Abstract
Cannabinoid type-1 receptors (CB1Rs) modulate synaptic neurotransmission by participating in retrograde signaling in the adult brain. Increasing evidence suggests that cannabinoids through CB1Rs play an important role in the regulation of motor activities in the striatum. In the present study, we used human brain samples to examine the relationship between CB1R and dopamine receptor density in case of Parkinson’s disease (PD).
Post mortem putamen, nucleus caudatus and medial frontal gyrus samples obtained from PD patients were used for CB1R and dopamine D2/D3 receptor autoradiography. [125I]SD7015, a novel selective CB1R inverse agonist, developed by a number of the present co-authors, and [3H]raclopride, a dopamine D2/D3 antagonist, were used as radioligands. Our results demonstrate unchanged CB1R density in the putamen and nucleus caudatus of deceased PD patients, treated with levodopa (l-DOPA). At the same time dopamine D2/D3 receptors displayed significantly decreased density levels in case of PD putamen (control: 47.97 ± 10.00 fmol/g, PD: 3.73 ± 0.07 fmol/g (mean ± SEM), p < 0.05) and nucleus caudatus (control: 30.26 ± 2.48 fmol/g, PD: 12.84 ± 5.49 fmol/g, p < 0.0005) samples. In contrast to the putamen and the nucleus caudatus, in the medial frontal gyrus neither receptor densities were affected.
Our data suggest the presence of an unaltered CB1R population even in late stages of levodopa treated PD. This further supports the presence of an intact CB1R population which, in line with the conclusion of earlier publications, may be utilized as a pharmacological target in the treatment of PD. Furthermore we found discrepancy between a maintained CB1R population and a decreased dopamine D2/D3 receptor population in PD striatum. The precise explanation of this conundrum requires further studies with simultaneous examination of the central cannabinoid and dopaminergic systems in PD using higher sample size.
Keywords: Parkinson’s disease, Endocannabinoid CB1 receptor, Dopamine D2/D3 receptor, Molecular imaging biomarker, Human brain autoradiography, Striatum
1. Introduction
The endocannabinoid (EC) system is commonly described as a neuromodulatory system that interacts with and regulates the functions of many neurotransmitter systems, including cholinergic (Ach), dopaminergic (DA), serotoninergic, adrenergic, opiate, glutamatergic and GABAergic systems [29,58,62]. The main contribution of ECs to the control of synaptic neurotransmission is to act as retrograde messengers through type 1 cannabinoid receptors (CB1R) [44,105]. Presynaptic CB1Rs are abundant in the adult mammalian brain [29]. CB1Rs are coupled to Gi/o proteins and, under specific conditions to Gs proteins [35,48,69]. CB1Rs regulate the activity of various plasma membrane proteins and signal transduction pathways, including ion channels, context-dependent recruitment of second messengers (Erks, STATs, etc.) and various kinases. In addition, CB1Rs activate G protein-independent pathways, as well [12,23,75]. Among various other functions, endocannabinoids have neuromodulatory functions, as well, [47] and play an important role in long term potentiation (e.g. [66]).
Multiple levels of evidence suggest that ECs have a potential to protect neurons under chronic degenerative conditions via CB1R-dependent and -independent mechanisms [19,35,73,78,95]. An increasing number of studies have demonstrated that CB1R density and binding is altered in the extrapyramidal system of humans in e.g., Huntington disease and PD [16,20,36,50,63,70,89,95].
However, the observed alterations in CB1R’s in various neurodegenerative diseases, such as HD or PD, may be of diverse origins. The GABAergic spiny neurons (MSNs) are the most populous neuronal cell type of the striatum (90–95% in rats and over 85% in humans), along with several small populations of interneurons [53,104]. CB1Rs are primarily expressed my MSNs. The HD brain is characterized by loss of the MSNs of the striatum, which results in robust down-regulation of CB1Rs. A severe loss of CB1R’s in the striatum has, consequently, been described as a landmark of HD [8,82]. On the other hand, the alteration of striatal CB1R population in PD is full of controversies and the most important striatal cells expressing the CB1R are affected in a lesser extent compared to HD and the EC systems shows a strong tendency for reorganization [18,84].
It is well known that a progressive degeneration of the dopaminergic system, especially the dopaminergic neurons of the substantia nigra pars compacta (SNc) [17,24], underlies the pathogenesis and clinical manifestations of PD. The decrease in striatal dopamine (DA) alters the regulation of synaptic dopamine levels, and dopamine receptor density and functional state [6,7,11,14,27,65,76,86,94,96]. Alterations in basal ganglia CB1R density or EC levels have been described in rat models of PD on the basis of which a strong functional connection between the striatal dopamine and endocannabinoid systems has been hypothesized [63,107]. However, experimental models in small animals are inconclusive regarding the direction of changes of CB1R density in Parkinson models: whereas there is evidence for the decrease of CB1Rs in the striatum, as a consequence of 6-hydroxydopamine-induced nigrostriatal terminal lesion in rats [101]; studies using postmortem human PD brain samples, 6-hydroxydopamine (6-OHDA) or reserpine-treated rat models of PD, MPTP-lesioned marmoset and mouse mutant models of PD indicate an up-regulation [10,30,63,70,80,89], no change [45,68,89,107] or down-regulation [50,95] of CB1Rs in Parkinson’s disease.
In order to investigate changes in CB1R: D2/D3 balance in PD in the human basal ganglia, we explore correlative alterations in dopamine D2/D3 receptors, key players in the disease process [52,61,93], and the alteration in CB1R using selective radioligands – [3H]raclopride [39,59], [125I]SD7015 [22] – in brain tissues obtained from PD and age-matched control subjects.
