Abstract
Background
Patients with an acute myocardial infarction (AMI) who have glucose abnormalities are at increased risk for death and adverse ischemic outcomes. The contemporary prevalence of glucose abnormalities among AMI patients in the U.S., as determined by HbA1c, is unknown.
Methods
Patients hospitalized with AMI in a 24-site U.S. AMI registry from 2005-2008 were examined for the presence of dysglycemia using HbA1c, which was analyzed at a core laboratory. Patients were categorized by American Diabetes Association guidelines as having diabetes (HbA1c ≥6.5%), pre-diabetes (HbA1c 5.7-6.4%), or normoglycemia. Baseline demographic, clinical, and metabolic characteristics, as well as long-term all-cause mortality, were compared among groups.
Results
Among 2853 patients with AMI, 1083 (38%) had diabetes, of which 196 (18%) were newly diagnosed. There were an additional 887 patients (31%) with pre-diabetes, and 883 patients (31%) who had normal glucose metabolism. Patients with metabolic abnormalities were older, more frequently female, and had higher prevalence of cardiac and non-cardiac comorbidities, including multivessel disease and left ventricular systolic dysfunction. Patients with increasing metabolic abnormalities had higher mortality over the 3-years after the AMI (8.6% in those with normoglycemia, 10.6% in pre-diabetes, 11.3% in newly diagnosed diabetes, and 20.3% in known diabetes; log rank p<0.001).
Conclusions
In a large U.S. AMI registry, we found that nearly 7 in 10 patients had dysglycemia, with 38% having diabetes, and an additional 31% with pre-diabetes based on HbA1c levels. Over half of the patients who did not have a known diagnosis of diabetes at the time of admission had either newly diagnosed diabetes or pre-diabetes. Progressively greater severity of dysglycemia was also associated with incremental increase in long-term mortality. These data highlight the AMI hospitalization as a key opportunity to screen for glucose abnormalities, so that appropriate interventions and patient education efforts can be implemented prior to discharge.
Keywords: diabetes mellitus, myocardial infarction, HbA1c
Advances in invasive and medical management have significantly improved outcomes in all patients presenting with an acute myocardial infarction (AMI).1 However, patients with diabetes (DM) continue to have a higher risk of recurrent adverse cardiac events after AMI, as well as higher short- and long-term mortality compared with patients without DM.2-3 Furthermore, patients with pre-DM are also at increased risk of these adverse events after AMI as compared with those having normal glucose values.4 While the prevalence of DM and pre-DM have been previously described in the AMI population, these data were based on oral glucose tolerance testing (which is rarely used in contemporary practice) and conducted over 10 years ago.5-6 Since that time, the profile of glucose abnormalities may have changed due to the rising prevalence of dysglycemia in the general population7 and the addition of HbA1c as a diagnostic criterion for DM and pre-DM.8 Accordingly, we sought to define the prevalence of dysglycemia among AMI patients enrolled in a multicenter U.S. registry from 2005-08, as assessed with HbA1c, in order to better understand the contemporary metabolic profiles of patients who present with AMI in the U.S. Finding high rates of DM and pre-DM would underscore the value of using an AMI hospitalization as an important opportunity to also address glucose metabolism and control.
METHODS
Study Population and Protocol
Details of the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) AMI registry have been previously published.9 Eligible patients had biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI. Baseline data were obtained through chart abstraction and a structured interview. Consenting patients had a fasting blood specimen collected prior to discharge, which was analyzed by a core laboratory (Clinical Reference Laboratory, Lenexa, KS). Known DM was defined as a chart-documented diagnosis of DM or glucose-lowering medications at the time of admission (except metformin or thiazolidinediones, as these may have been used for DM prevention [2 patients]). Congruent with the American Diabetes Association guidelines8, newly diagnosed DM was defined as HbA1c≥6.5%, pre-DM was defined as HbA1c 5.7-6.4%, and normal glucose metabolism was defined as HbA1c <5.7%. Mortality at 3-years post-AMI was assessed through a combination of follow-up interviews and a query of the Social Security Death Masterfile. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent.
