members of Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) (1,235 MS cases and 697 controls). Logistic regression models were used to estimate odds ratios (ORs) with 95% confidence intervals (95% CI). Body mass index (BMI) or body size was the primary predictor of each model. Both incident and prevalent MS cases were studied.
Results
In analyses stratified by gender, being overweight at age 10 and 20 were associated with MS in females (p<0.01). Estimates trended in the same direction for males, but were not significant. BMI in 20’s demonstrated a linear relationship with MS (p-trend=9.60 × 10−4), and a twofold risk of MS for females with a BMI ≥ 30 kg/m2 was observed (OR = 2.15, 95% CI 1.18, 3.92). Significant associations between BMI in 20’s and MS in males were not observed. Multivariate modeling demonstrated that significant associations between BMI or body size with MS in females persisted after adjusting for history of infectious mononucleosis and genetic risk factors, including HLA-DRB1*15:01 and established non-HLA risk alleles.
Interpretation
Results show that childhood and adolescence obesity confer increased risk of MS in females beyond established heritable and environmental risk factors. Strong evidence for a dose-effect of BMI in 20’s and MS was observed. The magnitude of BMI association with MS is as large as other known MS risk factors.
Search Terms: multiple sclerosis, childhood, adolescence, genetics, risk factors in epidemiology
INTRODUCTION
Multiple sclerosis (MS) is a severe and complex disease of the central nervous system (CNS) that affects over 400,000 Americans and 2.5 million people worldwide.[1] It remains the second leading cause of neurological disability in young to middle-aged adults.[2] While advances in clinical management have been made over the past 30 years, long-term prognosis for most individuals diagnosed with MS remains poor.[3] After 20 years from onset, more than 60% of individuals with MS require ambulatory assistance; very progressive MS occurs in approximately 5–10% of individuals.[2,4] Strong evidence supports the contribution of both genetic and environmental factors to disease susceptibility as demonstrated by increased, though incomplete, disease concordance among monozygotic twins (~25%) compared to dizygotic twins (~5%).[5–7] Substantial progress has been made towards the identification of several MS risk factors including the HLA-DRB1*15:01 allele within the major histocompatibility complex (MHC) and other non-HLA genetic variants,[8–10] as well as exposure to tobacco smoke, Epstein-Barr Virus (EBV) infection, and lower levels of vitamin D;[11–13] however, the mechanisms underlying disease pathogenesis are still undefined.
Recently, obesity has emerged as an important risk factor for MS. Association between body mass at age 18[14] or age 20[15] and MS onset later in life was observed in two studies, where obese participants demonstrated greater than a twofold increase in risk of MS compared to those at normal weight. Additionally, childhood obesity and risk of both pediatric[16] and later onset[17] MS was reported. In pediatric cases, an increased risk was more prominent amongst females, and extremely obese children had over three times the odds of developing disease compared to normal weight children.[16] In a Danish study, childhood obesity was associated with 1.75 increased risk of developing MS later in life when comparing BMI of girls ≥ 95th percentile to girls < 85th percentile.[17] While obesity has been shown convincingly to be associated with MS, previous studies have not investigated this relationship while accounting for effects of other established risk factors. As both childhood and adolescence may be critical exposure periods for MS,[18] this study aimed to examine whether body size/mass during childhood and adolescence were associated with MS while controlling for a number of environmental and genetic risk factors, including history of infectious mononucleosis and HLA-DRB1*15:01 status, the strongest genetic contributor to MS.[19]
METHODS
Participants
Data were collected from members of Kaiser Permanente Medical Care Plan, Northern California Region (KPNC). Both incident and prevalent MS cases were studied. KPNC is an integrated health services delivery system with a membership of 3.2 million that comprises about 25–30% of the population of a 22 county service area and is the largest healthcare provider in northern California. Comparisons with the general population have shown that the membership is objectively representative; however, persons in impoverished neighborhoods are underrepresented.[20] The membership is stable with 64% of all members, and over 72% of those aged 40 or more years, maintaining membership for five years or more. Individuals with chronic conditions such as MS have historically been more likely to remain members. The KPNC MS Research Program was recently established to support epidemiologic investigations of both genetic and environmental risk factors in a large, population-based study sample.
Eligible KPNC cases were defined as: individuals with a diagnosis of MS by a neurologist (multiple sclerosis, ICD9 code 340.xx; 95% had at least two MS diagnoses by a neurologist), current age of 18 through 69 years, and membership in KPNC at initial contact. The study was restricted to white (non-Hispanic) race/ethnicity, the population with the highest prevalence of MS. The treating neurologist of each MS case was contacted for approval to contact each case as a potential MS study participant. Eligibility for the study was confirmed at initial contact, and diagnoses were validated utilizing medical record review and published diagnostic criteria.[21–22]
Controls were KPNC current members without a diagnosis of MS or related condition (optic neuritis, transverse myelitis, or demyelination disease; ICD9 codes: 340, 341.0, 341.1, 341.2, 341.20, 341.21, 341.22, 341.8, 341.9, 377.3, 377.30, 377.39, and 328.82) confirmed through electronic records, and white (non-Hispanic) race/ethnicity. Potential study participants were contacted by mail with a follow-up phone call to explain the study and procedures. The average participation rate was 58% for controls and 79% for cases. A total of 1,932 individuals (1,235 MS cases and 697 controls) with data on body size were studied at the time of the data freeze (February 2013). Study protocols were approved by the Institutional Review Boards (IRB) of KPNC and the University of California, Berkeley.
