Abstract
A largely unilamellar epithelial layer lines body cavities and organ ducts such as the digestive tract and kidney tubules. This polarized epithelium is composed of biochemically and functionally separate apical and basolateral surfaces. The epidermal growth factor receptor (EGFR) signaling pathway is a critical regulator of epithelial homeostasis and is perturbed in a number of epithelial disorders. It is underappreciated that in vivo EGFR signaling is most often initiated by cell-surface delivery and processing of one of seven transmembrane ligands, resulting in release of the soluble form that binds EGFR. In polarized epithelial cells, EGFR is restricted largely to the basolateral surface, and apical or basolateral ligand delivery therefore has important biological consequences. In vitro approaches have been used to study the biosynthesis, cell-surface delivery, proteolytic processing, and release of soluble EGFR ligands in polarized epithelial cells. We review these results, discuss their relevance to normal physiology, and demonstrate the pathophysiological consequences of aberrant trafficking. These studies have uncovered a rich diversity of apico-basolateral trafficking mechanisms among the EGFR ligands, provided insights into the pathogenesis of an inherited magnesium-wasting disorder of the kidney (isolated renal hypomagnesemia), and identified a new mode of EGFR ligand signaling via exosomes.
Keywords: EGFR, basolateral, polarized trafficking, epithelial polarity, cancer
INTRODUCTION
In the nineteenth century, the French physiologist Claude Bernard proposed the concept milieu intérieur to describe the internal environment in which tissue elements live (1). He certainly did not intend to convey that this internal environment was divorced from external influences. In fact, he said that “external variations are at every instant compensated and brought into balance. In consequence, far from being indifferent to the external world, the higher animal is on the contrary in a close and wise relation with it, so that its equilibrium results from a continuous and delicate compensation” (1). This concept was largely ignored until the early twentieth century, when Walter B. Cannon (2) introduced the term homeostasis to describe the body’s ongoing adaptations to the external environment to maintain a steady state—albeit one that is continuously responding and adapting.
Epithelia, which line the body cavities, glands, and surfaces, straddle the interface between the external and internal environments; at this interface, homeostatic controls face their sternest test, as epithelia are exposed to a continuous barrage of environmental insults (mechanical, chemical, and pathogenic). It is thus not surprising that more than 90% of cancers are epithelially derived (3, 4). Most epithelia are unilamellar and exhibit an apico-basolateral polarity that is conserved across metazoans; its organizing principles may extend to lower organisms as far back as Dictyostelium (5). Apical and basolateral membranes are segregated by tight junctions, which prevent the mixing of macromolecules; as a result, these membranes have distinct protein and lipid compositions (6, 7). Tight junctions also limit free exchange of solutes and other molecules across the epithelium. Thus, the majority of transport across epithelia is regulated; transporters and channels on specific membranes allow for selective transport across the monolayer. The establishment of apico-basolateral polarity and trafficking pathways that determine preferential localization of proteins have been extensively reviewed (7–12).
The perspective of this review is that epidermal growth factor receptor (EGFR) signaling is critical to epithelial homeostasis. EGFR signaling is initiated by ligand binding to the receptor. EGFR ligands are synthesized as transmembrane precursors and are processed by metalloproteases to soluble ligands that bind to the receptor (Figure 1). In polarized epithelia, the regulated spatiotemporal delivery of ligands, metalloproteases, and receptors to the membrane creates a platform for autocrine EGFR signaling. As we show, the precision and fidelity of these highly orchestrated events are perturbed in a number of epithelial disorders, including cancer. We discuss the trafficking of EGFR ligands in polarized epithelial cells, the relevance to epithelial physiology and pathophysiology, and selected aspects of trafficking of ERBBs (where ERBB denotes erythroblastosis oncogene B) and metalloproteases.
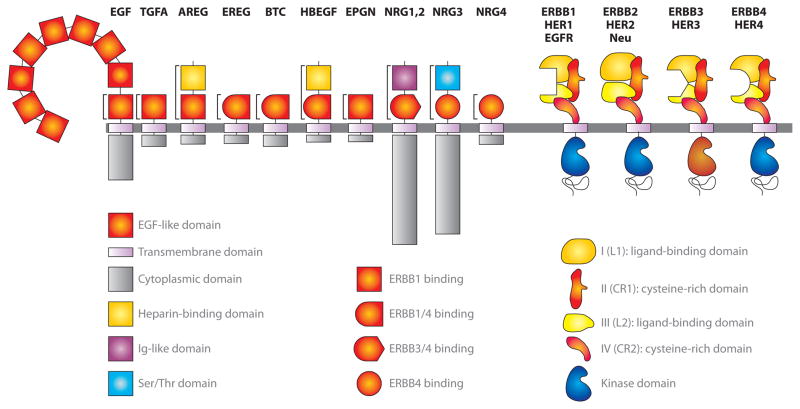
Figure 1.
Mammalian ERBBs and their ligands. Eleven mammalian EGF-like ligands bind to four ERBBs. The ligands are synthesized as type I transmembrane precursors except for NRG1 type III, which traverses the membrane twice (12a). With the exception of EGF, which has nine EGF-like domains in its extracellular region, all other ligands have one EGF-like domain. The first seven ligands depicted above (EGF, TGFA, AREG, EREG, BTC, HBEGF, and EPGN) bind to EGFR; EREG, BTC, and HBEGF also bind to ERBB4. All neuregulins bind to ERBB4; NRG1 and NRG2 also bind to ERBB3. In addition to the EGF-like domain, some ligands contain additional domains. Mature AREG and HBEGF also contain a heparin-binding domain proximal to the EGF-like domain. NRG1 and NRG2 possess an immunoglobulin-like domain, and NRG3 possesses a serine/threonine-rich domain. There is considerable variability in the length of the cytoplasmic domains of EGF-like ligands. These transmembrane ligands undergo proteolytic cleavage in their ectodomains by metalloproteases to release mature receptor-binding forms, indicated by lines. All four receptors are synthesized as type 1 transmembrane proteins. The extracellular region is divided into four domains. Ligand binding induces a conformational change in the receptor that exposes the dimerization region in cysteine-rich domain I; ERBB2 does not bind to any ligand and independently adopts this dimerization-competent conformation. Dimerization allows for the intrinsic cytoplasmic tyrosine kinase activity and subsequent transphosphorylation of the tyrosine residues (blue); ERBB3 kinase activity is severely compromised (brown). In the old nomenclature, heregulin-α, heregulin-β1 (a/b/c/d), and heregulin-β2 are neuregulin-1 isoforms; all of these contain the complete 375-amino-acid cytoplasmic domain. ERBB is derived from avian erythroblastosis oncogene B (ERBB1–4 are human homologs), and Neu is derived from the rat neuro/glioblastoma transforming gene neu. Abbreviations: AREG, amphiregulin; BTC, betacellulin; EGF, epidermal growth factor; EGFR, EGF receptor; EPGN, epigen; EREG, epiregulin; HBEGF, heparin-binding EGF-like growth factor; HER, human epidermal growth factor receptor; NRG, neuregulin; TGFA, transforming growth factor-α.
APICO-BASOLATERAL POLARITY: EGFR SIGNALING IN A POLARIZED EPITHELIUM
Cells have defined shapes and spatial orientations that are mediated, at least in part, by the asymmetric distribution of key sensors and effectors, allowing for differential responses to spatially restricted stimuli. EGFR asymmetry is widely observed; for example, EGFR asymmetric distribution during cell division is essential for diversification of central-nervous-system progenitor cells, and EGFR planar asymmetry governs bract cell fate in Drosophila (13, 14).
In addition to segregation of membrane components, there is also polarization of intracellular structures. For example, tight junctions organize at the apico-basolateral boundary; the nucleus positions near the base of the cell; and microtubules align parallel to the lateral membranes, with minus ends directed toward the apical surface. Such polarity may be recapitulated in vitro and has been extensively studied to understand the underlying principles of trafficking and polarity. Multiple studies have revealed that trafficking pathways are modular and may be applied to other aspects of cellular polarity. Axonal polarity in neurons, for example, displays numerous parallels to apico-basolateral polarity and employs common modules (15). Certain aspects of polarity are also hijacked by cancer cells and may be alternatively assembled or selectively amplified to their own advantage (16).
Epithelia are one of the most proliferative tissues in the body and are highly prone to injury and insult by external mechanical, chemical, and pathogenic factors. EGFR is critically important to the repair processes that reestablish polarity and barrier function after injury. Whereas EGFR signaling and subsequent endocytic trafficking following administration of exogenous EGFR ligands have been thoroughly investigated, much less is known about how endogenous EGFR ligands initiate EGFR signaling (17, 18). In a polarized epithelium, the proper delivery of ligands, proteases, and receptors in a spatiotemporal manner is critical for ensuring the proper activation of EGFR and downstream signaling. Polarized trafficking cannot be fully appreciated in 2D plastic cultures and requires higher-dimensional cultures, such as Transwell™ filters or Matrigel™/collagen cultures, that recapitulate apico-basolateral polarity.
POLARIZED TRAFFICKING PATHWAYS: MOTIFS, ROUTES, AND ADAPTORS
De novo building of a polarized epithelium is dependent upon evolutionarily conserved pathways that involve the Par protein family, PDZ protein complexes, proteins like Crumbs, and lipids that confer membrane identity (12, 19–21). Polarized trafficking pathways are layered on top of these polarity-establishing pathways. Initial studies with vectorial targeting of viral proteins like hemagglutinin and vesicular stomatitis virus glycoprotein uncovered important principles of trafficking (8). Proteins in the secretory pathway are synthesized in the ER; mature in the Golgi; and become packaged into exocytic vesicles, which then travel along microtubule tracks to specific cellular locations. Trafficking is modular, and the proteins involved may broadly be divided into cargoes, adaptors, and effectors, introduced briefly here.