2. Material and methods
2.1. Radioligand and chemicals
The detailed synthesis procedure of [125I]SD7015 has been recently described elsewhere [22,21]. The specific radioactivity of the ligand was 2175 Ci/mmol. [3H]raclopride was obtained commercially (specific activity 87 Ci/mmol, PerkinElmer Life Sciences Inc.). TRIS-HCl, bovine serum albumin (BSA), pargyline hydrochloride (selective MAO-B inhibitor), GBX Developer and Fixer Twin Pack were from Sigma–Aldrich (Budapest, Hungary), rimonabant (CB1R antagonist) was from Cayman Chemicals (Michigan, USA). Other chemicals were from commercial suppliers and at the analytical grade.
2.2. Brain tissue and processing
Putamen tissues (n = 2) were collected and stored in the human brain bank of the Department of Anatomy, Semmelweis University, Budapest (age: 72 and 74, post mortem time: 2 and 4 h). Nucleus caudatus (n = 3) and medial frontal gyrus (n = 3) specimens were obtained from The Netherlands Brain Bank (NBB), Netherlands Institute for Neuroscience, Amsterdam (age of death: age: 68, 74 and 77, postmortem delay: 3, 7 and 8 h). The causes of death were dehydration, pneumonia leading to septic shock, uremic coma, terminal circulatory and respiratory failure. All materials have been collected from donors for or from whom written informed consent for brain autopsy had been obtained. The study was approved by the Local Ethics Committee of the University of Debrecen (protocol number: DEOEC RKEB/IKEB M2547a-2006).
Age matched control samples of the same regions were obtained from deceased subjects (n = 5) with no documented history of neurological or psychiatric disorders. Neuropathological examination excluded any pathological finding in case of controls whereas all PD cases show advanced Lewy body disease pathology with severe neocortical involvement, consistent with Braak alpha-synuclein stage 5 or 6. The PD patients underwent long-time levodopa treatment (14 ± 6 years), started between 3 and 5 years after first diagnosis. Out of five at two patients we do not have exact data about disease duration and/or about treatment duration. Despite this fact, since these specimens were showing results concordant with those obtained in the specimens from patients with well know medical records, we included them in the final analysis.
Frozen brain samples were sectioned at 20 µm thickness using a cryomicrotome (Leica, CM 1850) at −20 °C. Sections were thaw mounted onto glass slides, air dried and stored at −20 °C for later use.
2.3. CB1R autoradiography with [125I]SD7015
Consecutive tissue sections in duplicates were used from the nucleus caudatus, putamen and medial frontal gyrus. Tissue were incubated with [125I]SD7015 (40 pM) using 0.174 mCi/ml RA concentration for 60 min in a TRIS buffer (50 mM, pH 7.4) containing sodium chloride (120 mM), potassium chloride (5 mM), calcium chloride (2 mM), magnesium chloride (1 mM), ascorbic acide (0.1% w/v), 10 µM pargyline and bovine serum albumin (BSA 0.1%). Non-specific binding was determined in the presence of 10 µM rimonabant (CB1R antagonist) [22]. The sections were then washed in the same buffer three times for thirty minutes each time and briefly dipped in ice cold distilled water. The sections were dried on a warm plate and afterwards exposed to γ-radiation sensitive film (Kodak Biomax MS, Sigma–Aldrich, Budapest, Hungary) for 24 h. Autoradiograms were digitized using a high-resolution scanner (Epson Perfection V750 Pro). Adobe Photoshop CS2 software was used for measurements and image processing. 14C-calibration scales (American Radiolabelled Chemicals Inc, St Louis, MO, USA) were used for quantification as described by Baskin and Wimpy [5]. Briefly, radioactivity of the [14C] plastic standards, supplied by the manufacturer, was transformed in tissue equivalent concentrations of [125I] expressed as disintegrations per minute per mm2 (DPM/mm2). The transformation was based on the following quadratic polynomial equation:
where x values represent [14C] radioactivity in µCi/g plastic. Mean pixel values were converted to DPM/mm2 applying the obtained [125I] DPM/mm2 values of the scale. Taking into consideration the standard specific radioactivity value of the radioligand (2200 Ci/mM), the 1 DPM = 451 fCi conversion factor and the slide thickness (20 µm), DPM/mm2 values were converted into fmol/g_tissue concentration of the radioligand bound to the receptors, i.e. the radioligand’s specific binding value. Statistical analysis was performed with Student’s t-test (two tailed, unequal variance). A p < 0.05 value was considered statistically significant.
2.4. Dopamine D2/D3 receptor autoradiography with [3H]raclopride
Autoradiography with [3H]raclopride was performed as described earlier [41,42]. [3H]raclopride was obtained commercially (specific activity 87 Ci/mmol, PerkinElmer Life Sciences Inc.). After 30 min of pre-incubation at room temperature in TRIS buffer containing 20 mM Hepes (2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid), pH 7.4, 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, and 10 mM NaOH, the sections were incubated for 15 min at room temperature in Krebs-Ringer-HEPES (KRH) buffer (HEPES 10 mM, pH 7.4, KCl 4.7 mM, CaCl2 2.2 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, NaCl 120 mM, dextrose 10 mM), supplemented with 0.05 mg/mL BSA (bovine serum albumin) and 1 nM [3H]raclopride. Nonspecific binding was determined in the presence of 10 µM cold raclopride [40]. The measurements were made in duplicates. The washing of labeled sections was carried out for 20-min in the ice-cold TRIS buffer (without ligand), followed by a brief dipping in ice-cold distilled water to remove salts. Finally, the sections were dried under a stream of cold air.