KPNC Exposure Assessment
KPNC participants completed a computer-assisted telephone interview (CATI) administered by trained staff interviewers and comprised of questions related to various events and exposures. Education level was defined as the self-reported highest education level attained on an 8-point scale: “none”; “grade school only (1–8)”; “some high school”; “not high school graduate”; “high school graduate or GED”; “some college or technical/trade/vocation school or associate’s degree”; “bachelor’s degree”; “master’s degree”; and “doctoral degree.” Smoking was classified as ever or never based on: “Have you ever smoked at least one cigarette per day for one month or more?” Sun exposure at 10 years of age was assessed by asking “At 10 years of age, how often did you sunbathe in the summer (lay in the sun with a bathing suit on between the hours of 10am–2pm)?” and categorized as: ““almost every day”; “2–5 days per week”; “at least once per week”; “1–2 times per month”; and “never”. Physical activity at 10 years of age was determined by inquiring: “Overall, as a young girl/boy at 10 years of age you were:” with possible responses being “not physically active”; “a little physically active”; “moderately physically active”; and “very physically active”. Additional information included residence at birth and age 10 (city, state), birth weight, having been breastfed as an infant, mother and father’s body size at age 30, and history of infectious mononucleosis as a proxy for pre-MS EBV serostatus, which was not available for study participants. EBV exposure can manifest as infectious mononucleosis[23] and previous epidemiologic studies have repeatedly shown association between this condition and the development of MS.[24] Onset year of MS, determined as year of first self-reported symptom, and age of diagnosis were determined based on a series of standardized CATI questions including: “How old were you when you had your first symptoms of MS?”, “How old were you when a doctor first told you that you had MS?” Year for symptom onset and age of diagnosis were calculated using date of birth provided in the electronic medical record (EMR). Additional questions such as “Prior to being diagnosed, which of the following describes your very first MS symptoms which lasted for 3 or more days?” (13 possible first symptoms were defined). When possible, EMR data were used to validate self-reported symptoms.
KPNC study participants reported their current weight and height at the time of the interview, as well as their highest and lowest (non-pregnancy) weight during their 20’s, and their 30’s. Self-reported height and weight have repeatedly been shown to be valid for identifying relationships in epidemiologic studies, as self-reported and measured weights show strong correlation.[25–26] Previous studies have also demonstrated that recalled weight at 18 years of age and self-reported height are highly valid,[27] including Troy et al. (1995) in which women aged 25–42 in the Nurses’ Health Study II were examined.[28]
Body mass index (BMI) for each participant was calculated by dividing weight in kilograms by height reported at time-of-interview in meters squared. Mean BMI of each participant was calculated by averaging the highest and lowest BMI during each period. BMI categories were divided according to the World Health Organization’s definitions: <18.5 kg/m2 (underweight), 18.5–<25 kg/m2 (normal weight), 25–<30 kg/m2 (overweight), and ≥ 30 kg/m2 (obese). The categories for normal and overweight were subdivided to measure smaller variations in MS risk analogous to previous studies: <18.5, 18.5–<21, 21–<23, 23–<25, 25–<27, 27–<30, and ≥ 30.[14–15]
KPNC participants were asked to recall body size at age 10 and age 20 from one of four categories (“underweight,” “just about right,” “little overweight,” or “very overweight”), with the two overweight categories combined for analyses. Participants also identified their body type at time-of-interview, age 10, age 20, and age 30 from one of nine body silhouettes, which ranged from very thin to extremely obese.[29] The largest four body type categories were combined for consistency with prior studies[14] and to avoid small sample size in these categories. At the time of the data freeze, silhouette body type information was obtained for 72.5% of the dataset (906 cases and 496 controls). Missing data were included as an indicator variable.
Whole blood was collected, processed and extracted for DNA using Gentra Puregene protocol. Saliva was collected using Oragene kits. Medium resolution HLA-DRB1 and genome-wide SNP genotyping was performed as previously described.[10,19,30] Additionally, a weighted genetic risk score (wGRS) was calculated for each individual that combines the weighted OR from each of 110 non-MHC MS susceptibility loci identified through recent GWAS and follow-up studies.[10,31,32] The wGRS was calculated by multiplying the number of risk alleles for each locus by the weight for that variant and then taking the sum across the 110 loci. Genetic data were available for 88% of study participants (86% of males; 89% of females).
Statistical Analyses
Demographic differences between cases and controls were compared using X2 test and independent sample t-test where appropriate; Fisher’s exact test was utilized in instances with small cell counts. Stratified analyses were performed for female and male cases and controls due to conflicting evidence regarding the relationship between BMI and MS by gender [15–17]. Logistic regression models were used to study multiple risk factors in addition to BMI in KPNC cases and controls. The primary predictor of each multivariate model was BMI/ body size at various ages, adjusted for year of birth, self-reported history of cigarette smoking, and college education. Tests for linear trend across BMI categories were assessed by modeling BMI categories as continuous, ordinal variables. Both crude and adjusted ORs with 95% CIs were estimated.
When individually added to the multivariate model, latitude at birth and age 10 (calculated from city and state of residence at these time points), physical activity at age 10, sun exposure at age 10, having been breastfed as an infant, birth weight, mother’s body size at 30, and father’s body size at 30 did not significantly contribute to the models (p>0.10) and thus were not included in subsequent analyses. Self-reported history of infectious mononucleosis, number of copies of (HLA)-DRB1*15:01, as well as individual wGRS, were included in final models to investigate if body size/mass contributed to MS risk after adjusting for these established risk factors.
To minimize the potential for reverse causality of MS on body mass and body size, analyses were restricted to MS cases where age of first symptom was > 15 years old for variables involving childhood (39 MS cases excluded; N=1,196 cases), ≥ 30 years old for variables involving BMI during 20’s (an additional 499 cases excluded; N= 697 cases), and ≥ 40 for variables involving BMI during 30’s (an additional 431 cases excluded; N= 266 cases). Spearman’s rank correlation coefficients were computed to measure the relationship between body size variables at various ages, and Wald tests were conducted to measure differences between full and restricted models. All analyses were conducted in Stata v11.2 (StataCorp, TX). This study was focused on a single hypothesis established a priori. A type I error of 5% (α=0.05) was utilized for significance. Attributable risk percent for measures of body size was calculated as: [(OR−1/OR)*100].