With regard to cargoes, the localization information for a specific protein is either encoded in its primary sequence or added posttranslationally. Basolateral sorting information usually resides in the cytoplasmic domain and is encoded mostly in the primary sequence. Discrete tyrosine-based (NPXY, YXXΦ) and leucine-based [LL, DXXLL, EEXXXL, (DE)XXXL(LI)] basolateral sorting motifs have been identified (22–24). For apical cargo, this information is less defined. However, GPI anchors and N- or O-glycosylation, as well as transmembrane and cytosolic regions of proteins, have been shown to confer apical specificity (25–27).
The best-characterized adaptor proteins are basolateral clathrin adaptors of the adaptor protein (AP) and Golgi-localized, γ adaptin ear-containing (GGA) families. GGAs are monomeric; APs are heterotetrameric, and usually the μ and σ subunits interact with cargo, whereas other subunits interact with clathrin and molecular motors, to facilitate packaging into vesicles and transport along microtubule tracks (22, 28). Apical sorting adaptors are not characterized, but raft association or interaction with galectins has been shown to direct apical trafficking (29, 30).
A specific cargo-adaptor interaction determines the packaging of cargo into exocytic vesicles. The cargo-adaptor complex establishes secondary and tertiary interactions with other components of transport machinery that are loosely termed effectors. For example, GTPases of the Rab family direct the route and fate of specific vesicles (31, 32). These Rabs toggle between active and inactive states (GTP bound and GDP bound), governed by cognate GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs). Owing to this on-off state, the cargo may be sequentially passed along trafficking routes, encountering GTPases at specific trafficking steps and compartments (32, 33). In the case of basolateral trafficking, an octameric exocyst complex, located near tight junctions, tethers vesicles to the plasma membrane, and fusion to the membrane is mediated by the interaction of v-SNAREs and t-SNAREs present on the vesicle and target membrane, respectively (34–36).
MODEL SYSTEMS FOR THE STUDY OF POLARIZED TRAFFICKING
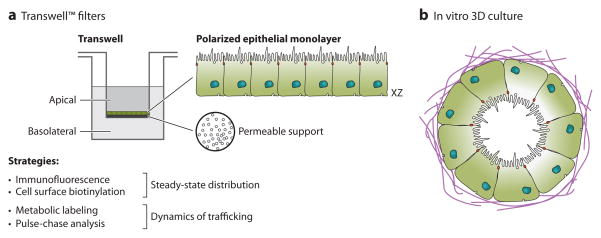
Epithelial cells do not polarize when grown on glass or plastic; under these culture conditions, cells at confluence reach, at best, a pseudopolarized state, which is not suitable for trafficking studies. When epithelial cells are grown on a permeable support, like perforated polycarbonate/polystyrene filters, a limited number of epithelial cell lines retain the ability to form a polarized monolayer (Table 1). This system has been optimized for trafficking studies: It allows for selective access to, manipulation of, and harvest from apical and basolateral surfaces (Figure 2). These filters come in various sizes (0.4–10-μm-diameter pores) with various coatings (uncoated or Matrigel/collagen coated). In polarized Madin-Darby canine kidney (MDCK) cells, approximately 40,000 EGFRs (>90% of total) are found at the basolateral surface (37).
Table 1.
Epithelial cell lines that display apico-basolateral polarity in vitro
| Cell line | Origin | Species | Transwell filters/3D |
|---|---|---|---|
| MDCK | Kidney | Dog | Either |
| LLC-PK1 | Kidney | Pig | Filters |
| IMCD3 | Kidney | Mouse | 3D |
| MA-104 | Kidney | Monkey | Filters |
| Caco-2 | Colon | Human | Either |
| HCA-7 | Colon | Human | Either |
| HT-29 | Colon | Human | Filters |
| T-84 | Colon | Human | Filters |
| SK-CO-15 | Colon | Human | Filters |
| MCF10A | Breast | Human | 3D |
| Eph4.9 | Breast | Mouse | Either |
| Calu-3 | Lung | Human | 3D |
| A549 | Lung | Human | Filters |
| H441 | Lung | Human | Filters |
| Ishikawa | Endometrium | Human | 3D |
| FRT | Thyroid | Rat | Filters |
| RPE | Retina | Human | Filters |
Figure 2.
Methods of culturing epithelial cells to study apico-basolateral polarity. (a) Selected epithelial cell lines are plated at high density on Transwell™ filters (0.4-μm pore size). Cells grow to confluence and spontaneously organize into a polarized monolayer with the apical surface facing the inner chamber and the basal surface in contact with the permeable support. Polarity of the epithelium is assessed indirectly by measuring transepithelial electrical resistance or permeability across the monolayer (with 3H-inulin or FITC-dextran) or directly by examining compartment-specific localization of cell-surface proteins by immunofluorescence [e.g., ZO-1, E-cadherin, gp135 (podocalyxin), Crumbs] or by cell-surface biotinylation. Permeability across the monolayer is restricted, so one can selectively add to or sample from the apical or the basolateral compartment. Metabolic labeling combined with cell-surface biotinylation allows for study of the dynamics of trafficking for selected proteins. (b) Epithelial cells from various tissue origins, when added as a single-cell suspension in Matrigel™ or collagen and allowed to grow over a number of days, organize into unilamellar polarized structures termed cysts or acini. Normally, the basolateral surface faces outward and is in contact with the extracellular matrix, whereas the apical surface faces inward, enclosing a central, hollow lumen. The introduction of oncogenes and/or the removal of tumor suppressor genes results in phenotypic alterations (39, 40).
When allowed to grow suspended in gelatinous extracellular matrix (ECM) components (collagen or Matrigel), some epithelial cell lines form unilamellar spherical structures, termed cysts or acini. These structures show a greater resemblance to in vivo epithelia, as their basal membranes are in contact with the ECM and the apical surface contacts a de novo–generated apical lumen. In many ways, cysts may be considered the basic unit of the epithelium in vitro. In addition to the structural specializations seen in Transwell cultures, these ECM cultures (often termed 3D cultures) recapitulate features of epithelia, such as branching morphogenesis, and certain aspects of cellular transformation (11, 39, 40). 3D culture systems are also being used increasingly for primary tissue explants and stem cell–based organotypic cultures (41).
OVERVIEW OF EGFR LIGANDS
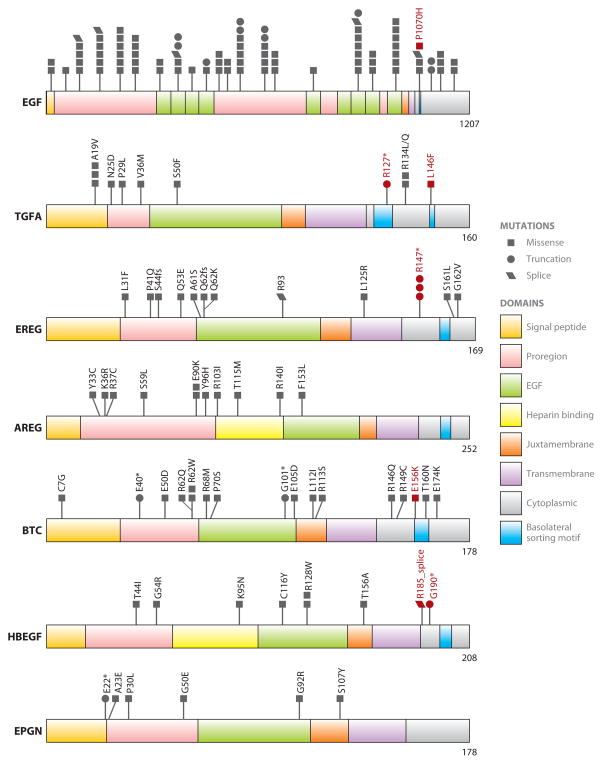
Eleven mammalian ligands bind to four members of the ERBB family of receptor tyrosine kinases (Figure 1). Four EGFR ligands are organized in a syntenic cluster in the human and mouse genomes on chromosomes 4 and 5, respectively, maintaining their order and orientation (Figure 3). Additional structural and sequence similarity indicates that the ligands arose as a result of duplication that occurred before the divergence of mammals (42).
Figure 3.
Syntenic clustering of EGFR ligands in the human and mouse genomes. Five of the seven human EGFR ligands are located on chromosome 4. Four of these ligands are organized in a syntenic cluster; the order and orientation of these four ligands are maintained in the mouse genome.
In cell culture conditions, different ligands have different affinities for EGFR and traffic ligand-associated EGFR through different endocytic trafficking pathways, resulting in differential biological outputs (43). We showed that, in mature polarized epithelia, transforming growth factor-α (TGFA) is rapidly cleaved and captured by basolateral EGFRs (43a); we believe that this TGFA-EGFR pair subserves homeostatic signaling. TGFA is a widely expressed, high-affinity ligand that outcompetes low-affinity ligands like amphiregulin (AREG) and epiregulin (EREG) for binding to EGFR.