Subsequently the readings, using tritium sensitive phosphorimager plates (Fujifilm Plate BAS-TR2025) were made in a Fujifilm BAS-500 phosphorimager (90 min) (Fujifilm, Tokyo). Quantitative densitometry was performed using Multi Gauge 3.2 phosphorimager software (Fujifilm, Tokyo). Autoradiographic [3H] microscales (RPA510, Batch 18, GE Bioscience), placed alongside the brain tissue sections, were used to quantify the data and render the quantitative values in fmol/g_tissue.
Results of multiple measurements in the same region were averaged for each subject. These values were then used to calculate the mean specific binding of the radioligand. Specific binding of radioligands ([125I]SD7015 and [3H]raclopride) was calculated as difference between mean total (specific and non-specific) and mean blocked (non-specific) binding. Student’s t-test (two-tailed, unequal variance) has been applied for statistical comparison between disease and control groups. A p < 0.05 value was considered statistically significant.
3. Results
In the present study we have performed autoradiography in control and PD brain specimens, with special regard to the nucleus caudatus and the putamen, using [125I]SD7015 and [3H]raclopride.
In Fig. 1, CB1R autoradiography is shown in the three regions under investigation. We did not find a significant difference between specific binding values (total binding − non-specific binding after block, expressed as fmol/g_tissue in the three regions of interest in PD and age matched control brains (Table 1).
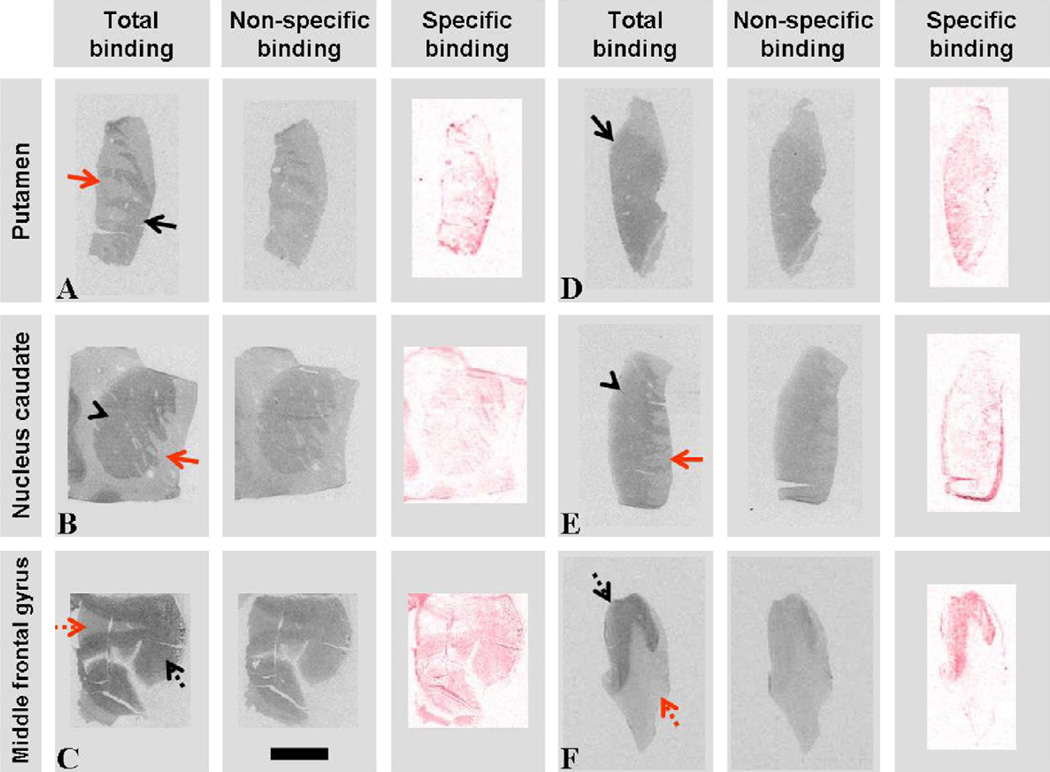
Fig. 1.
Images of CB1R receptor autoradiography obtained in control (A–C) and PD (D–F) putamen (black arrow), nucleus caudatus (black arrow head) and medial frontal gyrus sections. Red arrow, internal capsule; dotted black arrow, cortex; dotted red arrow, white matter. Specific binding represents subtracted, red weighted images of total binding − non-specific binding. Scale bar indicates 10 mm.
Table 1.
CB1R density expressed as specific binding values of [125I]SD7015 to CB1Rs (fmol/g_tissue). There was no significant difference between the average values belonging to control and PD brains in any region. Data are expressed as mean ± SEM; n: number of subjects. Measurements in all brains were made in duplicates.
| Region | Control | PD | ||
|---|---|---|---|---|
| Putamen | 157.54 ± 25.52 | n = 5 | 180.30 ± 36.80 | n = 2 |
| Nucleus caudatus | 170.77 ± 15.89 | n = 3 | 187.58 ± 21.22 | n = 3 |
| Medial frontal gyrus | 263.84 ± 41.51 | n = 3 | 286.70 ± 25.83 | n = 3 |
In Fig. 2 dopamine D2/D3 autoradiographic images are shown in the three anatomical brain regions. We found significant differences between specific binding values measured in the putamen and nucleus caudatus in PD brains, relative to age matched controls. However, there was no significant difference between the specific binding values in the medial frontal gyrus (Table 2).