RESULTS
Demographic differences between female MS cases and controls (986 cases, 585 controls) were found with respect to year of birth and education (Table I). Further, smoking status, ever having infectious mononucleosis, DRB1*15:01 status and wGRS were strongly associated with MS. There was no significant difference in BMI at time-of-interview in females. Within males, there were significant differences between cases and controls (249 cases, 112 controls) for BMI at time-of-interview, DRB1*15:01 status and wGRS.
Table I.
Demographic and disease characteristics of KPNC MS cases and controls by gendera
| Characteristic | Females
|
Males
|
||||
|---|---|---|---|---|---|---|
| MS Cases (N = 986) | Controls (N = 585) | Pb | MS Cases (N = 249) | Controls (N = 112) | Pb | |
| Year of birth | 1958 ± 8.88 | 1957 ± 8.24 | 0.02 | 1958 ± 9.03 | 1957 ± 8.50 | 0.13 |
| Disease type | -- | -- | -- | -- | ||
| Relapsing-Remitting | 692 (70.18) | 150 (60.24) | ||||
| Secondary-Progressive | 142 (14.40) | 44 (17.67) | ||||
| Primary-Progressive | 59 (5.98) | 31 (12.45) | ||||
| Progressive-Relapsing | 31 (3.14) | 3 (1.20) | ||||
| Unknown | 62 (6.29) | 21 (8.43) | ||||
| Disease duration | 12.07 ± 8.24 | -- | -- | 10.63 ± 8.31 | -- | -- |
| Age at first symptom | 31.18 ± 9.68 | -- | -- | 33.58 ± 9.09 | -- | -- |
| Smoker | 1.70 × 10−3 | 0.35 | ||||
| Never | 508 (51.63) | 348 (59.79) | 119 (47.79) | 59 (53.15) | ||
| Ever | 476 (48.37) | 234 (40.21) | 130 (52.21) | 52 (46.85) | ||
| College graduate | 1.44 × 10−4 | 0.29 | ||||
| Yes | 405 (41.08) | 298 (50.94) | 125 (50.20) | 63 (56.25) | ||
| No | 581 (58.92) | 287 (49.06) | 124 (49.80) | 49 (43.75) | ||
| Infectious mononucleosis | 2.80 × 10−8 | 0.06 | ||||
| Yes | 256 (26.31) | 83 (14.35) | 52 (21.05) | 14 (12.61) | ||
| No | 717 (73.69) | 498 (85.65) | 195 (78.95) | 97 (87.39) | ||
| HLA-DRB1*15:01 (N=1,708) | 1.53 × 10−21 | 6.50 × 10−4 | ||||
| 0 | 360 (41.33) | 357 (67.61) | 103 (49.05) | 69 (69.70) | ||
| 1–2 | 511 (58.67) | 171 (32.39) | 107 (50.95) | 30 (30.30) | ||
| wGRS (N=1,666) | 11.29 ± 0.70 | 11.11 ± 0.70 | 1.64 × 10−5 | 11.33 ± 0.70 | 10.97 ± 0.70 | 3.82 × 10−5 |
| BMI at Time-of-Interview | 0.46 | 0.05c | ||||
| <18.5 | 26 (2.71) | 9 (1.57) | 1 (0.72) | 0 (0.00) | ||
| 18.5–<21 | 121 (12.63) | 67 (11.69) | 5 (3.62) | 0 (0.00) | ||
| 21–<23 | 159 (16.60) | 95 (16.58) | 19 (13.77) | 5 (4.72) | ||
| 23–<25 | 137 (14.30) | 100 (17.45) | 27 (19.57) | 21 (19.81) | ||
| 25–<27 | 135 (14.09) | 73 (12.74) | 26 (18.84) | 30 (28.30) | ||
| 27–<30 | 115 (12.00) | 76 (13.26) | 30 (21.74) | 24 (22.64) | ||
| ≥30 | 265 (27.66) | 153 (26.70) | 30 (21.74) | 26 (24.53) | ||
Table values are mean ± SD for continuous variables and n (column %) for categorical variables.
P-value is for t-test (continuous variables) or χ2 test (categorical variables) between MS Cases vs. Controls
Fisher’s exact test was utilized due to small cell counts
Female report of being a little/very overweight during childhood was weakly correlated with report of being a little/very overweight in one’s twenties (Spearman’s rank correlation coefficient ρ = 0.34); mean BMI in one’s 20’s (ρ = 0.23), and mean BMI in one’s 30’s (ρ = 0.23). Body silhouette report at age 20 and at age 30 were also weakly correlated with body silhouette report at childhood (ages 10 & 20 ρ = 0.35; 10 & 30 ρ = 0.33; 20 & 30 ρ = 0.33). Wald tests determined that statistical models including body size variables at all ages were not significantly different from models including only a single body size variable (p>0.10), thus results were reported only for restricted models.
Being a little/very overweight at age 10 was significantly associated with MS (p=3.00 × 10−3), as was report of being a little/very overweight at age 20 in females (p=2.50 × 10−3) (Table II). Estimates trended in the same direction for males, but were not significant. For females, increased odds of MS were significantly associated with BMI in 20’s for categories 21–<23 kg/m2 (1.39, 95% CI 1.02, 1.91), 25–<27 kg/m2 (1.77, 95% CI 1.06, 2.97), and ≥ 30 kg/m2 (2.15, 95% CI 1.18, 3.92) (p-value for trend = 9.60 × 10−4). No associations between BMI in 30’s and MS were observed in females within any BMI category. Significant associations between BMI in 20’s and MS in males were not observed; however, some evidence of a protective effect for BMI in 30’s was revealed (p-value for trend = 0.04).
Table II.