MODES OF SIGNALING BY EGFR LIGANDS
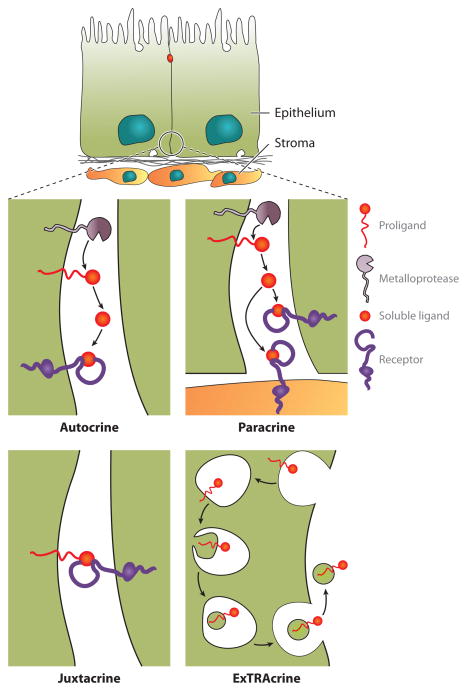
In epithelia, EGFR ligands activate EGFR by autocrine, paracrine, juxtacrine, and ExTRAcrine (exosomal targeted receptor activation) modes (Figure 4). Paracrine signaling occurs when soluble growth factors like epidermal growth factor (EGF) are released by one cell and act on adjacent or nearby cells (Figure 4). Autocrine signaling was a term introduced by Sporn & Todaro (45) to describe the release of transforming factors by virally transformed fibroblasts that conferred external growth factor independence, a property common to many neoplastic cells (45–47). In its strictest sense, autocrine signaling refers to a factor acting on the cell that produced it. However, this term is more loosely interpreted to include factors released by one cell that act on that cell or an adjacent cell of the same lineage. EGFR ligands act most often via paracrine and autocrine signaling modes. In the juxtacrine mode, a membrane-anchored ligand binds to a receptor on an adjacent cell. The most convincing evidence for juxtacrine signaling, among the EGFR ligands, exists for heparin-binding EGF-like growth factor (HBEGF) (42, 48, 49).
Figure 4.
Modes of EGFR signaling. In autocrine signaling, released ligands bind to and activate receptors on the same cell. In paracrine signaling, soluble ligands diffuse over a short distance and bind to and activate receptors on nearby cells. Juxtacrine signaling requires a transmembrane ligand binding to the receptor on an adjacent cell. ExTRAcrine (exosomal targeted receptor activation) signaling involves the packaging and release of transmembrane ligands in extracellular vesicles termed exosomes. We depict an EGFR ligand being endocytosed into an endosome that subsequently undergoes inward budding and fusion with late endosomes to generate a multivesicular body that contains intraluminal vesicles. These multivesicular bodies fuse with the plasma membrane to release their intraluminal vesicles, now termed exosomes, extracellularly.
We (50) recently described a new mode of EGFR ligand signaling via exosomes (Figure 4). This work was spurred by detection of full-length pro-HBEGF in conditioned medium of MDA-MB-231 cells and in the apical medium of MDCK cells expressing pro-HBEGF, without its corresponding presence at the apical plasma membrane. HBEGF was later found packaged in exosomes, which are membrane-enclosed vesicles 30–100 μm in size. Since then, we have detected full-length HBEGF, AREG, and TGFA in exosomes from human breast (MDA-MB-231) and colon (HCA-7) cancer cell lines. Compositional heterogeneity of individual exosomes was quantified by fluorescence-activated vesicle sorting (FAVS) (50a). After expressing individual EGFR ligands in MDCK cells that do not express measurable amounts of ligands, we found that AREG exosomes were fivefold more potent in enhancing the invasiveness of recipient cancer cells than TGFA or HBEGF exosomes. The rapid manner in which AREG exosomes were taken up by recipient cells was, at least in part, EGFR dependent. Thus, these exosomes may act as EGFR ligand payloads.
To assess differences in exosome composition and behavior between normal and transformed cells, we studied a human colon cancer cell line, DLD-1 (KRASWT/mut), and its isogenic derivatives in which wild-type KRAS (DKO-1) or mutant KRAS (DKs-8) was eliminated by homologous recombination (51). In contrast to DLD-1 and DKO-1 cells, wild-type KRAS DKs-8 cells no longer grow in soft agar or form tumors in nude mice. DKO-1-derived exosomes are highly enriched with AREG compared with DKs-8 exosomes and confer significantly greater invasiveness when added to recipient cells (52). Studies are under way to determine how AREG is delivered into exosomes. Full-length AREG is the most rapidly internalized of the EGFR ligands from the cell surface (H.S. Wiley, personal communication). Ubiquitylation of AREG is important, as mutation of the three lysyl residues in its cytoplasmic tail significantly reduces levels of exosomal AREG (R.J. Coffey, unpublished observation).
POLARIZED TRAFFICKING OF EGFR LIGANDS
We studied trafficking of EGFR ligands in polarized MDCK cells by using full-length constructs and followed trafficking by using specific antibodies. In most cases, we confirmed these results, examining trafficking of endogenous ligands in a battery of polarizing human colorectal cancer cell lines. We also tagged the ligands with fluorescent proteins. However, care must be taken that the addition of a tag does not alter trafficking. For example, TGFA interacts with multiple proteins via its C-terminal PDZ target motif (TVV); appending a tag at the C terminus would block PDZ-dependent interactions (52a). Although a C-terminal tag does not alter the route or destination of TGFA, it compromises the trafficking efficiency. Below, using EREG trafficking as an example, we include additional details that may be instructive for researchers embarking on trafficking studies of transmembrane or secreted proteins in a polarized setting.
EGF: Impaired Basolateral Sorting Manifests as an Inherited Magnesium-Wasting Disease
The EGF ligand family derives its name from EGF, the prototypic member of the family and the first polypeptide growth factor to be identified (53). Like other EGFR ligands, EGF is synthesized as a glycosylated transmembrane precursor (Figure 1). However, EGF is atypical in many ways. It is bigger (1,207 amino acids long) than other ligands, which are 150–252 amino acids long. Other ligands contain only one EGF repeat in their ectodomains, whereas EGF contains nine such repeats (Figure 1). Ectodomain cleavage produces an approximately 150–170-kDa product; fully processed, bioactive EGF is 6 kDa (54).
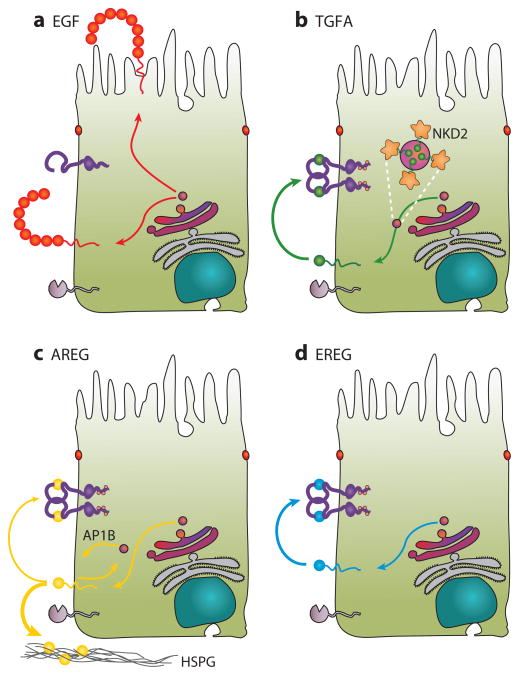
The EGF protein is detected in relatively few normal adult tissues, including the submaxillary glands, exocrine glands of the GI tract, and serous acini of the nasal cavity (42). Egf (and Tgfa) mRNA expression has been detected in intestinal Paneth cells, where Egf is thought to stimulate the proliferation of adjacent Lgr5+ stem cells (55). EGF immunoreactivity is detected on the luminal surface of epithelial cells in the distal convoluted tubule of the kidney (56). Likewise, we detected immunoreactive EGF at the apical membranes of polarized MDCK cells stably overexpressing EGF under steady-state conditions (54). However, metabolic labeling coupled to cell-surface biotinylation showed that newly synthesized EGF was delivered equally to the apical and basolateral cell surfaces but was selectively cleaved from the basolateral surface (Figure 5a) (54). These studies provide a novel mechanism for steady-state localization of a cell-surface transmembrane protein: selective cleavage rather than selective delivery or retention. Cleavage results in the release of the complete ectodomain of EGF that is unable to bind to EGFR; this cleavage could be blocked by the broad-specificity metalloprotease inhibitor BB94. Recently, a BB94-insensitive protease, RHBDL2, was shown to cleave human EGF (57).
Figure 5.
Trafficking of EGFR ligands in polarized epithelial cells. (a) EGF is delivered equally to both the apical and basolateral surfaces but is preferentially cleaved from the basolateral surface, resulting in apical localization under steady-state conditions. (b) TGFA is directly delivered to the basolateral surface, where it is cleaved chiefly by ADAM17 and is rapidly bound by EGFRs. NKD2 recognizes basolateral sorting determinants in the cytoplasmic tail of TGFA, coats TGFA-containing vesicles, and delivers these vesicles to the basolateral corner. These vesicles tether, dock, and fuse in a NKD2 myristoylation–dependent manner. (c) AREG is delivered to the basolateral surface, where it is cleaved by ADAMs to release soluble ligand, which may then bind to EGFR or be sequestered via its interaction with heparan sulfate proteoglycans (HSPG). A fraction of basolateral AREG is endocytosed and recycled to the basolateral membrane in an AP1B-dependent manner. (d) EREG is directly delivered to the basolateral surface in an AP1B-independent manner.
Further details of EGF trafficking and its basolateral sorting motif came unexpectedly from Bindels and coworkers (58, 59) while they were studying isolated renal recessive hypomagnesemia (IRH), an autosomal-recessive magnesium-wasting disorder. IRH patients harbor a germline EGF mutation (a P1070L substitution in the cytoplasmic domain); P1070 is the distal proline residue in a PXXP motif, which was confirmed to be the EGF basolateral sorting motif. In the distal convoluted tubule, basolateral EGF activity is required to activate the apical magnesium transporter TRPM6. These patients waste magnesium in the urine due to the failure of EGF basolateral delivery and the activation of basolateral EGFRs, which normally transmit a signal to apical TRPM6 transporters to reabsorb magnesium (58, 59). Therefore, selective trafficking of EGFR ligands is clinically relevant. Interestingly, hypomagnesemia is one of the most common side effects observed in cancer patients undergoing treatment with cetuximab, an EGFR-neutralizing monoclonal antibody that blocks binding of the ligand to its receptor (60).
