Abstract
The imidazoline I2 receptor ligand BU99006 binds to and attenuates effects mediated by I2 receptors in vitro, although its effects in vivo have not been studied. This study examined the effects of BU99006 in two behavioral assays in rats: hypothermia and 2-BFI discrimination. BU99006 (3.2 – 15 mg/kg, i.p.) produced a dose-dependent hypothermic effect (rectal temperature), which was antagonized by the I2 receptor antagonist idazoxan. BU99006 (3.2 or 10 mg/kg given 10 min or 2 hr before the session, respectively) did not significantly alter hypothermia produced by I2 receptor agonist 2-BFI (10 mg/kg). In rats discriminating 5.6 mg/kg 2-BFI, BU99006 (1.78 – 17.8 mg/kg, i.p.) produced 40% and 82% responding on the 2-BFI-associated lever when it was administered immediately or 2 hrs prior to the test sessions, respectively. BU99006 enhanced the discriminative stimulus and rate-suppressing effects of 2-BFI. Collectively, these data suggest that BU99006 is an imidazoline I2 receptor agonist with no evidence of I2 receptor antagonism in rats.
Keywords: BU99006, 2-BFI, hypothermia, drug discrimination, rats
Introduction
The concept of imidazoline binding sites was first proposed in 1984 (Bousquet et al., 1984); since then, research has focused on understanding the nature of these binding sites (Regunathan and Reis, 1996; Head and Mayorov, 2006). Initially, these binding sites were thought to recognize compounds with imidazoline moiety; however, we now know that some compounds with no imidazoline structure also have high affinities for these binding sites (Nicolik and Agbaba, 2012). In addition, there appears to be at least three different imidazoline receptor subtypes, each of which has unique pharmacological properties (Eglen et al., 1998). For example, I1 receptors have been shown to be involved in the central control of blood pressure, glucose balance and metabolism (Head and Mayorov, 2006; Sun et al., 2007), and a gene that encodes I1 receptors (imidazoline receptor antisera-selected gene/Nischarin) has been cloned (Sun et al., 2007). The molecular identities (e.g., related gene, protein and signaling substrates) of another subtype of imidazoline receptor (I3) have not been confirmed, although pharmacological studies suggest that I3 receptors might participate in pancreatic insulin secretion (Eglen et al., 1998; Morgan and Chan, 2001).
Imidazoline I2 receptors might be the most interesting of the imidazoline receptor subtypes because they are emerging as a possible drug target for some neurological and psychiatric disorders, such as pain, stroke and drug abuse (Li and Zhang, 2011; Garau et al., 2013). I2 receptors have not been cloned, although ligands selective for I2 receptors have been used to study this receptor system. I2 receptors were first thought to be nonadrenergic receptors that bind 3H-idazoxan with high affinity and 3H-para-aminoclonidine and 3H-clonidine at much lower affinity (Regunathan and Reis, 1996). When it became clear that these ligands were binding to both I2 receptors and α2 adrenoceptors, medicinal chemistry efforts focused on improving the ligand selectivity for I2 receptors over α2 adrenoceptors. Several highly selective I2 receptor ligands were developed and pharmacological studies suggest possible therapeutic benefits of these compounds (Hudson et al., 2003; Nikolic and Agbaba, 2012). For example, I2 receptor agonists consistently produce robust antinociceptive effects in various rodent models of acute and chronic pain (Ferrari et al., 2011; Li et al., 2011; Meregalli et al., 2012; Sampson et al., 2012). In both rats and mice, these agonists also produce effects similar to drugs used in humans for their antidepressant effects (Finn et al., 2003; Hudson et al., 2003; Meregalli et al., 2012; Tonello et al., 2012). Thus, I2 receptor agonists might be useful for treating a broad range of disorders, including some of the most treatment-resistant neurological and psychiatric disorders, such as neuropathic pain and depression. Additional studies aimed at improving our understanding of the I2 receptor system is warranted.