Adjusted odds ratios (OR) demonstrating the association between childhood and adult body size and increased susceptibility to MS by gender
| Characteristic | Females
|
Males
|
||||
|---|---|---|---|---|---|---|
| N (%MS) | Adjusted OR* (95% CI) | p-value | N (%MS) | Adjusted OR* (95% CI) | p-value | |
| Body size at age 10 | ||||||
| Underweight | 268 (60.07) | 1.02 (0.77, 1.35) | 0.90 | 73 (61.64) | 0.72 (0.41, 1.26) | 0.25 |
| Just about right (Ref) | 919 (59.85) | 1.00 | -- | 206 (68.93) | 1.00 | -- |
| Little – Very overweight | 339 (69.62) | 1.50 (1.15, 1.97) | 3.00 × 10−3 | 77 (75.32) | 1.30 (0.71, 2.38) | 0.39 |
| Body size at age 20 | ||||||
| Underweight | 126 (49.21) | 1.13 (0.77, 1.65) | 0.54 | 36 (47.22) | 0.57 (0.28, 1.17) | 0.13 |
| Just about right (Ref) | 761 (44.94) | 1.00 | -- | 207 (59.90) | 1.00 | -- |
| Little – Very overweight | 229 (56.33) | 1.60 (1.18, 2.16) | 2.50 × 10−3 | 30 (70.00) | 1.50 (0.65, 3.46) | 0.35 |
| BMI in 20’s | ||||||
| <18.5 | 107 (47.66) | 1.22 (0.79, 1.89) | 0.36 | 9 (88.89) | 3.79 (0.41, 34.64) | 0.24 |
| 18.5–<21 (Ref) | 371 (42.32) | 1.00 | -- | 32 (68.75) | 1.00 | -- |
| 21–<23 | 276 (49.64) | 1.39 (1.02, 1.91) | 0.04 | 68 (58.82) | 0.67 (0.27, 1.63) | 0.37 |
| 23–<25 | 150 (49.33) | 1.38 (0.94, 2.03) | 0.10 | 78 (52.56) | 0.53 (0.22, 1.27) | 0.15 |
| 25–<27 | 72 (55.56) | 1.77 (1.06, 2.97) | 0.03 | 40 (52.50) | 0.51 (0.19, 1.37) | 0.18 |
| 27–<30 | 52 (53.85) | 1.63 (0.90, 2.94) | 0.10 | 22 (63.64) | 0.79 (0.25, 2.49) | 0.68 |
| ≥30 | 53 (60.38) | 2.15 (1.18, 3.92) | 0.01 | 15 (66.67) | 0.86 (0.23, 3.30) | 0.83 |
| p-value trend | 9.60 × 10−4 | p-value trend | 0.33 | |||
| BMI in 30’s | ||||||
| <18.5 | 25 (28.00) | 1.22 (0.46, 3.25) | 0.69 | -- | -- | -- |
| 18.5–<21 (Ref) | 168 (23.81) | 1.00 | -- | 13 (69.23) | 1.00 | -- |
| 21–<23 | 202 (24.26) | 1.03 (0.63, 1.69) | 0.91 | 24 (33.33) | 0.25 (0.06, 1.10) | 0.07 |
| 23–<25 | 110 (27.27) | 1.21 (0.68, 2.15) | 0.52 | 49 (38.78) | 0.36 (0.09, 1.38) | 0.14 |
| 25–<27 | 90 (18.89) | 0.86 (0.44, 1.68) | 0.66 | 35 (40.00) | 0.37 (0.09, 1.47) | 0.16 |
| 27–<30 | 62 (32.26) | 1.87 (0.95, 3.69) | 0.07 | 31 (29.03) | 0.22 (0.05, 0.94) | 0.04 |
| ≥30 | 72 (27.78) | 1.69 (0.87, 3.30) | 0.12 | 13 (23.08) | 0.15 (0.03, 0.90) | 0.04 |
| p-value trend | 0.09 | p-value trend | 0.04 | |||
Adjusted for year of birth, history of smoking, and college education (see Methods for details)
Multivariate models examining body size at age 10 and susceptibility to MS in females, and restricted to individuals with complete genetic information, demonstrated a consistent and more pronounced association after controlling for conventional covariates (age, smoking, and education), history of infectious mononucleosis, and various genetic factors relating to MS predisposition (Table IIIa). Similarly, this association was evident for body size at age 20 (Table IIIb) and mean BMI in 20’s (Table IIIc) in females. There was no association between body size/BMI at any age period and MS in males when restricting to individuals with complete genetic information and controlling for conventional covariates. Associations remained insignificant when infectious mononucleosis and genetic risk factors were added to the model (data not shown).
Table IIIa.
Multivariate models assessing the association between body size during childhood and increased susceptibility to MS in females#
| Conventional Covariates* | Conventional Covariates and Infectious Mononucleosis | Conventional Covariates, Infectious Mononucleosis, and Genotype | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Overall body size at age 10 (0=Just about right, 1= Little/Very overweight) | 1.54 (1.15, 2.06) | 3.50 × 10−3 | 1.58 (1.18, 2.12) | 2.10 × 10−3 | 1.63 (1.21, 2.21) | 1.50 × 10−3 |
| Year of Birth | 1.01 (1.00, 1.02) | 0.12 | 1.01 (1.00, 1.02) | 0.14 | 1.01 (1.00, 1.03) | 0.08 |
| Smoker (0=no, 1=yes) | 1.23 (0.98, 1.54) | 0.08 | 1.23 (0.97, 1.54) | 0.08 | 1.25 (1.03, 1.59) | 0.06 |
| College (0=yes, 1=no) | 1.50 (1.20, 1.89) | 4.4 × 10−4 | 1.61 (1.27, 2.03) | 6.10 × 10−5 | 1.58 (1.24, 2.00) | 2.10 × 10−4 |
| Infectious Mononucleosis (0=no, 1=yes) | 2.26 (1.70, 3.00) | 1.80 × 10−8 | 2.17 (1.62, 2.91) | 2.00 × 10−7 | ||
| HLA-DRB1*15:01 positive (0=no, 1=yes) | 2.90 (2.29, 3.68) | 1.70 × 10−18 | ||||
| wGRS | 1.41 (1.19, 1.68) | 7.20 × 10−5 | ||||
Analyses restricted to individuals with complete genetic information and age of onset > 15 years (N=1327)
Conventional covariates include year of birth, history of smoking, and college education.