Statistical Analysis
Baseline characteristics and in-hospital treatments of patients with the 4 different levels of glucose metabolism (known DM, newly diagnosed DM, pre-DM, normoglycemia) were compared using ANOVA for continuous variables and chi-square tests for categorical variables. In addition, patients with known DM were compared with those with newly diagnosed DM using t-tests for continuous variables and chi-square tests for categorical variables. Finally, we compared 3-year mortality rates across the 4 groups using Kaplan-Meier curves. All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Patient Population
Among 4340 patients enrolled in TRIUMPH, 2853 (66%) consented to and provided blood samples that were analyzed for HbA1c. Patients who participated in the laboratory sub-study (vs. not) were more likely to be younger, male, white, current smokers, and have depressive symptoms, although the prevalence of known DM and chart-documented HbA1c, fasting glucose, and cholesterol levels were similar (Supplemental Table 1). Among the 2853 patients with AMI who participated in the laboratory sub-study, 1083 (38%) had DM, of which 196 (18%) had not been previously diagnosed as having DM. There were an additional 887 patients (31%) who had pre-DM, which left only 883 patients (31%) with normal glucose metabolism. Among the 1966 patients in TRIUMPH who did not have DM at the time of admission of their AMI, 10% had new DM (i.e., previously undiagnosed) and 45% had pre-DM.
Comparisons Across Metabolic Groups
There were multiple demographic and clinical differences among the 4 metabolic groups (Table 1). Patients with metabolic abnormalities were older, more frequently female, non-white race, and had greater prevalence of cardiac and non-cardiac comorbidities. Patients with DM and pre-DM were less likely to present with ST-elevations (vs. non-ST-elevation AMI) but were more likely to have multivessel disease and left ventricular systolic dysfunction. In addition, patients with increasing metabolic abnormalities had higher HbA1c levels, higher fasting glucose levels, higher insulin levels, and higher triglyceride levels (Table 2).
Table 1.
Demographic and clinical characteristics of AMI patients according to degree of glucose dysmetabolism
| Known DM n=887 | New DM n=196 | Pre-DM n=887 | Normal n=883 | |
|---|---|---|---|---|
| Age (years) | 60.0 ± 11.5 | 58.6 ± 10.7 | 59.5 ± 12.8 | 55.9 ± 12.4 |
| Male | 62.7% | 64.8% | 67.4% | 75.5% |
| White race | 59.8%* | 51.8%* | 71.2% | 78.2% |
| Currently working | 35.9%‡ | 53.8%‡ | 51.0% | 60.5% |
| Current smoking | 32.7%* | 42.5%* | 45.8% | 44.3% |
| Prior AMI | 26.8%† | 16.8%† | 19.3% | 15.9% |
| Prior angioplasty | 25.6%‡ | 12.8%‡ | 19.4% | 15.5% |
| Prior bypass graft surgery | 17.9%† | 10.2%† | 9.5% | 6.9% |
| Peripheral vascular disease | 6.4% | 5.1% | 3.7% | 2.7% |
| Prior stroke | 7.1%† | 4.1%† | 3.4% | 3.9% |
| Prior heart failure | 15.8%* | 10.2%* | 5.4% | 4.0% |
| Estimated GFR (mL/min/1.73 m2) | 70.7 ± 31.1‡ | 82.7 ± 26.3‡ | 77.2 ± 24.3 | 81.4 ± 24.1 |
| Depression | 26.7%‡ | 13.0%‡ | 16.9% | 19.9% |
| ST-elevation AMI | 31.6%* | 40.8%* | 47.8% | 50.8% |
| Peak troponin (ng/dL) | 21.6 ± 62.5 | 29.4 ± 106.4 | 30.7 ± 72.1 | 34.5 ± 79.1 |
| Multivessel disease | 61.1%† | 50.0%† | 46.3% | 40.9% |
| Left ventricular systolic dysfunction | 21.9% | 20.4% | 16.1% | 17.8% |
| GRACE mortality risk score | 106.5 ± 29.8† | 99.0 ± 26.8† | 99.3 ± 29.6 | 91.8 ± 29.0 |
All comparisons across the 4 groups are significantly different among groups at p<0.001 except LV systolic dysfunction (p=0.016) Pairwise comparisons of Known DM vs. New DM
p<0.05
p<0.01
p<0.001
Table 2.