One significant challenge when studying the I2 receptor system is that no selective I2 receptor antagonists are available. While some drugs, such as idazoxan (Sanchez-Blazquez et al., 2000; Thorn et al., 2012; Tonello et al., 2012) and BU224 (Sanchez-Blazquez et al., 2000; Bhalla et al., 2013) have been shown to attenuate the effects of I2 receptor agonists such as 2-BFI and CR4056, those compounds have also been shown to have agonist effects in some assays, suggesting that they have low efficacy at I2 receptors. For example, both 2-BFI and BU224 increase rotational behaviors in rats with nigrostriatal lesions (MacInnes and Duty, 2004). In drug discrimination studies, both idazoxan and BU224 produce responding predominantly on the 2-BFI-associated lever in rats (Jordan et al., 1996; MacInnes and Handley, 2002). Thus, idazoxan and BU224 appear to be agonists in some assays and antagonists in other assays, suggesting that these drugs have lower efficacy at I2 receptors as compared to other ligands. In this context, a neutral I2 receptor antagonist will be a useful tool to facilitate the understanding of I2 receptor pharmacology.
BU99006 is an analog of the prototypic I2 receptor ligand 2-BFI. In competition binding studies, BU99006 inhibits the binding of 3H-2-BFI (Tyacke et al., 2002) in both rats and mice (Tyacke et al., 2002; Garcia-Sevilla and Ferrer-Alcon, 2003). After peripheral administration, BU99006 readily enters the brain and binds in a pattern similar to that of 2-BFI (Paterson et al., 2007). Although BU99006 binds selectively to I2 receptors, its efficacy at these receptors has not been determined; however, to the extent that BU99006 has little or no efficacy, it might be a useful tool for selectively blocking other I2 receptor ligands (Tyacke et al., 2002). This study examined the effects of BU99006, alone and in combination with I2 receptor agonist 2-BFI, in two behavioral assays that are sensitive to I2 receptor agonists, hypothermia and 2-BFI discrimination (Thorn et al., 2012; MacInnes and Handley, 2002). If BU99006 is an I2 receptor antagonist, it is expected that it does not produce hypothermia or increase 2-BFI-associated lever responding as I2 receptor agonists do (Thorn et al., 2012; MacInnes and Handley, 2002). In contrast, it is expected that BU99006 should attenuate the effects of 2-BFI in these assays.
Methods
Subjects
A total of 49 adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) participated in the studies (body temperature studies: 41 rats [n=5–6 per group]; drug discrimination: 8 rats). Rats used in body temperature studies were housed individually on a 12/12-h light/dark cycle with free access to water and food except during experimental sessions, which were conducted during the light period. Body weights of the rats that participated in the drug discrimination study was maintained at 85% of their free-feeding body weight by adjusting the amount of standard rodent chow that was provided in the home cage after daily sessions. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the Guide for the Care and Use of Laboratory Animals (11th edition, Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Body temperature
Body temperature was measured in a quiet procedure room maintained under environmental conditions (temperature, humidity and lighting) that were identical to those in the animal colony room. Rats were handled for at least 3 days prior to testing drugs in order to habituate rats to the procedure, and they were habituated to the procedure room for at least 30 min prior to each test. Body temperature was measured by gently inserting a rectal probe (5.0 cm) and recording temperature from the digital thermometer (BAT7001H, Physitemp Instruments Inc., Clifton, NJ, USA) (Li et al., 2009; Thorn et al., 2012). Each rat was used to study only one drug dose or combination test. A test session in which saline was administered was always conducted prior to a test session in which drug was administered. For each test session, body temperature was measured immediately before drug administration and every 15 min after drug was given until the effect of the drug was no longer evident or until 3.5 h had elapsed since drug administration. BU99006 was studied alone and in combination with 2-BFI or idazoxan. For drug combination studies, BU99006 was administered 10 min (3.2 mg/kg) or 2 h (10 mg/kg) before the first measurement of body temperature which was followed immediately by the administration of either 2-BFI or idazoxan and body temperature was measured every 15 min as described above. The pretreatment time of 2 hours was chosen because it was necessary to wait until the hypothermic effect of BU99006 dissipated before 2-BFI administration.