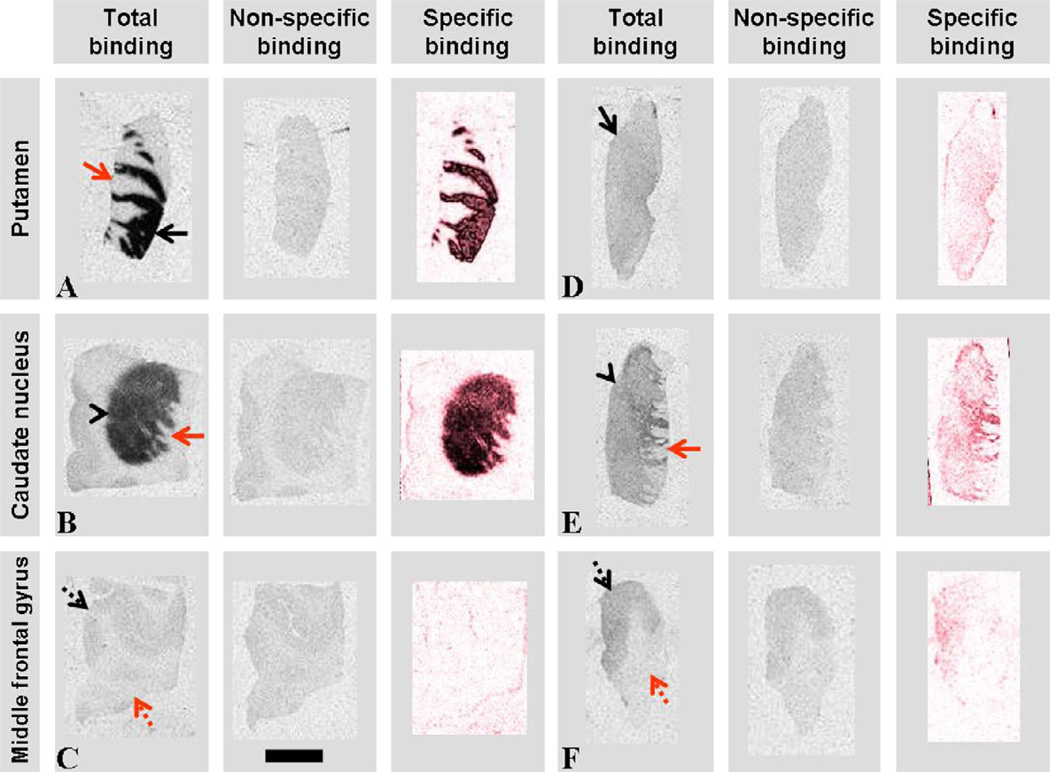
Fig. 2.
Images of D2/D3 receptor autoradiography obtained in control (A–C) and PD (D–F) putamen (black arrow), nucleus caudatus (black arrow head) and medial frontal gyrus sections. Red arrow, internal capsule; dotted black arrow, cortex; dotted red arrow, white matter. Specific binding represents subtracted, red weighted images of total binding −non-specific binding. Scale bar indicates 10 mm.
Table 2.
Dopamine D2/D3 receptor densities expressed in fmol/g_tissue. There was a significant difference between the average values belonging to control and PD brains in the putamen and the nucleus caudatus, whereas no significant difference was present in the medial frontal gyrus. Data were expressed as means ± SEM; n: number of subjects. Measurements in all brains were made in duplicates.
| Region | Control | PD | ||
|---|---|---|---|---|
| Putamen | 47.97 ± 10.00 | n = 2 | 3.73 ± 0.07 | n = 2 |
| Nucleus caudatus | 30.26 ± 2.48 | n = 3 | 12.84 ± 5.49 | n = 3 |
| Medial frontal gyrus | 3.29 ± 2.50 | n = 3 | 4.33 ± 1.45 | n = 3 |
4. Discussion
We investigated the relationship of CB1R and D2/D3 receptor densities in PD human brains by means of receptor autoradiography. [125I]SD7015, a novel CB1R agonist, [22] and [3H]raclopride, a dopamine D2/D3 receptor antagonist [59], were applied as radioligands.
CB1R densities in putamen, nucleus caudatus and frontal cortex samples seem to be unchanged in PD while in contrast, dopamine D2/D3 receptor density in PD putamen and nucleus caudatus decreases. The latter is in line with previous findings, namely this decrease of D2/D3 receptor density in PD putamen and nucleus caudatus could be the consequence of longterm antiparkinsonian treatment. It is generally agreed that dopaminergic denervation leads to striatal D2 dopamine receptor up-regulation as postsynaptic compensatory mechanism in response to deficiencies in synaptic dopamine signaling [1,3,24,67,85]. Treatment of PD patients with dopaminergic drugs returns the striatal dopamine D2 receptor expression to near normal levels [1,67,98]. Frontal cortex samples presented no difference between the subject cohorts.
The CB system in experimental PD models and PD patients has been extensively studied, yet with contradictory conclusions. In rat Parkinson models (reserpine treatment or 6-OHDA-lesion models) an increase in endogenous endocannabinoid levels was observed in the striatum [19,26,38,68] as it was also observed in the CNS of 16 untreated PD patients [83]. Other authors found significantly altered CB1R mRNA expression in animal models of PD or in postmortem human PD brain specimens. [50,89,95,107]. Finally, changes in CB1R binding sites [30,63,80] and activation of GTP-binding proteins in the basal ganglia of PD patients and of MPTP-treated marmosets were also reported [63]. On the other hand, using the 6-OHDA rat model Romero et al. [89] did not find significant changes in CB1 receptor binding, measured by [3H]WIN-55,212,2 autoradiography, or in the activation of signal transduction mechanisms, measured by WIN-55,212,2-stimulated [35S]GTPgammaS binding autoradiography, between the lesioned and non-lesioned sides at the level of the lateral caudate-putamen, globus pallidus and substantia nigra.
In the present study we did not find changes in CB1R densities in the striatum and frontal cortex of PD subjects. One of the explanations for the unaltered CB1R density found by us could be the unchanged density of high affinity CB1Rs in the investigated PD brain regions [63,89]. This may be possible due to the presence of the large reserve of CB1Rs and their likely inter-conversion between low and high affinity states [4,94]. Due to low sample size results are only suggestive in the aspect of an intact CB1R density. This statement requires further justification in the future by studies using more specimens and performing the detailed investigation of this problem.