Table IIIb.
Multivariate models assessing the association between body size in 20’s and increased susceptibility to MS in females#
| Conventional Covariates* | Conventional Covariates and Infectious Mononucleosis | Conventional Covariates, Infectious Mononucleosis, and Genotype | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Overall body size at age 20 (0=Just about right, 1= Little/Very overweight) | 1.62 (1.17, 2.24) | 3.80 × 10−3 | 1.62 (1.17, 2.25) | 4.00 × 10−3 | 1.70 (1.21, 2.39) | 2.50 × 10−3 |
| Year of Birth | 0.97 (0.96, 0.99) | 2.90 × 10−3 | 0.97 (0.96, 0.99) | 3.30 × 10−3 | 0.98 (0.96, 1.00) | 0.02 |
| Smoker (0=no, 1=yes) | 1.11 (0.86, 1.43) | 0.44 | 1.11 (0.86, 1.44) | 0.44 | 1.21 (0.88, 1.51) | 0.30 |
| College (0=yes, 1=no) | 1.50 (1.16, 1.94) | 2.10 × 10−3 | 1.59 (1.22, 2.07) | 5.20 × 10−4 | 1.53 (1.15, 1.98) | 3.20 × 10−3 |
| Infectious Mononucleosis (0=no, 1=yes) | 2.01 (1.46, 2.76) | 1.80 × 10−5 | 2.34 (1.45, 2.79) | 3.00 × 10−5 | ||
| HLA-DRB1*15:01 positive (0=no, 1=yes) | 2.83 (2.17, 3.72) | 3.40 × 10−14 | ||||
| wGRS | 1.43 (1.18, 1.73) | 2.30 × 10−4 | ||||
Analyses restricted to individuals with complete genetic information and age of onset ≥ 30 years (N=981)
Conventional covariates include year of birth, history of smoking, and college education.
Table IIIc.
Multivariate models assessing the association between mean BMI in 20’s and increased susceptibility to MS in females#
| Conventional Covariates* | Conventional Covariates and Infectious Mononucleosis | Conventional Covariates, Infectious Mononucleosis, and Genotype | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Mean BMI in 20’s (0= 18.5–<21 kg/m2 1= ≥ 30 kg/m2)| | 2.51 (1.29, 4.87) | 6.50 × 10−3 | 2.67 (1.36, 5.22) | 4.20 × 10−3 | 2.98 (1.49, 5.94) | 2.00 × 10−3 |
| Year of Birth | 0.97 (0.95, 0.99) | 7.20 × 10−4 | 0.97 (0.95, 0.99) | 6.50 × 10−4 | 0.97 (0.96, 0.99) | 5.20 × 10−3 |
| Smoker (0=no, 1=yes) | 1.14 (0.88, 1.49) | 0.32 | 1.15 (0.88, 1.50) | 0.29 | 1.20 (0.91, 1.58) | 0.20 |
| College (0=yes, 1=no) | 1.46 (1.12, 1.91) | 4.90 × 10−3 | 1.54 (1.18, 2.02) | 1.60 × 10−3 | 1.45 (1.09, 1.92) | 9.60 × 10−3 |
| Infectious Mononucleosis (0=no, 1=yes) | 2.08 (1.50, 2.88) | 1.10 × 10−5 | 2.12 (1.51, 2.96) | 1.30 × 10−5 | ||
| HLA-DRB1*15:01 positive (0=no, 1=yes) | 2.82 (2.14, 3.71) | 2.00 × 10−13 | ||||
| wGRS | 1.48 (1.22, 1.80) | 8.30 × 10−5 | ||||
Analyses restricted to individuals with complete genetic information and age of onset ≥ 30 years (N=952)
Conventional covariates include year of birth, history of smoking, and college education.
When variables of body size during childhood and mean BMI in twenties were considered in the same model and multivariate analyses restricted to female MS cases with age at onset between 20 and 30 years old, report of being a little/very overweight at age 10 was significantly different from controls (p=0.01), while being little/very overweight at age 20 was not (p=0.68) (Figure 1). In contrast, female MS cases with age at onset 30 years old or later, being a little/very overweight at age 10 was not significant (p=0.14), and report of being a little/very overweight at age 20 was significant (p=0.049).
Figure 1.
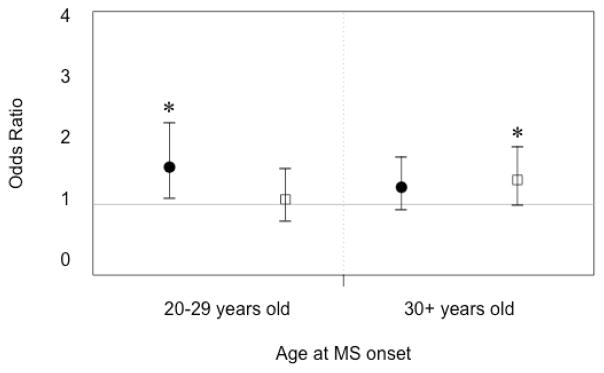
Multivariate adjusted odds ratios and 95% CIs for body size/mass and MS susceptibility in females at different time periods. Stratified odds ratios are presented for MS cases with age of onset between 20–29 years of age, and those with age at onset at 30 years old or later. Black circles reflect odds of MS amongst females reporting being a little/very overweight at age 10. White squares demonstrate odds of MS amongst females reporting being a little/very overweight at age 20. Asterisk indicates p-value <0.05.
Body size as reported by silhouette identification was not significantly different between female cases and controls at time-of-interview (Supplementary Table I). Report of larger body size at age 10, however, was associated with increased odds of MS (adjusted OR body size 5 vs. 3 = 1.59, 95% CI 1.01, 2.49); report of larger body size at age 20 also trended toward significance (adjusted OR body size 5 vs. 3 = 1.58, 95% CI 0.99, 2.52). No association between body size as reported by silhouette identification was found in KPNC males at any age (data not shown).