Drug discrimination
Drug discrimination studies were conducted in commercially available two-lever operant chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA). Data were collected through an interface using Graphic State 3.03 software (Coulbourn Instruments Inc.) (Li et al., 2013). The experimental protocol has been described in detail elsewhere (Li et al., 2013). Briefly, daily sessions consisted of a 10-min timeout period followed by a 10-min response period. During the timeout period, the chamber was dark and lever presses had no programmed consequence; during the response period, the stimulus lights above both levers were illuminated and 10 consecutive responses [fixed ratio (FR) 10] on the correct lever resulted in the delivery of a food pellet (45 mg; BioServ Inc., Frenchtown, New Jersey, USA). A response on the incorrect lever reset the FR requirement on the correct lever. The response period ended after 10 min or the delivery of 20 food pellets, whichever occurred first.
For training sessions, saline or 5.6 mg/kg 2-BFI was injected (i.p.) at the start of the timeout period, 10 min before the response period. When rats received saline, only responding on the saline lever resulted in food delivery. When rats received 2-BFI, only responding on the drug lever resulted in food delivery. Sessions were conducted 7 days/week with the order of training sessions generally following a double alternation schedule (e.g. saline, saline, drug, drug).
Test sessions began when rats satisfied the following criteria for five consecutive training sessions or for six out of seven training sessions: at least 90% of the total responses occurring on the correct lever, based on the injection given before the session, and fewer than 10 responses (1 FR) occurring on the incorrect lever before delivery of the first food pellet. After the first test session, these criteria were satisfied for the 2 consecutive training sessions immediately before each test. Test sessions were identical to training sessions, except that 10 consecutive responses on either lever resulted in the delivery of food; on separate occasions, varying doses of 2-BFI or BU99006 alone were administered immediately before the session. When the BU99006 and 2-BFI drug combinations were studied, BU99006 was administered 10 min or 2 h prior to 2-BFI. The order of tests with 2-BFI and BU99006 varied nonsystematically among animals.
Drugs
The compounds studied were 2-BFI hydrochloride (2-(2-benzofuranyl)-2-imidazoline hydrochloride) and BU99006 (5-isothiocyanato-2-benzofuranyl-2-imidazoline), which were synthesized at Research Triangle Institute and verified by HPLC [> 95% pure], NMR and elemental analysis, and idazoxan hydrochloride (Sigma-Aldrich, St. Louis, MO). Drugs were dissolved in 0.9% physiological saline and administered i.p. BU99006 powder was stored at ∓ 20 °C and the solution was prepared before tests. Doses are expressed as milligrams of the form indicated above per kilogram of body weight. Injection volumes were 1 ml/kg.
Data analyses
Changes in body temperature (°C, mean ± S.E.M) were calculated by subtracting the first measurement of body temperature during each test session from all the subsequent measurements, which were then plotted as a function of time. Drug interactions were analyzed using two-way repeated measures analysis of variance (ANOVA) (time × treatment) followed by Newman-Keuls’s post hoc test. P < 0.05 is considered statistically significant.
Two dependent variables were collected during drug discrimination sessions: (i) the percentage of responses on the 2-BFI-associated lever, calculated by dividing the number of responses on the 2-BFI-associated lever by the total number of responses on both levers and multiplying by 100; and (ii) response rate, calculated by dividing the total number of responses made on both levers by the duration of the response period in seconds. When a rat responded at a rate that was less than 20% of the vehicle control rate (the rate that was collected after saline was administered), percentage of responses on the 2-BFI-associated lever for that dose or dose combination was not included for further analysis, although the response rate data were included. The mean percentage of responses on the 2-BFI-associated lever ± 1 S.E.M. and the mean rate of responding ± 1 S.E.M. during test sessions were plotted as a function of dose. One-way (as compared to vehicle data) or two-way repeated measures ANOVA (2-BFI dose × BU99006 treatment) followed by Newman-Keuls’s post hoc test was used to analyze the effects of BU99006 on 2-BFI discrimination. Paired Student’s t test was used to compare the difference when only two data points were available. When appropriate, the ED50 values (95 % confidence intervals [CI]) were estimated using linear regression. P < 0.05 is considered statistically significant.