Whereas the basolateral sorting motif of EGF is known, the apical sorting motif remains undefined. EGF is N-glycosylated at a number of sites, but inhibition of N-glycosylation with tunicamycin does not interfere with its apical sorting (42, 54). EGF is also O-glycosylated at T801/S807 and S954/T955, but the role of these sites in apical trafficking remains to be determined (61).
Spatial restriction of signaling through ERBB members was first recognized in the polarized lung epithelium. There, heregulin-α/NRG1 (neuregulin 1) was localized to the apical surface, away from its cognate basolateral receptors, ERBB3 and -4. However, in an injury in which epithelial integrity was breached, the ligand could access and activate basolateral receptors. Thus, polarized trafficking pathways determine downstream signaling (62). Under steady-state conditions, EGF appears to be localized to the apical surface. However, EGF is delivered equally to both surfaces, and selective cleavage from the basolateral surface results in apical accumulation (54). A mechanistic understanding of familial IRH disease supports the conclusion that basolateral EGF is critical for the activation of basolateral EGFRs in the distal convoluted tubules (58).
Transforming Growth Factor-α: Homeostatic Signaling and Identification of a Novel Basolateral Sorting Adaptor
TGFA is synthesized as a 160-residue transmembrane precursor with a 39-residue cytoplasmic domain (Figure 6). Mouse Tgfa deletion results in hair follicle and eye abnormalities similar to those seen in Egfr hypomorphs, suggesting that the Tgfa-Egfr pair contributes to epithelial homeostasis (44). We performed a detailed analysis of TGFA trafficking in polarized epithelial cells by using MDCK cells stably expressing human TGFA (43a, 64–66).
Figure 6.
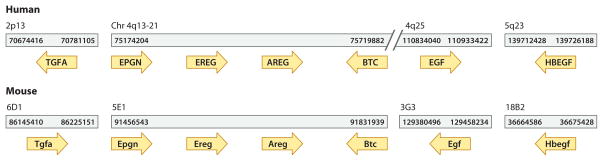
EGFR ligand mutations in human cancer. Domain organization of the EGFR ligands is depicted. The mutations reported in the cBioPortal for Cancer Genomics (http://www.cbioportal.org/public-portal/) are indicated. Mutations predicted to disrupt basolateral sorting are highlighted in red. EGF mutations are too numerous to be depicted here; they have been pooled together into domains. Individual mutations are as follows: K14Q, S21L, A34T, E94D, R100K, E109_splice, E115D, V130I, I152T, P160S, R170_splice, F173C, A185S, D211H, L214fs, N220S, R221T, S224C, G235E, 246D, L254F, P257L, W264S, F289Y, P300S, G323E, L347Q, R394*, Q397_splice, W459*, E473K, D492Y, R494Q, F498I, L517I, D518V, E552*, D573G, K576N, R588H, R588C, Q599H, E624*, Q629*, L631fs, R633S, S638C, G646E, F651L, A691T, V719L, G750*, K757R, D870_splice, C874Y, P883H, N890S, R898P, G902S, G907E, I908V, R943C, R943C, V969I, S979P, Y983C, V989L, N1002_splice, R1023H, S1064L, P1070H, S1075R, E1103*, E1103*, M1126I, R1132K, R1163Q, H1166Y, D144Y, S377Y, C380Y, R394*, C423S, Q510*, V554L, G942V, R961M, A1037S, S1064L, K1065E, G1119R.
The absence of TGFA in the basolateral medium of these polarized MDCK cells was surprising (43a). However, upon addition of a monoclonal antibody directed against the EGFR ectodomain that binds more strongly than TGFA, we detected high levels of TGFA in the basolateral medium. No such increase was observed in basolateral EGF or AREG after addition of the antibody to the basolateral compartment of MDCK cells stably expressing these ligands. We thus proposed that TGFA is a locally acting growth factor. Moreover, there was a threefold increase in serum TGFA 1 day after intravenous administration of cetuximab to a patient with Ménétrier’s disease. An earlier review discussed the biological relevance of this local capture for epithelial homeostasis (42).
In polarized MDCK cells, newly synthesized TGFA is delivered preferentially to the basolateral surface, where it is rapidly cleaved by ADAM17 (Figure 5b) (43a). The 39-residue cytoplasmic tail of TGFA contains a bipartite basolateral sorting motif consisting of basic juxtamembrane residues and a dileucine sequence (Table 2) (64). By yeast two-hybrid analysis using the TGFA cytoplasmic tail as bait, we found that NKD2, but not NKD1, binds to these basolateral sorting elements. NKD1 and NKD2 are mammalian orthologs of Drosophila naked cuticle, an inducible negative regulator of canonical Wnt signaling. NKD2 recognizes a Golgi-processed form of TGFA. NKD2 coats TGFA-containing exocytic vesicles and directs them to a basolateral corner of polarized MDCK cells, where the vesicles dock and fuse in a NKD2 myristoylation–dependent manner (Figure 5b) (65). The glycyl residue at position 2 in NKD2 undergoes myristoylation, a cotranslational modification that enables proteins to bind to membranes. Myristoylation-deficient G2A NKD2 still interacts with TGFA-containing exocytic vesicles and directs them to the plasma membrane, but the vesicles fail to fuse and accumulate at the basolateral corner of the cell, leaving TGFA trapped in the cytoplasm (66). We exploited the retention of basolaterally targeted, TGFA-containing exocytic vesicles in the presence of G2A NKD2 to isolate and characterize these vesicles by using FAVS (50a).
Table 2.
Human EGFR ligand cytoplasmic domains and their basolateral sorting motifsa

|

|
Cytoplasmic domain sequences of EGFR ligands are shown. Key trafficking residues are in bold red font. On the basis of sequence homology to AREG and BTC, the HBEGF residues in bold green font are predicted to confer basolateral sorting. Possible basolateral sorting motifs in NRGs are underlined. Abbreviations: AREG, amphiregulin; BTC, betacellulin; EGF, epidermal growth factor; EPGN, epigen; EREG, epiregulin; HBEGF, heparin-binding EGF-like growth factor; NRG, neuregulin; TGFA, transforming growth factor-α.
NKD2 has a short half-life of 1 to 2 h. It is degraded in the cytoplasm by the E3 ligase RNF25 (68). TGFA protects NKD2 from degradation. We mapped TGFA binding to residues 300–385 of NKD2 (65). RNF25 binds just distal to those residues. Although RNF25 and TGFA do not directly compete for binding, we speculate that binding of TGFA to NKD2 creates steric hindrance for RNF25 binding. Thus, NKD2 is stabilized by its cargo, TGFA. On the basis of the ability of NKD2 to recognize TGFA-containing vesicles and to direct them to the basolateral corner of polarized epithelial cells, we termed NKD2 a cargo recognition and targeting (CaRT) protein (66). In contrast to basolateral corner delivery of TGFA vesicles by NKD2, basolateral targeting of LDLR vesicles depends on a Sec6/8-containing octameric exocyst complex; however, these vesicles are recruited to the apical junctional complex (35).
TGFA also possesses a PDZ target motif (ETVV) at its C terminus. Fidelity of trafficking does not depend on the PDZ target motif, as ETVV-deficient TGFA sorts to the basolateral surface, although with less efficiency. We and others identified a number of proteins binding via this motif, including syntenin, GRASP55, MAGI-3, and PSD-95 (52a). We speculate that the PDZ target motif regulates TGFA maturation and transit through the early secretory pathway via sequential interactions with different compartment-restricted, PDZ-containing proteins.
Amphiregulin: Identification of a Novel Basolateral Sorting Motif
Human AREG is synthesized as a 252-residue, N-glycosylated transmembrane precursor that is approximately 50 kDa in size; proteolytic cleavage in its extracellular domain releases a soluble 43-kDa form (Figure 6) (70). AREG was isolated and cloned from the human breast cancer cell line MCF7 (71, 72). It is required for ductal growth and branching morphogenesis during mammary gland development (73). AREG is expressed at moderate levels in normal colonic mucosa but is overexpressed in colon cancer (74). AREG has lower binding affinity for EGFR than does EGF or TGFA. Therefore, in the presence of these ligands, AREG may not occupy EGFR. Mature soluble AREG contains a heparin-binding domain proximal to an EGF-like domain; in ECM, this domain enables an interaction with heparin that may limit AREG diffusion and increase its local concentration (70).
AREG is the most highly expressed EGF-like ligand in the polarizing human colorectal cancer cell lines Caco-2 and HCA-7. When stably expressed in polarized MDCK cells, AREG also localizes to the basolateral surface (Figure 5c) (75). Newly synthesized AREG is directly delivered to the basolateral surface with more than 95% efficiency. Tailless AREG was still efficiently delivered to the membrane, but basolateral targeting was lost. AREG was constitutively cleaved at both the apical and basolateral surfaces. However, basolateral cleavage was four times more efficient than apical cleavage. Constitutive AREG cleavage was less efficient than TGFA cleavage, and unlike TGFA, AREG was not consumed rapidly by basolateral EGFRs (in part owing to heparin interaction) (75).