DISCUSSION
The current study is the very first to demonstrate a significant association between self-report of being overweight at age 10, as well as at age 20, and MS in females after controlling for established genetic and environmental risk factors. Findings demonstrated a significant and two-fold increased risk of MS with increasing BMI similar to previous findings of prospective cohorts.[14] Additionally, the odds of MS in females increases linearly with BMI, providing strong evidence to support a dose effect. The observed association between body mass and MS persisted after controlling for history of infectious mononucleosis and smoking, HLA-DRB1*15:01 status, and the combined effects of known non-MHC risk alleles in females. Notably, the magnitude of BMI association with MS is strong and similar to other identified genetic and environmental MS risk factors. We also compared our findings to those from the Epidemiological Investigation of MS (EIMS), a large MS population-based case-control study in Sweden.[15,33] Association between BMI at age 20 and MS was examined in 1,571 MS cases and 3,371 controls matched on age, gender and residential area. Similar to the current study, an increased risk of MS was associated with increasing BMI at age 20 in females (Supplementary Figure 1), providing additional support for the importance of this time period in MS susceptibility.
The growing obesity epidemic significantly impacts public health at both local and global levels. Current estimates show more than one third of adults and approximately 17% of children in the United States are obese.[34] Common disorders such as cardiovascular and metabolic diseases, as well as many cancers have been convincingly linked to obesity.[35] Obesity also has been recently established as a risk factor for a number of chronic and autoimmune diseases, including MS.[36] Results indicate that given a causal relationship, approximately 33% of MS cases can be attributed to being a little/very overweight at age 10, amongst females reporting this weight status.
Our findings demonstrate that childhood, in addition to adolescence, is a particularly vulnerable period of exposure for MS risk, as has been suggested in the literature for obesity and other environmental factors such as sunlight exposure.[14,15,18] Results suggest that body size during the period immediately preceding onset of symptoms may be an important factor for MS susceptibility in females; however, further investigation is warranted.
Investigations of childhood obesity and risk of MS have reported conflicting findings. A recent study found that extremely obese children had over three times the odds of developing pediatric MS compared to normal weight children, with risk especially strong amongst females.[16] A previous study by Munger et. al (2009) did not find an association between obesity in childhood and risk of MS, though this may be due to utilization of silhouette data to characterize body size during childhood. While strong associations between MS and being a little/very overweight during childhood and adolescence were observed in the current study, results based on silhouette data were not consistent. This may be due to a more favorable perception of body silhouettes in overweight individuals,[37] which would bias results towards the null, and reduced available power to detect an association.
While childhood and adolescent body size in males suggested an increased risk of MS, results were not significant. Previous studies examining this relationship in males have not shown an association between obesity and pediatric MS,[16] or between childhood obesity and MS with a later onset;[17] however, risk of MS amongst males was greater with increasing BMI at age 20 in the EIMS study[15]. In contrast, our results demonstrated a null association between MS and BMI in 20’s, and an inverse relationship between MS and increasing BMI in 30’s. When analyses were restricted to males with genotype data, the inverse association was not significant and persisted after controlling for history of infectious mononucleosis and established genetic risk factors. Thus, further investigation of BMI and MS in males is needed. It should be noted that our sample size was small and wide confidence intervals were observed. Larger studies are needed to determine whether obesity is a strong independent risk factor for males, as demonstrated for females.
The biological mechanism through which obesity and MS may be related is unknown; however, several hypotheses are plausible. Obesity is characterized by a chronic, low-grade inflammatory response supported by growing experimental evidence. Recent literature suggests that integration of metabolic tissue and immune cells contribute to obesity and obesity-related inflammation by sharing a common cellular target.[38] Alterations in adipose tissue in human studies may occur as early as in childhood.[39] Obesity during childhood and adolescence is also associated with increased levels of C-reactive protein, interleukin-6, and leptin levels,[40,41] indicating a proinflammatory state that may be important in MS pathogenesis. Interestingly, adverse serum lipid profiles have been associated with MS disease progression, and statins may be beneficial in early MS by reducing the migration of immune cells across the blood brain barrier.[42,43] The gut microbiota have also been reported to shape immune response and may influence peripheral inflammation.[44] One study found that gut bacteria influences neurologic inflammation through induction of Th-17 responses in experimental autoimmune encephalomyelitis, a well-established animal model for MS studies.[45] Whether individual gut microbiota contribute directly to inflammation, or instead act causally to influence the development of obesity which in turn promotes inflammation, remains unknown.[46] A recent study also demonstrated an association between self-reported abuse during childhood and risk of severe obesity later in life.[47] Future studies examining obesity as a mediator of stressful life events and MS may be informative.
Additionally, adults and children with high body fat mass have lower circulating levels of vitamin D metabolites.[48,49] Lower levels of vitamin D have been associated with increased risk for MS[50] and more severe disease progression.[51] Therefore, overweight and obese individuals may be at particularly high risk for developing MS compared to normal weight individuals, especially during critical exposure periods of MS risk. The current study could not assess individual pre-disease levels of serum vitamin D; however, we do not see this as a limitation, similar to previous studies.[14] While information on time spent in the sun at age 10 was included as a potential proxy for sun exposure and resulting vitamin D levels, no association with MS was observed. One interview question was used in the current study to assess sun behavior at age 10. A more extensive index of sun exposure at this important time period might be more informative. Because vitamin D deficiency lies on the causal pathway between BMI and MS risk, further studies are needed to determine whether high BMI or obesity confers risk of MS exclusively through vitamin D deficiency, or whether other mechanisms related to obesity are involved.