Results
Body temperature
BU99006 dose dependently decreased body temperature in rats (left, Fig. 1). A dose of 3.2 mg/kg of BU99006 was ineffective whereas a larger dose markedly reduced body temperature. The maximum decrease produced by 10 mg/kg was −2.2 ± 0.12 °C (mean ± SEM) which was obtained 30 min after administration and the effect lasted for 2.5 hrs. A dose of 15 mg/kg BU99006 further decreased body temperature to −2.8 ± 0.15 °C 30 min after administration with the duration of action of 3 hrs. A dose of 10 mg/kg 2-BFI also markedly reduced body temperature (−2.0 ± 0.07 °C, middle, Fig. 1). That dose of 2-BFI was also studied in combination with BU99006. A small dose of BU99006 that did not alter body temperature when given alone also did not alter the hypothermic effects produced by 10 mg/kg 2-BFI. Two-way ANOVA revealed a significant main effect of interaction (F [11, 110] = 2.54, P < 0.01), but there was no significant main effect of BU99006 treatment (F [1, 110] = 0.90). Post hoc analysis revealed no significant differences across the time points studied. A larger dose of BU99006 was given 2 hrs before 2-BFI and this dose also did not significantly modify the hypothermic effects of 2-BFI (middle, Fig. 1). Two-way ANOVA revealed a significant main effect of interaction (F [11, 110] = 2.34, P < 0.05), but there was no significant main effect of BU99006 treatment (F [1, 110] = 1.50). Post hoc analysis revealed no significant differences across the time points studied. A dose of 3 mg/kg of the adrenergic α2 receptor/imidazoline I2 receptor antagonist idazoxan significantly attenuated the hypothermic effects of 10 mg/kg BU99006 (right, Fig. 1). Two-way ANOVA revealed a significant main effect of interaction (F [9, 81] = 2.80, P < 0.01) and a significant main effect of idazoxan treatment (F [1, 81] = 11.0, P < 0.01). Post hoc analysis revealed that the hypothermic effects of BU99006 were significantly smaller in rats that received a combination of 3 mg/kg idazoxan and 10 mg/kg BU99006 as compared to rats that received BU99006 alone at 30 – 90 min time period.
Fig. 1.
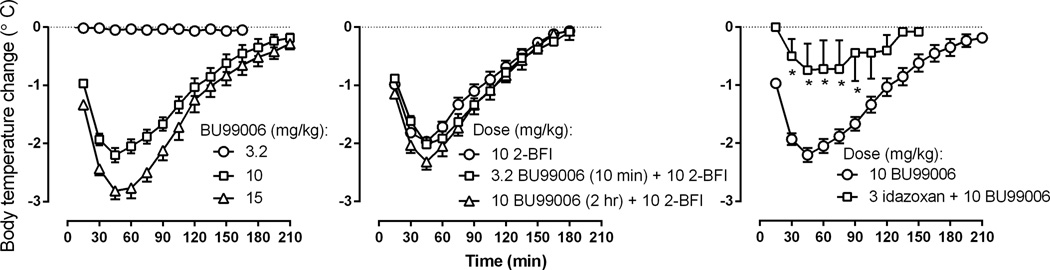
Hypothermic effects of BU99006 alone (left) or in combination with 2-BFI (middle) or idazoxan (right). Ordinates, body temperature changes ± SEM (°C). Abscissa, time after drug administration (min). * P < 0.05 as compared to 10 mg/kg BU99006 alone. Unit of drug doses: mg/kg.