On the other hand, functional relationship have been reported between CB1Rs and both dopamine D1 and D2 receptors [33,34,46,51,60,63,70,75,89, 99]. For instance, Giuffrida et al. [33] proved that striatal administration of D2 agonist results in release of endocannabinoids. Furthermore, activation of CB1 or dopamine D2 receptors alone resulted in inhibition of cAMP accumulation whereas simultaneous activation of both receptors increased cAMP levels [34]. Kreitzer and Malenka [60] reported in animal models of Parkinson’s disease that DA depletion blocked the generation of endocannabinoid-mediated long-term depression (eCB-LTD) in indirect striatal pathway but administration of dopamine D2 receptor agonist together with inhibitors of endocannabinoid degradation rescued indirect-pathway eCB-LTDs and in vivo reduced parkinsonian motor deficits. Dopamine receptor antagonists inhibited cannabinoid induced striatal mitogen activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) activation [99].
Administration of CB1R agonists increased dopamine turnover and release [15,31,32,87], excited dopaminergic neurons in the ventral tegmental area and substantia nigra [28], decreased the tremor associated with overactivity of the subthalamic nucleus and improved motor impairment [2,72,92], at the same time a CB1 antagonist (rimonabant) alleviated hypokinesia in animal models of Parkinson’s disease [37,54], probably effecting on the lateral globus pallidus. It has been demonstrated that glutamatergic and GABAergic terminals strongly express CB1Rs and CB1R agonists significantly inhibited glutamate and GABA release, however, cannabinoids in vitro, directly did not affect the release of dopamine [29,58,97]. Yin et al. [106] observed that presynaptic reduction in glutamate release was the consequence of a retrograde signal through eCBs; this eCB synthesis and release from the postsynaptic cell results from cooperating, convergent glutamate and dopamine inputs. A potential indirect dopamine–CB1R interaction through the cannabinoid induced regulation of the upper mentioned neurotransmitters (GABA, glutamate) of striatal neuronal pathways could be the basis of cannabinoid effect on motor activity. However, the findings of [79] that dopaminergic cells also express CB1R as well as observations about functional interactions between CB1Rs and both dopamine D1 and D2 receptors [33,34,46,51,60,63,70,74,89,99]. could contribute to the upper mentioned effect as well. Due to the complexity of this cannabinoid–dopamine receptor conundrum further researches are required with well designed study protocols. Although our results base on relatively small sample size, they refer to the presence of an apparently intact CB1 receptor population may be usable in PD therapy, even in advanced PD.
On the other hand, it is accepted that classical neuroinflammatory diseases such as multiple sclerosis present aspects of neurodegeneration, while classical degenerative disorders such as Alzheimer’s disease, Parkinson’s disease are demonstrably affected by inflammation [90]. In CNS CB1 receptors exist in all types of neural cells, in astrocytes [9,91], microglia [100,103], and oligodendrocytes [77] whereas CB2 receptors are expressed on cells of immune system and microglia [103]. Studies report neuroprotective [43,71,88] and anti-inflammatory effects through CB receptors [13,55,56,57,81,102]; CBs protected against dopaminergic cell death, as well [64]. Thus CB1 and CB2 receptors could provide substrate for neuroprotective and anti-inflammatory actions of cannabinoids in neurodegenerative diseases, however, the effects through CB1Rs are more relevant to neuroprotection, whereas CB2Rs modulate the immune response primarily, although a potential overlap as well as non CB1/CB2-mediated mechanisms may exist [90].
In this study we used PD putamen, nucleus caudatus, medial frontal gyrus samples in order to correlate CB1 receptor density with dopamine D2/D3 receptor density. Our results refer to an unchanged CB1R and decreased dopamine D2/D3 receptor density in nucleus caudatus and putamen of PD patients whereas medial frontal gyrus sections did not show any alteration. Our data suggest that in case of long-term l-DOPA treatment and long disease progression CB1R density does not fall under control levels, although, dopamine D2/D3 receptor density is significantly decreased. Various explanations could exist: (1) neurodegeneration induced affinity or sensitivity increase of ‘reserve’ CB1Rs could compensate CB1R density changes, (2) reactive changes of CB system could go along with PD progression, until more effective compensatory mechanisms come into action, (3) despite the decreased density, dopamine D2 receptor signal transduction seems functionally intact [25]) and possible physiological interactions with CB1 receptors could be maintained even in later stages of PD, which could result in unchanged CB1R density; however, functional receptor crosstalks between these receptor types are, yet, unequivocally unproven. Nevertheless the combination up to various degree of the aforementioned or other, yet, unknown mechanisms is the most probable phenomenon.
We concluded that an intact CB1R population could represent alternative target for treatment of PD; additionally it could open new perspectives in neuroprotection and anti-inflammatory therapy of this neurodegenerative disease. Better understanding and further exploration of central cannabinoid system and related questions need further in vivo and in vitro detailed designed studies, emphasizing study population/sample group homogeneity regarding to data about PD neuropathological stage, disease duration and l-DOPA substitution/dopamine agonist therapy. This is suggested since one of the limitation of this study is the low sample number and consequently the questionable reliability of statistical calculations on these data. By the parallel investigation of CB1 and DA D2/3 receptors in PD striatum we wished to base and pioneer future researches aiming this field of neuroscience.
Acknowledgements
The study was supported by the Hungarian National Science Found (OTKA) (K 68864) and was performed partly within a collaborative master research agreement between Karolinska Institutet, Mediso Medical Imaging Systems and CROmed Translational.