The cytoplasmic domain of AREG contains a dominant-acting basolateral sorting motif (76). This novel basolateral sorting motif is composed of a single leucine downstream of an acidic cluster (EEXXXL) (76). Basolateral delivery of AREG depends on this motif, as mutants of this motif are localized and delivered to both apical and basolateral surfaces to an approximately equal extent. Intriguingly, loss of AP1B led to apical localization of only a small fraction of AREG. Delivery of wild-type AREG continued to be basolateral in AP1B-deficient cells. We showed that AREG is endocytosed from the basolateral surface and is recycled back to the originating surface; AP1B is involved in this recycling stage of AREG trafficking (Figure 5c). In the absence of AP1B, newly synthesized AREG continues to be delivered to the basolateral surface, but after internalization, its recycling back to the basolateral surface is compromised, and part of the protein is misrecycled to the apical surface. Thus, steady-state basolateral localization of AREG depends on two factors: (a) basolateral delivery that depends on a novel basolateral sorting motif and (b) recycling that depends on AP1B (76). We previously showed that endogenous AREG also localizes to the basolateral surface (76a).
Epiregulin: Apical Mistrafficking Leads to Transformation
EREG is synthesized as a 169-residue precursor that is processed by ADAM17; soluble ligand then binds to EGFR or ERBB4 (Figure 6) (77, 78). Along with Areg and betacellulin (Btc), Ereg is required in cumulus-oocyte complex maturation and blastocyst implantation in the uterus (79, 80). Expression in adults is restricted, with low-level expression in the epidermis, colon, lung, and peripheral blood macrophages (81, 82). Ereg-null mice are viable and show no apparent phenotype. However, Ereg is required for protection from dextran sodium sulfate–induced intestinal damage (83). EREG is overexpressed in a number of cancers and cancer cell lines (84–87). Massagué and coworkers (88) identified EREG as one of the most overexpressed genes in MDA-MB-231 breast cancer cells trained in vivo to metastasize to the lung. Silencing of EREG, along with that of COX-2 and metalloproteases 1 and 2, in these metastatic lung derivatives led to decreased metastasis. High EREG and AREG mRNA expression in wild-type KRAS colorectal tumors correlates with clinical responsiveness to EGFR-directed therapy, a finding that has been interpreted as growth factor addiction (89, 90).
EREG localizes to the basolateral surface of polarized MDCK cells under steady-state conditions, as determined by immunofluorescence and selective cell-surface biotinylation (Figure 5d) (37). The EREG C terminus ends in a putative PDZ target motif (PQV). Using two fluorescently tagged and untagged EREG constructs, we observed identical localization, indicating that the PDZ target motif does not affect the fidelity of trafficking (37). We then removed the cytoplasmic domain of EREG, which led to its complete relocalization to the apical surface. Next, we swapped the cytoplasmic domain of NGFR, an apically localized protein, with that of EREG and showed that this chimera now relocalizes to the basolateral surface (26, 37). These results led us to conclude that the cytoplasmic domain of EREG contains a basolateral sorting motif that is autonomous, transplantable, and dominant acting. Thus, this motif is necessary and sufficient for basolateral localization.
To precisely identify the basolateral sorting motif, two strategies are often employed. Known basolateral sorting motifs in the cytoplasmic domain may be mutated to determine their effect on trafficking. Alternatively, sequential deletions can be performed. Given that there are two possible sorting motifs (YXXΦ and PXXP) in the cytoplasmic domain of EREG, we elected to perform sequential deletions from the C terminus (22, 58). By this approach, we narrowed down the basolateral sorting motif of EREG to five amino acids (EYERV), which contain a tyrosine-based sorting motif (YXXΦ). YXXΦ sorting motifs are usually recognized by the AP family of clathrin adaptor complexes via their μ subunits; μ1A of AP1B is most often associated with basolateral sorting. Interestingly, μ1A is lost in LLC-PK1 (Lilly Laboratories cell porcine kidney 1) cells, a polarizing pig kidney cell line; thus, an AP1B-dependent cargo would mistraffic in these cells (28). Surprisingly, EREG is basolateral in LLC-PK1 cells, indicating that EREG basolateral sorting is AP1B independent (37). Furthermore, EREG retained its basolateral localization in MDCK cells where the AP1B complex was disrupted by knocking down μ1A, confirming the above result. The role of other adaptors that recognize this motif for basolateral sorting (e.g., AP1A, AP4) or other trafficking routes like endocytosis and recycling have not been determined (76, 91, 92).
Studying the dynamics of EREG trafficking revealed that the motif operates at the level of biosynthetic delivery. Using metabolic labeling with cell-surface biotinylation, we showed that newly synthesized EREG was delivered directly to the basolateral surface and that the trafficking mutant was delivered directly and completely to the apical surface (37). In contrast, removal of the cytoplasmic domains of TGFA and AREG resulted in approximately equal delivery to the two surfaces (64, 76). Thus, the extracytoplasmic domain of EREG likely contains a recessive apical sorting motif. EREG is N-glycosylated only at N47, but removal of glycosylation either by mutation or tunicamycin treatment did not yield an equal distribution on both surfaces in the absence of a basolateral sorting motif. In an analogous experiment with EGFR, removal of the cytoplasmic domain led to predominant apical localization, but additional inhibition of N-glycosylation with tunicamycin redistributed tailless EGFR approximately equally between the apical and basolateral surfaces (96, 97). This putative apical sorting motif for EREG remains to be determined.
As seen for IRH mutations in EGF, loss of delivery to the basolateral surface is pathogenic. However, unlike EGF, which does not get cleaved from the apical surface, EREG is efficiently cleaved from both apical and basolateral surfaces and is thus biologically active on both surfaces (37). MDCK cells were not transformed and did not form tumors in nude mice, but interestingly, EREG-expressing cells formed tumors in nude mice. Even more striking, apical EREG-expressing MDCK tumors were up to seven times larger and invaded into muscle and nerves. Thus, EREG mistrafficking to the apical surface resulted in a gain-of-function transformation phenotype. Absence of the cognate receptors, EGFR and ERBB4, which are predominantly basolateral, argues against transformation by apical EREG. However, a small subset (1–10%) of EGFR is consistently found on the apical surfaces of MDCK cells (37). When we stimulated each surface selectively with EREG, we observed that apical stimulation was qualitatively different from basolateral stimulation. In contrast to transient phosphorylation after basolateral stimulation, EGFR phosphorylation was sustained after apical stimulation. We hypothesized that because most ligands are secreted basolaterally, basolateral EGFR activity is strictly controlled. Apical EGFRs may have fewer negative regulatory constraints, possibly due to a different lipid environment, which may also alter EGFR activity or downstream signaling (98).
One caveat to this explanation is the presence of EGF in luminal media; why does luminal EGF not induce transformation? Perhaps autocrine stimulation of EGFR is required, or protective mucins on the apical surface occlude luminal EGF binding to apical EGFR; these same mucins may trap autocrine ligands, increasing their effective concentration at the apical surface. Nevertheless, the transformation that we observed by apical EREG mutants, combined with EREG mutations that are found in human cancer and that disrupt the EREG basolateral sorting motif, supports a role for EREG mistrafficking in cancer (Figure 6) (37). Finally, even wild-type EREG may mistraffic apically under the following conditions: (a) The basolateral trafficking adaptor, unknown as of yet, may be mutated or lost in cancer; (b) EREG overexpression may saturate basolateral trafficking routes; or (c) basolaterally delivered and cleaved EREG may be apically transcytosed, as has been observed previously for EGF (99). Studies have shown significant differences between apical EGFR activity and basolateral EGFR activity.
Betacellulin
The 178-amino-acid-long transmembrane precursor of BTC undergoes ectodomain metalloprotease cleavage (usually by ADAM10 or ADAM17) to release the soluble ligand, which binds to EGFR and ERBB4 (100). Human BTC was first cloned from MCF7 breast cancer cells (101). Initially isolated from mouse cancerous pancreatic β-cells (hence the name beta-cellulin), Btc is also a strong mitogen for these tumors (102). Btc is expressed in many tissues, including smooth muscle cells, the liver, the kidney, and the small intestine; its expression is particularly high in the pancreas, where it is thought to play a role in β-cell differentiation (103). Surprisingly, however, Btc knockout mice are viable and are apparently normal; a Btc/Hbegf double knockout only exacerbates Hbegf-associated heart defects (104). ADAM10 cleaves the ectodomain of BTC to generate soluble ligand and a stable membrane-anchored fragment containing all the transmembrane and cytoplasmic domains. This membrane-anchored fragment then undergoes intramembrane cleavage by γ-secretase to generate an intracellular domain fragment that then traffics to the nuclear membrane in a palmitoylation-dependent manner (105, 106).
Recently, we found that BTC localizes to the basolateral membranes of polarized MDCK cells under steady-state conditions (B. Singh, G. Bogatcheva & R.J. Coffey, manuscript in preparation). We performed basolateral necessity-and-sufficiency testing as described; removal of the cytoplasmic domain resulted in equivalent apical and basolateral distribution. The 39-residue cytoplasmic domain of BTC has high homology across species (103). It contains an arginine- and lysine-rich region (a putative nuclear localization signal) N-terminal to a putative monoleucine-based basolateral sorting motif (EEXXXL) that is similar to the basolateral sorting motif of AREG (76). Sequential tail truncations and amino acid substitutions in this region showed that the EEXXXL region governs steady-state basolateral localization of BTC. As described above, the BTC cytoplasmic domain is palmitoylated, a reversible posttranslational modification that enhances membrane association (106). Basolateral sorting of BTC may thus depend on two motifs: the juxtamembrane palmitoylation cysteine site, which increases membrane association, and the monoleucine-based basolateral sorting motif, which imparts basolateral membrane specificity. Both of these motifs may act in concert to ensure effective and accurate polarized membrane trafficking of BTC.