Drug discrimination
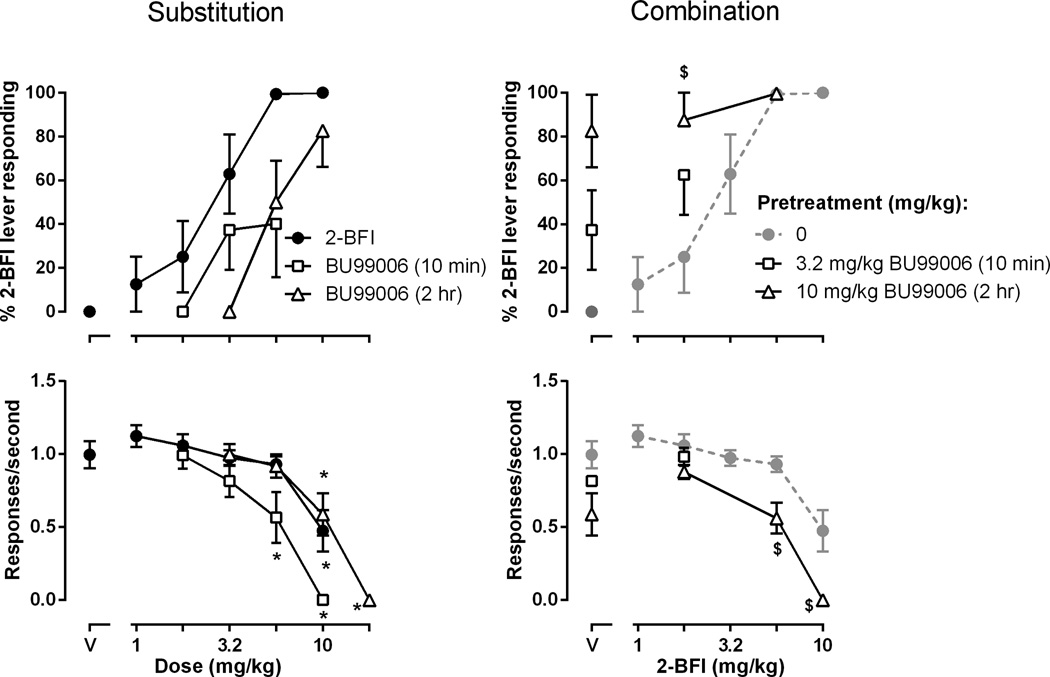
In rats discriminating 5.6 mg/kg 2-BFI from vehicle, 2-BFI dose dependently increased responding on 2-BFI-associated lever (top left, Fig. 2), with the training dose generating an average of 99.4 ± 0.6 % 2-BFI-associated lever responding (ED50 [95% CI] = 2.26 [1.63, 3.12] mg/kg). One-way repeated measures ANOVA analysis on response rate revealed that 2-BFI dose dependently decreased response rate (F (5, 47) = 12.66, P < 0.001) with rates significantly suppressed at a dose of 10 mg/kg. When given immediately before test sessions, BU99006 dose dependently increased responding on the 2-BFI-associated lever up to an average of 40.0 ± 24.5 % at a dose of 5.6 mg/kg. One-way repeated measures ANOVA analysis on response rate revealed that BU99006 dose dependently decreased response rate (F (4, 39) = 21.37, P < 0.001) with rates significantly suppressed at doses of 5.6 and 10 mg/kg. When given 2 hrs before test sessions, BU99006 dose dependently increased responding on the 2-BFI-associated lever up to a maximum of 82.6 ± 16.5 % at a dose of 10 mg/kg. One-way repeated measures ANOVA analysis on response rate revealed that BU99006 dose dependently decreased response rate (F (4, 39) = 27.52, P < 0.001) with rates significantly suppressed at doses of 10 and 17.8 mg/kg (bottom left, Fig. 2). When given 10 min before 2-BFI, 3.2 mg/kg BU99006 enhanced the effects of 1.78 mg/kg 2-BFI, increasing responding on the drug lever from 25.0 ± 16.4 % to 62.5 ± 18.3 %, although this increase did not reach statistical significance. Similarly, when given 2 hrs before sessions, 10 mg/kg BU99006 significantly increased the effects of 1.78 mg/kg 2-BFI (t [7] = 3.4, P < 0.05) (top right, Fig. 2). A dose of 10 mg/kg BU99006 significantly enhanced the rate-decreasing effects of 2-BFI. Two-way ANOVA revealed a significant main effect of BU99006 treatment (F [1, 28] = 16.06, P < 0.01) and post hoc analysis found that BU99006 significantly enhanced the rate-decreasing effects of 5.6 and 10 mg/kg 2-BFI (bottom right, Fig. 2).
Fig. 2.
Discriminative stimulus (top panel) and response rate-altering (bottom panel) effects of 2-BFI and BU99006, alone (left panels) or in combination (right panels), in rats discriminating 5.6 mg/kg 2-BFI from saline (n = 8). Ordinates: top panels, average percentage of responses on the 2-BFI-associated lever ± SEM; bottom panels, average response rate ± SEM in responses per second. Abscissa: dose in mg/kg body weight. ‘V’ indicates vehicle. * P < 0.05 as compared to Vehicle; $ P < 0.05 as compared to 2-BFI alone. See Fig. 1 for other details.