Metabolic characteristics of AMI patients according to degree of glucose dysmetabolism
| Known DM n=887 | New DM n=196 | Pre-DM n=887 | Normal n=883 | |
|---|---|---|---|---|
| Hemoglobin A 1c (%) | 8.1 ± 2.1† | 7.6 ± 1.7† | 6.0 ± 0.2 | 5.3 ± 0.3 |
| Fasting glucose (mg/dL) | 179.7 ± 82.6† | 146.2 ± 51.9† | 114.7 ± 27.4 | 108.5 ± 22.5 |
| Insulin level (μIU/mL) | 21.4 ± 37.3 | 21.1 ± 24.0 | 17.7 ± 26.4 | 15.3 ± 22.1 |
| Diabetes medications on arrival | 69.8%‡ | 0.0%‡ | 0.2% | 0.0% |
| Diabetes medications at discharge | 83.4%‡ | 25.6%‡ | 2.3% | 0.8% |
| Body mass index (kg/m2) | 31.9 ± 7.4* | 30.7 ± 5.9* | 29.1 ± 5.9 | 27.9 ± 5.6 |
| History of hypertension | 83.9%‡ | 62.2%‡ | 65.2% | 49.4% |
| Systolic blood pressure (mmHg) | 145.7 ± 30.4 | 146.5 ± 27.3 | 140.7 ± 29.8 | 140.3 ± 29.8 |
| Diastolic blood pressure (mmHg) | 83.2 ± 19.5† | 86.8 ± 17.6† | 82.2 ± 18.6 | 82.7 ± 18.7 |
| History of dyslipidemia | 62.2%‡ | 41.8%‡ | 46.4% | 38.1% |
| Total cholesterol (mg/dL) | 152.1 ± 42.6† | 162.0 ± 36.8† | 159.9 ± 36.2 | 153.9 ± 35.6 |
| Fasting triglycerides (mg/dL) | 168.8 ± 130.8 | 170.3 ± 115.2 | 150.1 ± 99.6 | 140.6 ± 81.2 |
| Fasting HDL-C (mg/dL) | 38.7 ± 10.1 | 39.7 ± 9.9 | 40.9 ± 11.1 | 40.1 ± 10.7 |
| LDL-C (mg/dL) | 92.0 ± 35.2† | 100.6 ± 29.8† | 98.4 ± 30.8 | 93.9 ± 30.2 |
All comparisons across the 4 groups are significantly different among groups at p<0.001 except diastolic blood pressure (p=0.02) Pairwise comparisons of Known DM vs. New DM
p<0.05
p<0.01
p<0.001
Compared with known DM, newly-diagnosed DM patients were more likely to be current smokers (new vs. known: 43% vs. 33%, p<0.001), had fewer non-cardiac comorbidities, and lower proportion of those with a history of prior coronary disease (Table 1). Patients with newly diagnosed DM were less likely to have multivessel disease (50% vs. 61%, p=0.006) and more likely to present with an ST-elevation AMI (41% vs. 32%, p=0.013). Patients with newly-diagnosed DM, on average, had milder glucose abnormalities compared with known DM, with lower mean HbA1c levels (7.6 vs. 8.1%, p=0.002) and fasting glucose levels (123 vs. 145 mg/dL, p=0.001), although fasting insulin levels were similar (21.1 vs. 21.4, p=0.912; Table 2).
Regarding long-term outcomes, patients with increasing metabolic abnormalities had increasing risk of mortality over the 3-years after the AMI. The Kaplan-Meier estimated rates of death at 3 years after AMI were 8.6% among those with normoglycemia, 10.6% in those with pre-DM, 11.3% in those with newly diagnosed DM, and 20.3% among those with known DM (log rank p<0.001; Figure 1).