References
- 1.Alexander GM, Schwartzman RJ, Grothusen JR, Brainard L, Gordon SW. Change in brain dopamine receptor in MPTP parkinsonian monkeys following L-DOPA treatment. Brain Res. 1993;625:276–282. doi: 10.1016/0006-8993(93)91069-5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LA, Anderson JJ, Chase TN, Walters JR. The cannabinoid agonists WIN 55,212-2 and CP 55,940 attenuate rotational behavior induced by a dopamine D1 but not a D2 agonist in rats with unilateral lesions of the nigrostriatal pathway. Brain Res. 1995;691:106–114. doi: 10.1016/0006-8993(95)00645-7. [DOI] [PubMed] [Google Scholar]
- 3.Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]raclopride. Mov. Disord. 1997;12:33–38. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- 4.Barbier P, Colelli A, Maggio R, Bravi D, Corsini GU. Pergolide binds tightly to dopamine D2 short receptors and induces receptor sequestration. J. Neural Transm. 1997;104:867–874. doi: 10.1007/BF01285554. [DOI] [PubMed] [Google Scholar]
- 5.Baskin DG, Wimpy H. Calibration of [14C] plastic standards for quantitative autoradiography of [125I] labeled ligands with Amersham Hyperfilm β-max. Neurosci. Lett. 1989;104:171–177. doi: 10.1016/0304-3940(89)90350-9. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard V, Chritin M, Vyas S, Savasta M, Feuerstein C, Agid Y, Javoy-Agid F, Raisman-Vozari R. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain. J. Neurochem. 1995;64:1669–1679. doi: 10.1046/j.1471-4159.1995.64041669.x. [DOI] [PubMed] [Google Scholar]
- 7.Blanchet PJ, Calon F, Martel JC, Bddard PJ, Di Paolo T, Waiters RR, Piercey MF. Continuous administration decreases and pulsatile administration increases behavioral sensitivity to a novel dopamine D2 agonist (U-91356A) in MPTP-exposed monkeys. J. Pharmacol. Exp. Ther. 1995;272:854–859. [PubMed] [Google Scholar]
- 8.Blázquez C, Chiarlone A, Sagredo O, Aguado T, Pazos MR, Resel E, Palazuelos J, Julien B, Salazar M, Börner C, Benito C, Carrasco C, Diez-Zaera M, Paoletti P, Díaz-Hernández M, Ruiz C, Sendtner M, Lucas JJ, de Yébenes JG, Marsicano G, Monory K, Lutz B, Romero J, Alberch J, Ginés S, Kraus J, Fernández-Ruiz J, Galve-Roperh I, Guzmán M. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain. 2011;134:119–136. doi: 10.1093/brain/awq278. [DOI] [PubMed] [Google Scholar]
- 9.Bouaboula M, Bourrie B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J. Biol. Chem. 1995;270:13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- 10.Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr. Opin. Pharmacol. 2003;3:54–61. doi: 10.1016/s1471-4892(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 11.Cai G, Wang HY, Friedman E. Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J. Pharmacol. Exp. Ther. 2002;302:1105–1112. doi: 10.1124/jpet.102.036673. [DOI] [PubMed] [Google Scholar]
- 12.Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn. Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chefer SI, Kimes AS, Matochik JA, Horti AG, Kurian V, Shumway D, Domino EF, London ED, Mukhin AG. Estimation of D2-like receptor occupancy by dopamine in the putamen of hemiparkinsonian Monkeys. Neuropsychopharmacology. 2008;33:270–278. doi: 10.1038/sj.npp.1301404. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psycopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 16.Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol. Dis. 1998;5:534–551. doi: 10.1006/nbdi.1998.0220. [DOI] [PubMed] [Google Scholar]
- 17.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 18.Di Filippo M, Picconi B, Tozzi A, Ghiglieri V, Rossi A, Calabresi P. The endocannabinoid system in Parkinson’s disease. Curr. Pharm. Des. 2008;14:2337–2347. doi: 10.2174/138161208785740072. [DOI] [PubMed] [Google Scholar]
- 19.Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB1 cannabinoid receptor knockout mice: evidence for non-CB1, non-CB2 receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- 21.Donohue SR. PhD Thesis. 2008. Development of Cannabinoid Subtype-1 (CB1) Receptor Ligands for PET, Karolinska Institutet. [Google Scholar]
- 22.Donohue SR, Varnäs K, Jia Z, Gulyás B, Pike VW, Halldin C. Synthesis and in vitro autoradiographic evaluation of a novel high-affinity radioiodinated ligand for imaging brain cannabinoid subtype-1 receptors. Bioorg. Med. Chem. Lett. 2009;19:6209–6212. doi: 10.1016/j.bmcl.2009.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Elsworth JD, Brittan MS, Taylor JR. Upregulation of striatal D2 receptors in the MPTP-treated vervet monkey is reversed by grafts of fetal mesencephalon: an autoradiographic study. Brain Res. 1998;795:55–62. doi: 10.1016/s0006-8993(98)00252-2. [DOI] [PubMed] [Google Scholar]
- 25.Farkas S, Nagy K, Jia Z, Hortobágyi T, Varrone A, Halldin C, Gulyás B, Csiba L. Signal transduction pathway activity compensates dopamine D2/D3 receptor density changes in Parkinson’s disease. Brain Res. doi: 10.1016/j.brainres.2012.03.014. (in press). [DOI] [PubMed] [Google Scholar]
- 26.Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur. J. Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 28.French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 29.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 30.García-Arencibia M, García C, Kurz A, Rodríguez-Navarro JA, Gispert-Sáchez S, Mena MA, Auburger G, de Yébenes JG, Fernández-Ruiz J. Cannabinoid CB1 receptors are early downregulated followed by a further upregulation in the basal ganglia of mice with deletion of specific park genes. J. Neural Transm. Suppl. 2009;73:269–275. doi: 10.1007/978-3-211-92660-4_22. [DOI] [PubMed] [Google Scholar]