Discussion
The primary finding of this study was that BU99006 appears to be an I2 receptor agonist with no evidence that it has antagonist activity. BU99006 produced a dose-dependent hypothermia which was antagonized by the adrenergic α2 receptor/imidazoline I2 receptor antagonist idazoxan, suggesting that the effect was primarily mediated by I2 receptors. In contrast, under conditions where BU99006 did not alter body temperature, it did not antagonize the hypothermic effects of 2-BFI, which argues against the notion that BU99006 might have antagonist activity. BU99006 significantly increased responding on the 2-BFI-associated lever and enhanced the discriminative stimulus effects of 2-BFI, which further supports the notion that BU99006 has I2 receptor positive efficacy. Taken together, these results provide behavioral evidence that BU99006 has agonist activity at imidazoline I2 receptors and shares behavioral effects with the prototypical I2 receptor agonist, 2-BFI.
BU99006 has high affinity and selectivity for I2 receptors. Once BU99006 occupies these receptors, it produces a conformational change which prevents binding of other I2 receptor ligands such as 2-BFI (Tyacke et al., 2002; Kimura et al., 2009), which has been demonstrated following in vitro pre-incubation treatment or intravenous injection (Tyacke et al., 2002). While the binding property of BU99006 for I2 receptors has been examined, the functional effects of BU99006 is not known. In contrast, it is clear that the prototypic I2 receptor agonist 2-BFI can produce a variety of effects. For example, 2-BFI increases dopamine levels and facilitates dopamine release in the rat striatum (Sastre-Coll et al., 2001). In rats with lesions in the nigrostriatal tract, 2-BFI increases the rotational behaviors (MacInnes and Duty, 2004). Rats also discriminate 2-BFI and this assay has high pharmacological selectivity such that only drugs with high imidazoline I2 receptor binding affinity and positive efficacy produce responding on the 2-BFI-associated lever (Jordan et al., 1996; MacInnes and Handley, 2002, 2003). 2-BFI produces antinociceptive effects in a rat writhing test which are antagonized by idazoxan (Li et al., 2011). In addition, seven I2 receptor agonists, including 2-BFI, have been shown to produce marked hypothermia in rats, an effect that is attenuated by the adrenergic α2 receptor antagonist/I2 receptor ligand idazoxan and not by the adrenergic α2 receptor antagonist yohimbine, suggesting that the hypothermic effects of 2-BFI are mediated by imidazoline I2 receptors (Thorn et al., 2012). These data suggest that I2 receptors mediate various behavioral effects. As such, the primary goal of this study was to examine the hypothesis that BU99006, as claimed in previous studies (Tyacke et al., 2002; Kimura et al., 2009), could serve as an I2 receptor antagonist.
BU99006 produced a dose-dependent hypothermic effect, which is similar to effects obtained with other I2 receptor agonists (Thorn et al., 2012). In the current study, the hypothermic effect produced by 10 mg/kg BU99006 was strikingly similar to that produced by 10 mg/kg 2-BFI and this hypothermia was significantly attenuated by 3 mg/kg idazoxan. Because BU99006 has very low affinity at adrenergic α2 receptors (Tyacke et al., 2002), the fact that this hypothermic effect was blocked by the adrenergic α2 receptor antagonist/I2 receptor ligand idazoxan suggests a role of I2 receptors. In order to examine the possibility that BU99006 might antagonize the effects of 2-BFI, a small dose of BU99006 (3.2 mg/kg) was studied in combination with 10 mg/kg 2-BFI. This dose alone did not significantly change body temperature in rats, and did not change the hypothermic effects of 2-BFI. The lack of effect of BU99006 on hypothermia produced by 2-BFI might be because this dose is too small to substantially decrease the available I2 receptors. Consequently, a larger dose of 10 mg/kg BU99006 was also studied in combination with 2-BFI; this dose interacts with I2 receptors, as evidenced by hypothermic effects that are attenuated by idazoxan. BU99006 was given 2 hrs before 2-BFI treatment to wait until the hypothermic effect of BU99006 dissipated. Because it binds irreversibly to I2 receptors, BU99006 should remain on the receptors even after the hypothermic effect wanes; however, it did not significantly change the effects of 2-BFI. Thus, BU99006 does not antagonize the effects of 2-BFI; rather it appears to be an I2 receptor agonist with effects similar to those of 2-BFI.