Heparin-Binding EGF-Like Growth Factor: Exosome-Mediated Delivery of Ligands
The 208-amino-acid-long transmembrane precursor of HBEGF contains a heparin-binding domain in the extracellular domain. This heparin-binding domain, along with an EGF-like domain, is processed into the soluble form (Figure 1). HBEGF binds to EGFR and ERBB4 (Figure 1) (42). Interaction with heparan sulfate proteoglycans through this motif is functionally required for cardiac valve development, which when dysregulated may lead to cardiac hypertrophy (107). Global and targeted HBEGF deletion shows that HBEGF is required for cardiac development, vulvogenesis, and epidermal wound healing (104, 108, 109). In addition, HBEGF is a potent growth factor for epithelial, endothelial, and smooth muscle cells and is induced in response to injury (110, 111). Overexpression and increased processing of HBEGF are functionally relevant in cancer (112, 113). Like the involvement of EREG in breast-to-lung metastasis, HBEGF is also involved in breast cancer metastasis to the brain (114). HBEGF is able to induce autocrine, paracrine, juxtacrine, and (as recently discovered) ExTRAcrine signaling (50, 115, 116). Whether HBEGF is selectively present at the cell surface is thus an important physiological and pathological question.
HBEGF is localized to the basolateral surfaces of polarized epithelial cells (116; R.J. Coffey, unpublished observation). HBEGF contains a putative monoleucine-based basolateral sorting motif (EEXXXL) that is characterized for AREG and BTC (76; B. Singh, G. Bogatcheva & R.J. Coffey, manuscript in preparation). The dependence on this motif, however, needs to be formally tested. As mentioned above, HBEGF is also packaged into exosomes, presumably by endocytic routing of cell-surface HBEGF into multivesicular bodies. O-Glycosylation of HBEGF may affect its polarized trafficking; this modification may also regulate HBEGF packaging into exosomes (61, 117). In addition, ubiquitylation is involved in packaging of cargo into exosomes; HBEGF has one lysyl residue in its cytoplasmic domain that may be ubiquitylated.
As noted above, among the EGFR ligands, the strongest evidence exists for HBEGF to be a paracrine-acting ligand. HBEGF also acts as a receptor (118). The B fragment of diphtheria toxin (DT) binds to human and monkey pro-HBEGF, enabling the A fragment to enter these receptor-bearing cells and kill them. This fact has also been exploited in genetically engineered mice to eliminate select populations of cells (119).
Epigen
Epigen (EPGN) was the last EGFR ligand to be identified and cloned (120). The 187-residue transmembrane precursor has a 23-amino-acid-long cytoplasmic tail (Figure 6). ADAM17 cleaves the extracellular domain of EPGN (78). Epgn knockout mice had no obvious phenotype, indicating redundancy with other ligands, which showed compensatory increased expression (121). EPGN has low affinity for EGFR but is a strong mitogen, and Epgn-overexpressing transgenic mice display enlarged sebaceous glands (122, 123). EPGN is an understudied ligand, and its preferential localization in polarized epithelial cells is unknown; a cursory analysis of the 23-amino-acid-long cytoplasmic domain, the shortest of all known EGFR ligands, does not reveal any putative sorting motif.
POLARIZED TRAFFICKING OF ERBBS
All four human ERBBs are basolaterally localized (62, 124). The basolateral sorting of EGFR has been characterized in detail; it is mediated by a bipartite motif in the juxtamembrane region, which is composed of a dileucine motif proximal to a PXXP motif (Figure 7) (96, 97, 125). Additionally, basolateral sorting is also controlled by posttranslational phosphorylation within this region (126). These components display a sorting hierarchy, indicating that EGFR may switch between various sorting pathways in a context-dependent manner (126, 127). A similar basolateral sorting motif has been described for ERBB2 (128). The basolateral sorting of EGFR and ERBB2 is dependent partly on AP1B, possibly via its interaction with the dileucine motif (127, 128). Interestingly, ERBB3 and ERBB4 share a high degree of homology in this juxtamembrane region, and the basic features of this basolateral sorting motif are conserved in all four receptors (Figure 7). This region of EGFR is also its activation domain, but there is minimal overlap between the sorting motif and the activation domain (129).
Figure 7.
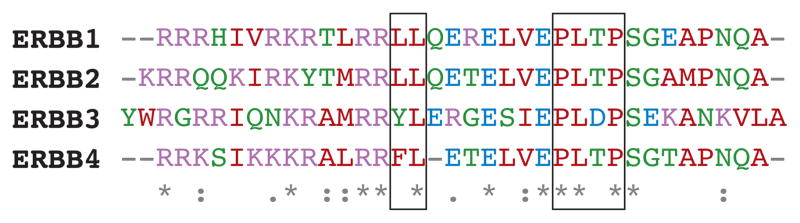
Alignment of juxtamembrane regions of human ERBBs. The established bipartite basolateral sorting motifs in EGFR and ERBB2 are aligned with putative basolateral sorting motifs of ERBB3 and ERBB4 in the black boxes.
TRAFFICKING OF ERBBS AND LIGANDS TO OTHER CELLULAR LOCATIONS
Apart from being found in cell membrane endosomal compartments during transit, ERBBs and their ligands are also found in other subcellular compartments, such as the nucleus and mitochondria (130, 131). The mechanism of nuclear translocation is best characterized, and both full-length and cytoplasmic domain fragments may be trafficked to the nucleus (132). For example, ERBB4 undergoes ADAM17-mediated ectodomain cleavage after ligand binding; the transmembrane fragment is subsequently cleaved by γ-secretase to release an intracellular domain fragment that then can enter the nucleus (133). EGFR also follows a similar route, except that the second cleavage is performed by rhomboid proteases instead of by γ-secretase. Full-length EGFR is also trafficked to the nucleus, where Sec61β, a component of the Sec61 translocon, facilitates its transit through the cytoplasm (134, 135). ERBB2 and ERBB3 have also been found in the nucleus (136, 137). Ligands are also reported to localize to the nucleus. An intracellular fragment of BTC, for example, is generated by sequential cleavage by ADAM10 and γ-secretase and subsequently localizes to the nucleus (106). EGF, HBEGF, TGFA, AREG, and NRG1 have also been reported in the nucleus (138). In the nucleus, the ligand and receptor intracellular fragments may act as transcription factors; receptors may, in addition, phosphorylate nuclear proteins. The signaling pathways induced as a result of nuclear translocation are important in normal and disease processes (106, 131).
POLARIZED TRAFFICKING OF METALLOPROTEASES
The ADAM family of metalloproteases primarily cleaves EGFR ligands; ADAM10 and -17 cleave six of the seven EGFR ligands and may be the major proteases in vivo (78). RHBDL2 cleavage of EGF is mentioned above. A number of these integral membrane proteases display spatial preference. ADAM9, -10, and -17 localize preferentially to the basolateral membranes (139–141). The basolateral sorting motif of ADAM10 is best characterized. The 708–715 amino acid region in the 55-amino-acid cytoplasmic domain of ADAM10 contains a putative SH3-binding motif, and P708 and P715 govern the basolateral sorting. Immediately downstream of this motif, L716 and P717 mutations disrupt basolateral sorting of ADAM10. Thus, the basolateral sorting motif of ADAM10 overlaps with the putative SH3-binding motif, extending to the distal leucyl and prolyl residues (139). Some features of this motif are also found in the cytoplasmic domains of other ADAMs. The interaction of a MAGUK (membrane-associated guanylate kinase) family member, SAP97 (synapse-associated protein-97), with ADAM10 is responsible for ADAM10 delivery to the neuronal postsynaptic membranes in response to NMDA receptor activation (142). iRhom2/RHBDF2, an inactive member of the rhomboid family, regulates trafficking of ADAM17 in macrophages, although its role in polarized trafficking has not been examined (143).
CLINICAL RELEVANCE
In epithelia, the EGFR signaling axis is localized to the basolateral compartment, where TGFA continuously engages EGFR for normal physiological actions, establishing a basolateral signaling platform. Loss of the TGFA adaptor NKD2 is a common event in colorectal cancer (R.J. Coffey, unpublished observation). Loss of NKD2 compromises cell surface delivery of TGFA, which results in a relatively unoccupied EGFR to which tumor-promoting ligands like EREG and AREG may bind. Additionally, as shown recently for EREG, mistrafficking of these ligands may help crystallize an apical signaling platform that may be tumorigenic, in part owing to the absence of negative controls. Trafficking pathways and domains that ensure basolateral delivery and establishment of the homeostatic EGFR basolateral signaling platform thus possess tumor-suppressive functions.
Importantly, the membrane-anchored ligands are not constitutively cleaved, with the possible exception of TGFA. Cleavage is highly regulated and integrates a number of stimuli. For example, G protein–coupled receptor (GPCR) ligands induce EGFR activation via regulated proligand cleavage by metalloproteases (112, 148). In addition to GPCR stimulation, EGFR transactivation activates EGFR in response to a number of physical stimuli such as osmotic stress, UV irradiation, and shear stress (149–151). Cancer cells are especially adept at integrating diverse stimuli to drive growth, survival, or angiogenic pathways through EGFR transactivation. EGFR transactivation is regulated in epithelia in which the selective presence of stimuli leads to specific spatiotemporal activation. In an epithelium that mistraffics the components of the EGFR signaling axis to the apical surface, EGFR transactivation may be controlled by apically localized stimuli (like shear forces) rather than by basolateral stimuli and, in turn, elicit differential signaling. There is a need to understand signaling derived from different membrane compartments in response to diverse stimuli.
SUMMARY POINTS.
In polarized epithelia, all EGFR ligands and ERBBs studied to date show preferential delivery to the basolateral surface, except for EGF, which is delivered equally to both apical and basolateral surfaces.
Most of the signaling through these ligands, including EGF, is elicited at the basolateral surface. A germline mutation in the basolateral sorting motif of EGF results in an inherited magnesium-wasting disorder in the kidney (isolated renal hypomagnesemia).