Limitations of this study include the potential for inaccurate recall of body size, though we would expect this to bias results towards the null, as women that classify as overweight often underreport weight[52] and identify with a more favorable perception of body silhouette.[37] There is also the potential for selection bias, if controls participating in the study are healthier than nonparticipants with respect to body size/mass. Such differences could bias results away from the null. However, no association between BMI at time-of-interview or BMI in 30’s and MS in females was detected. Additionally, 27% of the control population in our study classified as obese at time-of-interview, comparable to 26% of the California population using estimates of reported BMI in 2009.[53]
An additional limitation in our study was the small number of male MS cases and controls. Our recruitment pool is drawn from the eligible KPNC study participants meeting inclusion criteria and shows the female to male MS patient ratio in the overall KPNC membership is closer to 3:1; whereas, to date, our recruitment efforts indicate a 4:1 ratio. Females thus far have been more likely to participate, similar to what has been observed in other epidemiologic studies,[54] and extra efforts are currently being made to recruit more male participants. Stratified analyses of BMI and body size variables based on gender were performed in the current study, as well as separate power analyses by gender, which indicated that reduced power was available for males (Supplementary Data). We also studied white, non-Hispanic individuals, which may limit the external validity of our findings. Further, study of this relationship in African-Americans, as well as other populations, is needed.
While self-reported weight for a specific age has been shown to be valid, [25–28] we asked participants about the highest and lowest weight during a ten-year interval, which has not been specifically validated. However, it would appear that the same process that enables an individual to reliably recall their weight at a particular age would also enable an individual to review their weight at each age during a specified and limited interval, and be able to report fairly reliably their highest and lowest weights during that interval. Average BMI over the interval may be more accurate because it captures potential variability, rather than relying on recall of only one weight at a specific time. Weight is important to individuals in their 20’s and the highest and lowest weights are likely to be remembered because of their salience in terms of body image. In the current study, the measure (average of highest and lowest BMI in the 20’s) is significantly associated with MS, in line with what has been shown for recalled weight at age 18[14] and at age 20[15] in other studies. Many potential confounders have been assessed and controlled for, as described in the manuscript. We can think of no plausible unmeasured confounder that provides an alternative explanation for the association that we have found.
Finally, while a prospective cohort design can be used to fully establish temporality and minimize potential recall bias, a case-control design for a less prevalent disease such as MS, as described here, is essential to rigorously pursue a combined study of genetic and environmental risk factors with reasonable statistical power. Data were uniformly collected from all participants by trained interviewers or using standardized surveys. The observed association between BMI and MS in females within our study is very similar with respect to direction and magnitude to a previously published cohort study.[14] Similarity in results using both study designs underscores the utility of case-control studies to identify and model effects of multiple MS risk factors. Within our study specifically, we found nearly identical reporting of childhood and adolescent body size variables between cases with greater than 10 years of disease duration as compared to cases with less than 10 years of disease duration. We therefore do not expect results to vary between prevalent and incident MS cases.
In summary, the etiology of MS is very complex. The importance in MS susceptibility of both genetic and environmental factors, including obesity, is shown convincingly in our models. MS is a disease with high burden on society and quality of life, due primarily to disability.[2] Given the growing obesity epidemic in the U.S. and worldwide, these findings add to the increasing body of evidence for the involvement of obesity and related mechanisms in chronic diseases, including MS. Our results demonstrate obesity as a potentially modifiable factor that could influence risk associated with developing MS in the population.
Supplementary Material
Acknowledgments
This work was supported by NIH R01NS049510, NIH R01NS0495103, NIH R01AI076544, NIH R01ES017080. FBSB is a National MS Society Postdoctoral Fellow. The Swedish group has received grant support from the Swedish research council, Knut and Alice Wallenbergs foundation, the AFA foundation, the Swedish Council for Working Life and Social Research and the Swedish Brain Foundation. We also thank all members and staff of Kaiser Division of Research and UC Berkeley Genetic Epidemiology and Genomics Laboratory, especially Diana Quach, Candi Farlice, Adam Boroian, and Carol Rabello.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadovnick AD, Baird PA, Ward RH. Multiple sclerosis: updated risks for relatives. Am J Med Genet. 1988;29(3):533–541. doi: 10.1002/ajmg.1320290310. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Atlas: Multiple Sclerosis Resources in the World. Multiple Sclerosis International Federation; 2008. [Google Scholar]
- 3.Confavreux C, Vukusic S, Moreau T, et al. Relapses and Progression of Disability in Multiple Sclerosis. N Eng J Med. 2000;343:1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 4.Cottrell DA, Kremenchutzky M, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999 Apr;122(4):625–39. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- 5.Sadovnick AD, Baird PA. The familial nature of multiple sclerosis: age-corrected empiric recurrence risks for children and siblings of patients. Neurology. 1988;38(6):990–991. doi: 10.1212/wnl.38.6.990. [DOI] [PubMed] [Google Scholar]
- 6.Robertson NP, Clayton D, Fraser M, et al. Clinical concordance in sibling pairs with multiple sclerosis. Neurology. 1996;47(2):347–352. doi: 10.1212/wnl.47.2.347. [DOI] [PubMed] [Google Scholar]
- 7.Favorova OO, Kulakova OG, Boiko AN. Multiple sclerosis as a polygenic disease: an update. Genetika. 2010 Mar;46(3):302–13. [PubMed] [Google Scholar]
- 8.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007 Aug 30;357(9):851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 9.Patsopoulos NA, Esposito F, Reischl J, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Annals of neurology. 2011 Dec;70(6):897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011 Aug 11;476(7359):214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol. 2008 Feb;28(1):17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- 12.Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurology. 2008 Mar;7(3):268–277. doi: 10.1016/S1474-4422(08)70042-5. [DOI] [PubMed] [Google Scholar]