A second attempt to examine the potential antagonist activity of BU99006 was conducted in rats discriminating 2-BFI. When given alone immediately prior to the sessions, BU99006 produced 40% responding on the 2-BFI-associated lever up to the dose that markedly decreased response rates. When given 2 hr before sessions, BU99006 produced greater than 80% responding on the 2-BFI-associated lever. Not surprisingly, the potency of BU99006 for suppressing response rates was decreased 2-fold when BU99006 was administered 2 hrs before the test sessions. Doses of 3.2 mg/kg (administered 10 min prior to test sessions) and 10 mg/kg BU99006 (administered 2 hrs prior to test sessions) only enhanced the discriminative stimulus effects of 2-BFI. Overall, the interaction between 2-BFI and BU99006 in 2-BFI discrimination rats seemed to be additive and no evidence of antagonism was observed. It should be noted that in both experiments, a maximum of 2 hrs was used as the pretreatment time, and this period might not be enough to reveal slowly-emerging antagonist effects. There are examples, notably irreversible mu opioid receptor antagonist beta-funaltrexamine and pseudo-irreversible mu receptor antagonist buprenorphine, that the antagonist effects are not apparent until 24–48 hrs after the drugs are administered (Ward et al., 1982; Walker et al., 1995). A longer pretreatment time would be necessary to reveal a similar slowly-emerging antagonist effect.
In summary, in two behavioral assays that are sensitive to imidazoline I2 receptor agonists, the effects of BU99006 are similar to those of 2-BFI and there is no evidence of antagonism. In male Wistar rats, the half-life of BU99006 binding (5 mg/kg administered intravenously) is 4.3 hrs (Paterson et al., 2007), which is consistent with the duration of the hypothermic effects observed in the current study and does not seem to support the claim of irreversible binding. Because the hypothermic effect of BU99006 was antagonized by idazoxan, these data suggest that BU99006 is an imidazoline I2 receptor agonist with similar efficacy as 2-BFI and higher efficacy than idazoxan.
Acknowledgements
This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Awards no. R01DA034806, R21DA033426 and R21DA032837, and by National Natural Science Foundation of China (81373390). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Lisa Gerak at University of Texas Health Science Center at San Antonio for her expert editorial assistance to this paper.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- Bhalla S, Ali I, Lee H, Andurkar SV, Gulati A. Potentiation of oxycodone antinociception in mice by agmatine and BMS182874 via an imidazoline I2 receptor-mediated mechanism. Pharmacol Biochem Behav. 2013;103:550–560. doi: 10.1016/j.pbb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984;230:232–236. [PubMed] [Google Scholar]
- Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, et al. 'Seeing through a glass darkly': casting light on imidazoline 'I' sites. Trends Pharmacol Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, et al. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP, Martí O, Harbuz MS, Vallès A, Belda X, Márquez C, et al. Behavioral, neuroendocrine and neurochemical effects of the imidazoline I2 receptor selective ligand BU224 in naive rats and rats exposed to the stress of the forced swim test. Psychopharmacology (Berl) 2003;167:195–202. doi: 10.1007/s00213-003-1392-3. [DOI] [PubMed] [Google Scholar]
- Garau C, Miralles A, García-Sevilla JA. Chronic treatment with selective I2-imidazoline receptor ligands decreases the content of pro-apoptotic markers in rat brain. J Psychopharmacol. 2013;27:123–134. doi: 10.1177/0269881112450785. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Ferrer-Alcon M. In vivo effects of the I(2)-alkylating agent BU99006 on the immunodensity of imidazoline receptor proteins in the mouse brain. Ann N Y Acad Sci. 2003;1009:323–331. doi: 10.1196/annals.1304.041. [DOI] [PubMed] [Google Scholar]
- Head GA, Mayorov DN. Imidazoline receptors, novel agents and therapeutic potential. Cardiovasc Hematol Agents Med Chem. 2006;4:17–32. doi: 10.2174/187152506775268758. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Tyacke RJ, Lalies MD, Davies N, Finn DP, Marti O, et al. Novel ligands for the investigation of imidazoline receptors and their binding proteins. Ann N Y Acad Sci. 2003;1009:302–308. doi: 10.1196/annals.1304.039. [DOI] [PubMed] [Google Scholar]