Ligands can engage and activate the EGFR via autocrine, paracrine, juxtacrine, and ExTRAcrine modes of signaling.
TGFA is a widely expressed, high-affinity EGFR ligand that is rapidly cleaved and captured by basolateral EGFRs; these properties enable it to contribute to epithelial homeostasis.
Naked2 (NKD2) acts as CaRT protein to deliver TGFA-containing exocytic vesicles to the basolateral surfaces of polarized epithelial cells, where vesicles dock and fuse in a NKD2 myristoylation–dependent manner.
Mistrafficking of EREG to the apical surface leads to the transformation of polarized MDCK cells.
Human cancers contain mutations in the cytoplasmic domains of EGFR ligands; such mutations would disrupt their basolateral trafficking.
Acknowledgments
The work on trafficking of EGFR ligands was supported by National Institutes of Health (NIH) grants R01-CA046413 and R01-CA163563 and GI Special Program of Research Excellence (SPORE) P50-CA095103 (to R.J.C.). The authors thank Emily J. Poulin for critical review of the manuscript.
KEY TERMS AND DEFINITIONS
- AREG
amphiregulin
- BTC
betacellulin
- CaRT
cargo recognition and targeting
- EGF
epidermal growth factor
- EGFR transactivation
mode of EGFR activation in response to stimuli other than its ligands. Various physical and chemical stimuli elicit EGFR signaling via activating metalloproteases that cleave the transmembrane EGFR ligands, which then diffuse in the extracellular medium and subsequently bind to the receptor
- EGFR
epidermal growth factor receptor
- EPGN
epigen
- EREG
epiregulin
- Exosomal targeted receptor activation (ExTRAcrine)
mode of signaling involving growth factors that are packaged in extracellular vesicles termed exosomes. Packaging of ligands into exosomes protects them from degradation and allows prolonged action at local or distant sites
- HBEGF
heparin-binding EGF-like growth factor
- Homeostasis
property of a system (especially one living) that regulates its internal environment to maintain a relatively stable state that constantly adjusts in response to external environmental perturbations
- LLC-PK1
Lilly Laboratories cell porcine kidney 1
- Madin-Darby canine kidney cells (MDCK cells)
MDCK-I cells have higher transepithelial electrical resistance than do MDCK-II cells; the latter are more widely used for trafficking studies. Our studies were performed in MDCK-II cells, referred to here as MDCK for simplicity
- Matrigel™
trade name for a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells; the chief proteins in Matrigel, ™ are laminin, entactin, and collagen IV
- NRG
neuregulin
- TGFA
transforming growth factor-α
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Bhuminder Singh, Email: bhuminder.singh@vanderbilt.edu.
Robert J. Coffey, Email: robert.coffey@vanderbilt.edu.
LITERATURE CITED
- 1.Bernard C. Leçons sur les phénomènes de la vie commune aux animaux et aux végétaux. Paris: Baillière; 1878. [Google Scholar]
- 2.Cannon WB. The Wisdom of the Body. New York: Norton Libr; 1932. [Google Scholar]
- 3.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by β- and α-catenin predates cadherin and metazoan origins. Science. 2011;331:1336–39. doi: 10.1126/science.1199633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerl MJ, Sampaio JL, Urban S, Kalvodova L, Verbavatz JM, et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol. 2012;196:213–21. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–25. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 8.Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–47. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–34. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol. 2012;14:1235–43. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaffrey LM, Macara IG. Signaling pathways in cell polarity. Cold Spring Harb Perspect Biol. 2012;4:a009654. doi: 10.1101/cshperspect.a009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–86. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Han C, Axelrod JD. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev Cell. 2012;23:507–18. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 16.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–57. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. BioEssays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–96. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–22. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 21.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 22.Bonifacino JS, Dell’Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–26. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- 24.Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–91. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 25.Scheiffele P, Peränen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 26.Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–40. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am J Physiol Cell Physiol. 2006;290:C1–10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 28.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–98. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 29.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Ren Physiol. 2009;296:F459–69. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, Surma MA, Simons K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012;22:793–805. doi: 10.1038/cr.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–10. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–59. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–7. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–77. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–70. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oztan A, Silvis M, Weisz OA, Bradbury NA, Hsu SC, et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–92. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh B, Bogatcheva G, Washington MK, Coffey RJ. Transformation of polarized epithelial cells by apical mistrafficking of epiregulin. Proc Natl Acad Sci USA. 2013;110:8960–65. doi: 10.1073/pnas.1305508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deleted in proof
- 39.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung CT, Brugge JS. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature. 2012;482:410–13. doi: 10.1038/nature10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–94. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 42.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 43.Roepstorff K, Grandal M, Henriksen L, Knudsen S, Lerdrup M, et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–27. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Dempsey PJ, Coffey RJ. Basolateral targeting and efficient consumption of transforming growth factor-α when expressed in Madin-Darby canine kidney cells. J Biol Chem. 1994;269:16878–89. [PubMed] [Google Scholar]
- 44.Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGFα deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–78. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 45.Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–80. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 46.Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–78. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 47.Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, et al. Production and auto-induction of transforming growth factor-α in human keratinocytes. Nature. 1987;328:817–20. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- 48.Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, et al. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–38. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takemura T, Kondo S, Homma T, Sakai M, Harris RC. The membrane-bound form of heparin-binding epidermal growth factor–like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem. 1997;272:31036–42. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- 50.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–86. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Cao Z, Li C, Higginbotham JN, Franklin JL, Tabb DL, et al. Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis. Mol Cell Proteomics. 2008;7:1651–67. doi: 10.1074/mcp.M700155-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 52.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–55. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Franklin JL, Yoshiura K, Dempsey PJ, Bogatcheva G, Jeyakumar L, et al. Identification of MAGI-3 as a transforming growth factor-α tail binding protein. Exp Cell Res. 2005;303:457–70. doi: 10.1016/j.yexcr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 54.Dempsey PJ, Meise KS, Yoshitake Y, Nishikawa K, Coffey RJ. Apical enrichment of human EGF precursor in Madin-Darby canine kidney cells involves preferential basolateral ectodomain cleavage sensitive to a metalloprotease inhibitor. J Cell Biol. 1997;138:747–58. doi: 10.1083/jcb.138.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–18. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salido EC, Barajas L, Lechago J, Laborde NP, Fisher DA. Immunocytochemical localization of epidermal growth factor in mouse kidney. J Histochem Cytochem. 1986;34:1155–60. doi: 10.1177/34.9.2426343. [DOI] [PubMed] [Google Scholar]
- 57.Adrain C, Strisovsky K, Zettl M, Hu L, Lemberg MK, Freeman M. Mammalian EGF receptor activation by the rhomboid protease RHBDL2. EMBO Rep. 2011;12:421–27. doi: 10.1038/embor.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Investig. 2007;117:2260–67. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thebault S, Alexander RT, Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20:78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrag D, Chung KY, Flombaum C, Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97:1221–24. doi: 10.1093/jnci/dji242. [DOI] [PubMed] [Google Scholar]
- 61.Halim A, Nilsson J, Ruetschi U, Hesse C, Larson G. Human urinary glycoproteomics; attachment site specific analysis of N- and O-linked glycosylations by CID and ECD. Mol Cell Proteomics. 2012;11:M111 013649. doi: 10.1074/mcp.M111.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–26. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 63.Deleted in proof
- 64.Dempsey PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factor-α precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Exp Cell Res. 2003;285:159–74. doi: 10.1016/s0014-4827(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Franklin JL, Graves-Deal R, Jerome WG, Cao Z, Coffey RJ. Myristoylated Naked2 escorts transforming growth factor α to the basolateral plasma membrane of polarized epithelial cells. Proc Natl Acad Sci USA. 2004;101:5571–76. doi: 10.1073/pnas.0401294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C, Hao M, Cao Z, Ding W, Graves-Deal R, et al. Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-α–containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells. Mol Biol Cell. 2007;18:3081–93. doi: 10.1091/mbc.E07-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deleted in proof
- 68.Ding W, Li C, Hu T, Graves-Deal R, Fotia AB, et al. EGF receptor–independent action of TGF-α protects Naked2 from AO7-mediated ubiquitylation and proteasomal degradation. Proc Natl Acad Sci USA. 2008;105:13433–38. doi: 10.1073/pnas.0806298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deleted in proof
- 70.Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem. 1998;273:17258–68. doi: 10.1074/jbc.273.27.17258. [DOI] [PubMed] [Google Scholar]
- 71.Shoyab M, McDonald VL, Bradley JG, Todaro GJ. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate–treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA. 1988;85:6528–32. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, et al. The amphiregulin gene encodes a novel epidermal growth factor–related protein with tumor-inhibitory activity. Mol Cell Biol. 1990;10:1969–81. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 74.Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52:3224–27. [PubMed] [Google Scholar]