- 13.Lauer K. Environmental risk factors in multiple sclerosis. Expert review of neurotherapeutics. 2010 Mar;10(3):421–440. doi: 10.1586/ern.10.7. [DOI] [PubMed] [Google Scholar]
- 14.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009 Nov 10;73(19):1543–1550. doi: 10.1212/WNL.0b013e3181c0d6e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. 2012 Sep;18(9):1334–1336. doi: 10.1177/1352458512436596. [DOI] [PubMed] [Google Scholar]
- 16.Langer-Gould A, Brara SM, Beaber BE, et al. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013 Feb 5;80(6):548–552. doi: 10.1212/WNL.0b013e31828154f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munger KL, Bentzen J, Lauresen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler. 2013 Apr 2; doi: 10.1177/1352458513483889. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam T, Gauderman WJ, Cozen W, et al. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology. 2007 Jul 24;69(4):381–8. doi: 10.1212/01.wnl.0000268266.50850.48. [DOI] [PubMed] [Google Scholar]
- 19.Barcellos LF, Sawcer S, Ramsay PP, et al. Heterogeneity at the HLA-DRB1 locus and risk for multiple sclerosis. Hum Mol Genet. 2006 Sep 15;15(18):2813–24. doi: 10.1093/hmg/ddl223. [DOI] [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001 Jul;50(1):121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 22.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005 Dec;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 23.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010 May 27;362(21):1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- 24.Handel AE, Williamson AJ, Disanto G, et al. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001–2006. BMC Public Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willet WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Park JY, Mitrou PN, Keogh RH, Luben RN, Wareham NJ, Khaw KT. Self-reported and measured anthropometric data and risk of colorectal cancer in the EPIC-Norfolk study. Int J Obes (Lond) 2012 Jan;36(1):107–118. doi: 10.1038/ijo.2011.61. [DOI] [PubMed] [Google Scholar]
- 28.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willet WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 29.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–120. [PubMed] [Google Scholar]
- 30.Barcellos LF, May SL, Ramsay PP, et al. High-density SNP screening of the major histocompatibility complex in systemic lupus erythematosus demonstrates strong evidence for independent susceptibility regions. PLoS Genet. 2009 Oct;5(10):e1000696. doi: 10.1371/journal.pgen.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jager PL, Chibnik LB, Cui J, et al. Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurology. 2009 Dec;8(12):1111–1119. doi: 10.1016/S1474-4422(09)70275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Multiple Sclerosis Genetics Consortium (IMSGC) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013 Sep; doi: 10.1038/ng.2770. <Epub ahead of print>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedstrom AK, Sundqvist E, Baarnhielm M, et al. Smoking and two HLA genes interact to increase the risk for multiple sclerosis. Brain. 2011;134:653–664. doi: 10.1093/brain/awq371. [DOI] [PubMed] [Google Scholar]; Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012 Feb 1;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003 Jan 1;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Burden: mortality, morbidity and risk factors. Global status report on noncommunicable diseases 2010. 2011 Available online < http://www.who.int/nmh/publications/ncd_report_full_en.pdf>.
- 36.Procaccini C, Carbone F, Galgani M, et al. Obesity and susceptibility to autoimmune diseases. Expert Rev Clin Immunol. 2011 May;7(3):287–294. doi: 10.1586/eci.11.18. [DOI] [PubMed] [Google Scholar]
- 37.Tehard B, van Liere MJ, Com Nougue C, et al. Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc. 2002 Dec;102(12):1779–1784. doi: 10.1016/s0002-8223(02)90381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim J, Iyer A, Liu L, et al. Diet-induced obesity, adipose inflammation, and metabolic dysfunction correlating with PAR2 expression are attenuated by PAR2 antagonism. FASEB. 2013;27:4757–4767. doi: 10.1096/fj.13-232702. [DOI] [PubMed] [Google Scholar]
- 39.Sbarbati A, Osculati F, Silvagni D, et al. Obesity and inflammation: evidence for an elementary lesion. Pediatrics. 2006 Jan;117(1):220–223. doi: 10.1542/peds.2004-2854. [DOI] [PubMed] [Google Scholar]
- 40.Chu NF, Chang JB, Shieh SM. Plasma leptin, fatty acids, and tumor necrosis factor-receptor and insulin resistance in children. Obes Res. 2003 Apr;11(4):532–540. doi: 10.1038/oby.2003.75. [DOI] [PubMed] [Google Scholar]
- 41.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012 Aug;13(8):707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 42.Weinstock-Guttman B, Zivadinov R, Mahfooz N, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Inflamm. 2011;8:127. doi: 10.1186/1742-2094-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ifergan I, Wosik K, Cayrol R, et al. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: Relevance to multiple sclerosis. Ann Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 44.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011 Jun 16;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YK, Menezes JS, Umesaki Y, et al. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011 Mar 15;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai F, Coyle WJ. The Microbiome and Obesity: Is Obesity Linked to Our Gut Flora? Current Gastroenterology Reports. 2009;11:307–313. doi: 10.1007/s11894-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 47.Richardson AS, Dietz WH, Gordon-Larsen P. The association between childhood sexual and physical abuse with incident adult severe obesty across 13 years of the National Longitudinal Study of Adolescent Health. Pediatr Obes. 2013 Sep; doi: 10.1111/j.2047-6310.2013.00196.x. <Epub ahead of print>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004 Mar;89(3):1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 49.Smotkin-Tangorra M, Purushothaman R, Gupta A, et al. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007 Jul;20(7):817–823. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 50.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 51.Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012 Aug;72(2):234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engstrom JL, Paterson SA, Doherty A, et al. Accuracy of self-reported height and weight in women: an integrative review of the literature. J Midwifery Womens Health. 2003 Sep-Oct;48(5):338–345. doi: 10.1016/s1526-9523(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 53.Center for Disease Control. Behavioral Risk Factor Surveillance System. Office of Surveillance, Epidemiology, and Laboratory Services; Prevalence and Trends Data: California – 2009. < http://apps.nccd.cdc.gov/brfss/>. [Google Scholar]
- 54.Galea S, Tracy M. Participation Rates in Epidemiologic Studies. Ann Epidemiol. 2007 Sep;17(9):643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