- Jordan S, Jackson HC, Nutt DJ, Handley SL. Discriminative stimulus produced by the imidazoline I2 site ligand, 2 -BFI. J Psychopharmacol. 1996;10:273–278. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- Kimura A, Tyacke RJ, Robinson JJ, Husbands SM, Minchin MC, Nutt DJ, et al. Identification of an imidazoline binding protein: creatine kinase and an imidazoline-2 binding site. Brain Res. 2009;1279:21–28. doi: 10.1016/j.brainres.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP. Food restriction and streptozotocin differentially modify sensitivity to the hypothermic effects of direct- and indirect-acting serotonin receptor agonists in rats. Eur J Pharmacol. 2009;613:60–63. doi: 10.1016/j.ejphar.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I2 receptor ligands. Eur J Pharmacol. 2011;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Jin C. The GPR88 receptor agonist 2-PCCA does not alter the behavioral effects of methamphetamine in rats. Eur J Pharmacol. 2013;698:272–277. doi: 10.1016/j.ejphar.2012.10.037. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br J Pharmacol. 2002;135:1227–1234. doi: 10.1038/sj.bjp.0704579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Potential serotonergic and noradrenergic involvement in the discriminative stimulus effects of the selective imidazoline I2-site ligand 2-BFI. Pharmacol Biochem Behav. 2003;75:427–433. doi: 10.1016/s0091-3057(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Macinnes N, Duty S. Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway. Br J Pharmacol. 2004;143:952–959. doi: 10.1038/sj.bjp.0706019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, et al. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NG, Chan SL. Imidazoline binding sites in the endocrine pancreas: can they fulfil their potential as targets for the development of new insulin secretagogues? Curr Pharm Des. 2001;7:1413–1431. doi: 10.2174/1381612013397366. [DOI] [PubMed] [Google Scholar]
- Nikolic K, Agbaba D. Pharmacophore development and SAR studies of imidazoline receptor ligands. Mini Rev Med Chem. 2012;12:1542–1555. doi: 10.2174/138955712803832636. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Robinson ES, Nutt DJ, Hudson AL. In vitro and in vivo effect of BU99006 (5-isothiocyanato-2-benzofuranyl-2-imidazoline) on I2 binding in relation to MAO: evidence for two distinct I2 binding sites. Neuropharmacology. 2007;52:395–404. doi: 10.1016/j.neuropharm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Annu Rev Pharmacol Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- Sampson C, Zhang Y, Del Bello F, Li JX. Effects of imidazoline I2 receptor ligands on acute nociception in rats. Neuroreport. 2012;23:73–77. doi: 10.1097/WNR.0b013e32834e7db3. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Boronat MA, Olmos G, García-Sevilla JA, Garzón J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Coll A, Esteban S, Miralles A, Zanetti R, García-Sevilla JA. The imidazoline receptor ligand 2-(2-benzofuranyl)-2-imidazoline is a dopamine-releasing agent in the rat striatum in vivo. Neurosci Lett. 2001;301:29–32. doi: 10.1016/s0304-3940(01)01599-3. [DOI] [PubMed] [Google Scholar]
- Sun Z, Chang CH, Ernsberger P. Identification of IRAS/Nischarin as an I1-imidazoline receptor in PC12 rat pheochromocytoma cells. J Neurochem. 2007;101:99–108. doi: 10.1111/j.1471-4159.2006.04413.x. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I2 receptor agonists in rats. Br J Pharmacol. 2012;166:1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonello R, Villarinho JG, da Silva Sant'Anna G, Tamiozzo L, Machado P, Trevisan G, et al. The potential antidepressant-like effect of imidazoline I2 ligand 2-BFI in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:15–21. doi: 10.1016/j.pnpbp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Tyacke RJ, Robinson ES, Nutt DJ, Hudson AL. 5-Isothiocyanato-2-benzofuranyl-2-imidazoline (BU99006) an irreversible imidazoline(2) binding site ligand: in vitro and in vivo characterisation in rat brain. Neuropharmacology. 2002;43:75–83. doi: 10.1016/s0028-3908(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of the mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Ward SJ, Portoghese PS, Takemori AE. Pharmacological characterization in vivo of the novel opiate, β-funaltrexamine. J Pharmacol Exp Ther. 1982;220:494–498. [PubMed] [Google Scholar]