- 31.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol. Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 32.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur. J. Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 33.Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 34.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 36.Glass M, Dragunow M, Faull RLM. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA-A receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 37.González S, Scorticati C, García-Arencibia M, de Miguel R, Ramos JA, Fernández-Ruiz J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 2006;1073–1074:209–219. doi: 10.1016/j.brainres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agrò A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J. Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall H, Farde L, Sedvall G. Human dopamine receptor subtypes–in vitro binding analysis using 3H-SCH 23390 and 3H-raclopride. J. Neural Transm. 1988;73:7–21. doi: 10.1007/BF01244618. [DOI] [PubMed] [Google Scholar]
- 40.Hall H, Halldin C, Dijkstra D, Wikström H, Wise LD, Pugsley TA, Sokoloff P, Pauli S, Farde L, Sedvall G. Autoradiographic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD. Psychopharmacology (Berl) 1996;128:240–247. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- 41.Hall H, Halldin C, Farde L, Sedvall G. Whole hemisphere autoradiography of the postmortem human brain. Nucl. Med. Biol. 1998;25:715–719. doi: 10.1016/s0969-8051(98)00053-5. [DOI] [PubMed] [Google Scholar]
- 42.Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse. 2000;38:421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- 45.Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howlett AC. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- 47.Hu W, Zhang M, Czéh B, Zhang W, Flügge G. Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res. Bull. 2011;85:374–379. doi: 10.1016/j.brainresbull.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Hudson BD, Hébert TE, Kelly ME. Ligand- and heterodimer-directed signaling of the CB(1) cannabinoid receptor. Mol. Pharmacol. 2010;77:1–9. doi: 10.1124/mol.109.060251. [DOI] [PubMed] [Google Scholar]
- 50.Hurley MJ, Mash DC, Jenner P. Expression of cannabinoid CB1 receptor mRNA in basal ganglia of normal and parkinsonian human brain. J. Neural Transm. 2003;110:1279–1288. doi: 10.1007/s00702-003-0033-7. [DOI] [PubMed] [Google Scholar]
- 51.Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 52.Kehne JH, Andree TH, Heinrich JN. D2 receptor partial agonists: treatment of CNS disorders of dopamine function. Curr. Top. Med. Chem. 2008;8:1068–1888. doi: 10.2174/156802608785161394. [DOI] [PubMed] [Google Scholar]
- 53.Kelly CM, Dunnett SB, Rosser AE. Medium spiny neurons for transplantation in Huntington’s disease. Biochem. Soc. Trans. 2009;37:323–328. doi: 10.1042/BST0370323. [DOI] [PubMed] [Google Scholar]
- 54.Kelsey JE, Harris O, Cassin J. The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson’s disease. Behav. Brain Res. 2009;203:304–307. doi: 10.1016/j.bbr.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 55.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 56.Klein TW, Friedman H, Specter SC. Marijuana, immunity and infection. J. Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- 57.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 58.Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J. Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Köhler C, Hall H, Ogren SO, Gawell L. Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem. Pharmacol. 1985;34:2251–2259. doi: 10.1016/0006-2952(85)90778-6. [DOI] [PubMed] [Google Scholar]
- 60.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R, Riddle LR, Griffin SA, Chu W, Vangveravong S, Neisewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 dopamine receptor selective compounds on L-DOPA-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:956–969. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert DM, Fowler CJ. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005;48:5059–5087. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 63.Lastres-Becker I, Cebeira M, de Ceballos M, Zeng B-Y, Jenner P, Ramos JA, Fernández-Ruiz JJ. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s disease and MPTP-treated marmosets. Eur. J. Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 64.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol. Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Lee CS, Samii A, Sossi V. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann. Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- 66.Lin QS, Yang Q, Liu DD, Sun Z, Dang H, Liang J, Wang YX, Chen J, Li ST. Hippocampal endocannabinoids play an important role in induction of long-term potentiation and regulation of contextual fear memory formation. Brain Res. Bull. 2011;86:139–145. doi: 10.1016/j.brainresbull.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Linazasoro G, Obeso JA, Gómez JC, Martínez M, Antonini A, Leenders KL. Modification of dopamine D2 receptor activity by pergolide in Parkinson’s disease: an in vivo study by PET. Clin. Neuropharmacol. 1999;22:277–280. [PubMed] [Google Scholar]
- 68.Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D, Bernardi G, Finazzi-Agro A, Calabresi P. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 2003;85:1018–1025. doi: 10.1046/j.1471-4159.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 69.Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005;77:1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Mailleux P, Vanderhaeghen JJ. Distribution of the neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–688. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- 71.Maneuf YP, Nash JE, Croosman AR, Brotchie JM. Activation of the cannabinoid receptor by D9-THC reduces GABA uptake in the globus pallidus. Eur. J. Pharmacol. 1996;308:161–164. doi: 10.1016/0014-2999(96)00326-3. [DOI] [PubMed] [Google Scholar]