Figure 1.
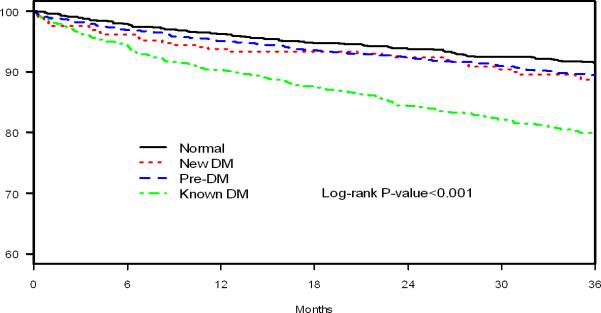
Kaplan Meier survival curves by degree of glucose dysmetabolism
DISCUSSION
In a large, contemporary, multicenter U.S. registry, we found that nearly 70% of patients who present with an AMI have abnormal glucose metabolism. Nearly 2 out of every 5 patients with an AMI have overt DM, with 18% of these patients without an established DM diagnosis. Furthermore, an additional 31% of patients with AMI have pre-DM. Over half of patients without a known diagnosis of DM at admission for AMI had either DM or pre-DM. These results highlight the epidemic of glucose abnormalities in the US and the very high prevalence of dysglycemia in patients with coronary artery disease—a trend that is likely to be accelerated in the future.7 In addition, increasing dysglycemia was associated with incremental increased risk of long-term mortality. Our findings suggest that the AMI hospitalization may be a key opportunity to screen for glucose abnormalities, which may strengthen secondary prevention efforts (including medical and lifestyle interventions) in this high-risk patient population.
Prior studies have found varying levels of abnormal glucose metabolism among patients with coronary artery disease. Among European patients, the prevalence of DM ranges from 20-30% (with higher prevalence in AMI patients5) and ~35% having pre-DM.6 In the China Heart Study, the prevalence of abnormal glucose metabolism was even higher, with DM estimated in ~53% and pre-DM in 24%.10 However, each of these studies focused on screening via oral glucose tolerance testing. Previously recommended by the European Society of Cardiology, this was not included as the primary method of screening in the most recent guideline statement11 due to the inconvenience of the multi-step testing and the emergence of HbA1c as the primary standard of screening, diagnosis, and management of patients with DM. Oral glucose tolerance testing is likely more sensitive than HbA1c for identifying glucose abnormalities12-14; however, there remains some controversy regarding its accuracy in the acute setting of the myocardial infarction.15 In contrast, although there are well known limitations to the use of HbA1c (e.g., blood transfusions, hemoglobinopathies, prolonged stress hyperglycemia, etc.), the representation of chronic glycemic control makes it particularly attractive in the setting of the acute adrenergic surge of an AMI. Given the widespread use of HbA1c in the diagnosis and management of patients with DM, we believe that the use of HbA1c is a key strength of our study.
There are potential limitations to our study that merit further discussion. First, although TRIUMPH included 24 rural, suburban, and urban hospitals across the U.S. and the patients represented a broad range of socioeconomic and demographic characteristics, it is uncertain if our findings are representative of the U.S. AMI population. However, we are unaware of any large studies examining the prevalence of glucose abnormalities in U.S. patients presenting with an AMI, which provides important epidemiologic insight into the prevalence of this risk factor. Second, not all TRIUMPH patients participated in the laboratory sub-study. While there were demographic and clinical differences between those who did and those who did not participate, the frequency of known DM did not differ between groups nor did any of the metabolic factors that were available by chart abstraction, supporting the generalizability of our findings.