- 72.Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 1997;148:265–270. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- 73.Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci. STKE. 2002;129:RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- 74.Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 75.Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007;56:382–392. doi: 10.1016/j.phrs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Minuzzi L, Cumming P. Agonist binding fraction of dopamine D2/3 receptors in rat brain: a quantitative autoradiographic study. Neurochem. Int. 2010;56:747–752. doi: 10.1016/j.neuint.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatylinositol-3 kinase/Akt signaling. J. Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulder J, Zilberter M, Pasquaré SJ, Alpár A, Schulte G, Ferreira SG, Köfalvi A, Martín-Moreno AM, Keimpema E, Tanila H, Watanabe M, Mackie K, Hortobágyi T, de Ceballos ML, Harkany T. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- 80.Orgado JM, Fernández-Ruiz J, Romero J. The endocannabinoid system in neuropathological states. Int. Rev. Psychiatry. 2009;21:172–180. doi: 10.1080/09540260902782828. [DOI] [PubMed] [Google Scholar]
- 81.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pazos MR, Sagredo O, Fernández-Ruiz J. The endocannabinoid system in Huntington’s disease. Curr. Pharm. Des. 2008;14:2317–2325. doi: 10.2174/138161208785740108. [DOI] [PubMed] [Google Scholar]
- 83.Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agrò A, Bernardi G, Brusa L, Pierantozzi M, Stanzione P, Maccarrone M. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- 84.Pisani V, Madeo G, Tassone A, Sciamanna G, Maccarrone M, Stanzione P, Pisani A. Homeostatic changes of the endocannabinoid system in Parkinson’s disease. Mov. Disord. 2011;26:216–222. doi: 10.1002/mds.23457. [DOI] [PubMed] [Google Scholar]
- 85.Reches A, Wagner HR, Jackson-Lewis V, Yablonskaya-Alter E, Fahn S. Chronic levodopa or pergolide administration induces down-regulation of dopamine receptors in denervated striatum. Neurology. 1984;34:1208–1212. doi: 10.1212/wnl.34.9.1208. [DOI] [PubMed] [Google Scholar]
- 86.Rinne JO, Laihinen A, Ruottinen H, Ruotsalainen U, Nagren K, Lehikoinen P. Increased density of dopamine D2 receptors in the putamen, but not in the nucleus caudatus nucleus in early Parkinson’s disease: a PET study with [11C]raclopride. J. Neurol. Sci. 1995;132:156–161. doi: 10.1016/0022-510x(95)00137-q. [DOI] [PubMed] [Google Scholar]
- 87.Romero J, García L, Cebeira M, Zadrozny D, Fernández-Ruiz JJ, Ramos JA. The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci. 1995;56:2033–2040. doi: 10.1016/0024-3205(95)00186-a. [DOI] [PubMed] [Google Scholar]
- 88.Romero J, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. The activation of cannabinoid receptors in striatonigral neurons inhibited GABA uptake. Life Sci. 1998;62:351–363. doi: 10.1016/s0024-3205(97)01117-x. [DOI] [PubMed] [Google Scholar]
- 89.Romero J, Berrendero F, Peǐrez-Rosado A, Manzanares J, Rojo A, Fernandez-Ruiz JJ, de Yeǐbenes JG, Ramos JA. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–494. doi: 10.1016/s0024-3205(99)00618-9. [DOI] [PubMed] [Google Scholar]
- 90.Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp. Neurol. 2010;224:92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 91.Sánchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Δ9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- 92.Sañudo-Peña MC, Patrick SL, Khen S, Patrick RL, Tsou K, Walker JM. Cannabinoid effects in basal ganglia in a rat model of Parkinson’s disease. Neurosci. Lett. 1998;248:171–174. doi: 10.1016/s0304-3940(98)00368-1. [DOI] [PubMed] [Google Scholar]
- 93.Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 94.Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK. Dopamine supersensitivity correlates with D2 High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silverdale MA, McGuire S, McInnes A, Crossman AR, Brotchie JM. Striatal cannabinoid CB1 receptor mRNA expression is decreased in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 2001;169:400–406. doi: 10.1006/exnr.2001.7649. [DOI] [PubMed] [Google Scholar]
- 96.Sossi V, Dinelle K, Topping GJ, Holden JE, Doudet D, Schulzer M, Ruth TJ, Stoessl AJ, de la Fuente-Fernandez R. Dopamine transporter relation to levodopa-derived synaptic dopamine in a rat model of Parkinson’s: an in vivo imaging study. J. Neurochem. 2009;109:85–92. doi: 10.1111/j.1471-4159.2009.05904.x. [DOI] [PubMed] [Google Scholar]
- 97.Szabo B, Müller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J. Neurochem. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- 98.Thobois S, Vingerhoets F, Fraix V, Xie-Brustolin J, Mollion H, Costes N, Mertens P, Benabid AL, Pollak P, Broussolle E. Role of dopaminergic treatment in dopamine receptor down-regulation in advanced Parkinson disease: a positron emission tomographic study. Arch. Neurol. 2004;61:1705–1709. doi: 10.1001/archneur.61.11.1705. [DOI] [PubMed] [Google Scholar]
- 99.Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 100.Waksman Y, Olson J, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- 101.Walsh S, Mnich K, Mackie K, Gorman AM, Finn DP, Dowd E. Loss of cannabinoid CB1 receptor expression in the 6-hydroxydopamine-induced nigrostriatal terminal lesion model of Parkinson’s disease in the rat. Brain Res. Bull. 2010;81:543–548. doi: 10.1016/j.brainresbull.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walter L, Stella N. Cannabinoids and neuroinflammation. Br. J. Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wictorin K. Anatomy and connectivity of intrastriatal striatal transplants. Prog. Neurobiol. 1992;38:611–639. doi: 10.1016/0301-0082(92)90044-f. [DOI] [PubMed] [Google Scholar]
- 105.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 106.Yin H, Lovinger M. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. PNAS. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeng BY, Dass B, Owen A, Rose S, Cannizzaro C, Tel BC, Jenner P. Chronic L-DOPA treatment increases striatal cannabinoid CB1 receptor mRNA expression in 6-hydroxydopamine-lesioned rats. Neurosci. Lett. 1999;276:71–74. doi: 10.1016/s0304-3940(99)00762-4. [DOI] [PubMed] [Google Scholar]