In conclusion, in a large, contemporary, U.S. population of AMI patients, we found that nearly 7 in 10 patients had dysglycemia, with 38% having diabetes, and an additional 31% with pre-diabetes based on HbA1c levels. Over half of the patients who did not have a known diagnosis of diabetes at the time of admission had either a new diagnosis of diabetes or pre-diabetes—emphasizing the importance of screening AMI patients with a HbA1c to detect these potentially modifiable risk factors. In addition, progressively greater severity of dysglycemia was associated with incremental increase in long-term mortality. These data improve our understanding of the extremely high prevalence of dysglycemia among patients who present with an AMI and suggest that the AMI hospitalization is an important opportunity to screen for dysglycemia, which could improve secondary prevention efforts in these high-risk patients.
Acknowledgments
Sources of Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): SCCOR Grant #P50HL077113-01. This study was sponsored by a research grant from Genentech, South San Francisco, CA. The funding organizations did not play a role in the conduct of the study or in the collection, management, analysis, and interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: DKM: Consultant honoraria: Genentech, F Hoffmann LaRoche, Pfizer, Daiichi Sankyo, NovoNordisk, Sanofi Aventis, Regeneron, Tethys Bioscience. Clinical trial leadership honoraria: Boehringer Ingelheim, Takeda, Orexigen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Daiichi Sankyo, Merck Schering Plough. JAS: Research grants: NHLBI, AHA, ACCF, Gilead, Lilly, EvaHeart, Amorcyte. Consultation: United Healthcare, Genentech, Amgen. MK: Research grants: American Heart Association, Genetech, Sanofi-Aventis, Gilead, Medtronic Minimed, Glumetrics, Maquet, Eisai; Consultant honoraria: Genentech, Gilead, F Hoffmann LaRoche, Medtronic Minimed, AstraZeneca, Abbvie, Regeneron, Edwards Lifesciences, Eli Lilly. The other authors report no conflicts of interest.
REFERENCES
- 1.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, et al. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995-2006. JAMA. 2009;302(7):767–73. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–75. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SV, Lipska KJ, Li Y, Goyal A, Maddox TM, McGuire DK, et al. The reliability and prognosis of in-hospital diagnosis of metabolic syndrome in the setting of acute myocardial infarction. J Am Coll Cardiol. 2013;62(8):704–8. doi: 10.1016/j.jacc.2013.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallander M, Malmberg K, Norhammar A, Ryden L, Tenerz A. Oral glucose tolerance test: a reliable tool for early detection of glucose abnormalities in patients with acute myocardial infarction in clinical practice: a report on repeated oral glucose tolerance tests from the GAMI study. Diabetes Care. 2008;31(1):36–8. doi: 10.2337/dc07-1552. [DOI] [PubMed] [Google Scholar]
- 6.Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25(21):1880–90. doi: 10.1016/j.ehj.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4(4):467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu DY, Pan CY, Yu JM. The relationship between coronary artery disease and abnormal glucose regulation in China: the China Heart Survey. Eur Heart J. 2006;27(21):2573–9. doi: 10.1093/eurheartj/ehl207. [DOI] [PubMed] [Google Scholar]
- 11.Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 12.Hage C, Lundman P, Ryden L, Mellbin L. Fasting glucose, HbA1c, or oral glucose tolerance testing for the detection of glucose abnormalities in patients with acute coronary syndromes. Eur J Prev Cardiol. 2013;20(4):549–54. doi: 10.1177/2047487312444371. [DOI] [PubMed] [Google Scholar]
- 13.de Mulder M, Oemrawsingh RM, Stam F, Boersma E, Umans VA. Comparison of diagnostic criteria to detect undiagnosed diabetes in hyperglycaemic patients with acute coronary syndrome. Heart. 2012;98(1):37–41. doi: 10.1136/heartjnl-2011-300163. [DOI] [PubMed] [Google Scholar]
- 14.Bartnik M, Ryden L, Malmberg K, Ohrvik J, Pyorala K, Standl E, et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007;93(1):72–7. doi: 10.1136/hrt.2005.086975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen EC, Seljeflot I, Abdelnoor M, Eritsland J, Mangschau A, Arnesen H, et al. Abnormal glucose regulation in patients with acute ST- elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol. 2009;8:6. doi: 10.1186/1475-2840-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