- 75.Brown CL, Coffey RJ, Dempsey PJ. The proamphiregulin cytoplasmic domain is required for basolateral sorting, but is not essential for constitutive or stimulus-induced processing in polarized Madin-Darby canine kidney cells. J Biol Chem. 2001;276:29538–49. doi: 10.1074/jbc.M102114200. [DOI] [PubMed] [Google Scholar]
- 76.Gephart JD, Singh B, Higginbotham JN, Franklin JL, Gonzalez A, et al. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic. 2011;12:1793–804. doi: 10.1111/j.1600-0854.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76a.Damstrup L, Kuwada SK, Dempsey PJ, Brown CL, Hawkey CJ, et al. Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. Br J Cancer. 1999;80:1012–19. doi: 10.1038/sj.bjc.6690456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komurasaki T, Toyoda H, Uchida D, Morimoto S. Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene. 1997;15:2841–48. doi: 10.1038/sj.onc.1201458. [DOI] [PubMed] [Google Scholar]
- 78.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–79. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–61. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 80.Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–84. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 81.Toyoda H, Komurasaki T, Uchida D, Morimoto S. Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. Biochem J. 1997;326(Pt. 1):69–75. doi: 10.1042/bj3260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukk M, Kapushesky M, Nikkila J, Parkinson H, Goncalves A, et al. A global map of human gene expression. Nat Biotechnol. 2010;28:322–24. doi: 10.1038/nbt0410-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–16. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baba I, Shirasawa S, Iwamoto R, Okumura K, Tsunoda T, et al. Involvement of deregulated epiregulin expression in tumorigenesis in vivo through activated Ki-ras signaling pathway in human colon cancer cells. Cancer Res. 2000;60:6886–89. [PubMed] [Google Scholar]
- 85.Zhu Z, Kleeff J, Friess H, Wang L, Zimmermann A, et al. Epiregulin is up-regulated in pancreatic cancer and stimulates pancreatic cancer cell growth. Biochem Biophys Res Commun. 2000;273:1019–24. doi: 10.1006/bbrc.2000.3033. [DOI] [PubMed] [Google Scholar]
- 86.Shigeishi H, Higashikawa K, Hiraoka M, Fujimoto S, Mitani Y, et al. Expression of epiregulin, a novel epidermal growth factor ligand associated with prognosis in human oral squamous cell carcinomas. Oncol Rep. 2008;19:1557–64. [PubMed] [Google Scholar]
- 87.Thogersen VB, Sorensen BS, Poulsen SS, Orntoft TF, Wolf H, Nexo E. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001;61:6227–33. [PubMed] [Google Scholar]
- 88.Gupta G, Nguyen D, Chiang A, Bos P, Kim J, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 89.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–37. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs B, De Roock W, Piessevaux H, Van Oirbeek R, Biesmans B, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27:5068–74. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 91.Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–59. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 92.Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, et al. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXΦ motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci USA. 2012;109:3820–25. doi: 10.1073/pnas.1117949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deleted in proof
- 94.Deleted in proof
- 95.Deleted in proof
- 96.Hobert M, Carlin C. Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J Cell Physiol. 1995;162:434–46. doi: 10.1002/jcp.1041620316. [DOI] [PubMed] [Google Scholar]
- 97.Hobert ME, Kil SJ, Medof ME, Carlin CR. The cytoplasmic juxtamembrane domain of the epidermal growth factor receptor contains a novel autonomous basolateral sorting determinant. J Biol Chem. 1997;272:32901–9. doi: 10.1074/jbc.272.52.32901. [DOI] [PubMed] [Google Scholar]
- 98.Coskun U, Grzybek M, Drechsel D, Simons K. Regulation of human EGF receptor by lipids. Proc Natl Acad Sci USA. 2011;108:9044–48. doi: 10.1073/pnas.1105666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brandli AW, Adamson ED, Simons K. Transcytosis of epidermal growth factor. The epidermal growth factor receptor mediates uptake but not transcytosis. J Biol Chem. 1991;266:8560–66. [PubMed] [Google Scholar]
- 100.Riese DJ, 2nd, Bermingham Y, van Raaij TM, Buckley S, Plowman GD, Stern DF. Betacellulin activates the epidermal growth factor receptor and erbB-4, and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-β. Oncogene. 1996;12:345–53. [PubMed] [Google Scholar]
- 101.Sasada R, Ono Y, Taniyama Y, Shing Y, Folkman J, Igarashi K. Cloning and expression of cDNA encoding human betacellulin, a new member of the EGF family. Biochem Biophys Res Commun. 1993;190:1173–79. doi: 10.1006/bbrc.1993.1173. [DOI] [PubMed] [Google Scholar]
- 102.Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, et al. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993;259:1604–7. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- 103.Dunbar AJ, Goddard C. Structure-function and biological role of betacellulin. Int J Biochem Cell Biol. 2000;32:805–15. doi: 10.1016/s1357-2725(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 104.Jackson L, Qiu T, Sunnarborg S, Chang A, Zhang C, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–16. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanderson MP, Erickson SN, Gough PJ, Garton KJ, Wille PT, et al. ADAM10 mediates ectodomain shedding of the betacellulin precursor activated by p-aminophenylmercuric acetate and extracellular calcium influx. J Biol Chem. 2005;280:1826–37. doi: 10.1074/jbc.M408804200. [DOI] [PubMed] [Google Scholar]
- 106.Stoeck A, Shang L, Dempsey PJ. Sequential and gamma-secretase-dependent processing of the betacellulin precursor generates a palmitoylated intracellular-domain fragment that inhibits cell growth. J Cell Sci. 2010;123:2319–31. doi: 10.1242/jcs.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iwamoto R, Mine N, Kawaguchi T, Minami S, Saeki K, Mekada E. HB-EGF function in cardiac valve development requires interaction with heparan sulfate proteoglycans. Development. 2010;137:2205–14. doi: 10.1242/dev.048926. [DOI] [PubMed] [Google Scholar]
- 108.Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA. 2003;100:3221–26. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–70. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 110.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–99. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 111.Freeman MR, Yoo JJ, Raab G, Soker S, Adam RM, et al. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Investig. 1997;99:1028–36. doi: 10.1172/JCI119230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–88. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 113.Braun AH, Coffey RJ. Lysophosphatidic acid, a disintegrin and metalloprotease-17 and heparin-binding epidermal growth factor-like growth factor in ovarian cancer: the first word, not the last. Clin Cancer Res. 2005;11:4639–43. doi: 10.1158/1078-0432.CCR-05-0973. [DOI] [PubMed] [Google Scholar]
- 114.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–93. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor–associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell-cell contact sites. J Cell Biol. 1995;129:1691–705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–12. [PubMed] [Google Scholar]
- 118.Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–61. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 119.Tian H, Biehs B, Warming S, Leong KG, Rangell L, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–59. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strachan L, Murison JG, Prestidge RL, Sleeman MA, Watson JD, Kumble KD. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J Biol Chem. 2001;276:18265–71. doi: 10.1074/jbc.M006935200. [DOI] [PubMed] [Google Scholar]
- 121.Dahlhoff M, Schafer M, Wolf E, Schneider MR. Genetic deletion of the EGFR ligand epigen does not affect mouse embryonic development and tissue homeostasis. Exp Cell Res. 2013;319:529–35. doi: 10.1016/j.yexcr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 122.Kochupurakkal BS, Harari D, Di-Segni A, Maik-Rachline G, Lyass L, et al. Epigen, the last ligand of ErbB receptors, reveals intricate relationships between affinity and mitogenicity. J Biol Chem. 2005;280:8503–12. doi: 10.1074/jbc.M413919200. [DOI] [PubMed] [Google Scholar]
- 123.Dahlhoff M, Muller AK, Wolf E, Werner S, Schneider MR. Epigen transgenic mice develop enlarged sebaceous glands. J Investig Dermatol. 2010;130:623–26. doi: 10.1038/jid.2009.251. [DOI] [PubMed] [Google Scholar]
- 124.Shelly M, Mosesson Y, Citri A, Lavi S, Zwang Y, et al. Polar expression of ErbB-2/HER2 in epithelia: bimodal regulation by Lin-7. Dev Cell. 2003;5:475–86. doi: 10.1016/j.devcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 125.He C, Hobert M, Friend L, Carlin C. The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, including a dominant signal with a polyproline core. J Biol Chem. 2002;277:38284–93. doi: 10.1074/jbc.M104646200. [DOI] [PubMed] [Google Scholar]
- 126.Ryan S, Verghese S, Cianciola NL, Cotton CU, Carlin CR. Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways. Mol Biol Cell. 2010;21:2732–45. doi: 10.1091/mbc.E09-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cotton CU, Hobert ME, Ryan S, Carlin CR. Basolateral EGF receptor sorting regulated by functionally distinct mechanisms in renal epithelial cells. Traffic. 2013;14:337–54. doi: 10.1111/tra.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dillon C, Creer A, Kerr K, Kumin A, Dickson C. Basolateral targeting of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal but independent of the C-terminal ERBIN-binding domain. Mol Cell Biol. 2002;22:6553–63. doi: 10.1128/MCB.22.18.6553-6563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, et al. The juxtamembrane region of the EGF receptor functions as an activation domain. Mol Cell. 2009;34:641–51. doi: 10.1016/j.molcel.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318:124–34. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang YN, Hung MC. Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family. Cell Biosci. 2012;2:13. doi: 10.1186/2045-3701-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carpenter G, Liao HJ. Trafficking of receptor tyrosine kinases to the nucleus. Exp Cell Res. 2009;315:1556–66. doi: 10.1016/j.yexcr.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 134.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–72. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liao HJ, Carpenter G. Regulated intramembrane cleavage of the EGF receptor. Traffic. 2012;13:1106–12. doi: 10.1111/j.1600-0854.2012.01371.x. [DOI] [PubMed] [Google Scholar]
- 136.Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929–39. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen QQ, Chen XY, Jiang YY, Liu J. Identification of novel nuclear localization signal within the ErbB-2 protein. Cell Res. 2005;15:504–10. doi: 10.1038/sj.cr.7290320. [DOI] [PubMed] [Google Scholar]
- 138.Wang YN, Yamaguchi H, Hsu JM, Hung MC. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene. 2010;29:3997–4006. doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wild-Bode C, Fellerer K, Kugler J, Haass C, Capell A. A basolateral sorting signal directs ADAM10 to adherens junctions and is required for its function in cell migration. J Biol Chem. 2006;281:23824–29. doi: 10.1074/jbc.M601542200. [DOI] [PubMed] [Google Scholar]
- 140.Merchant NB, Voskresensky I, Rogers CM, LaFleur B, Dempsey PJ, et al. TACE/ADAM-17: a component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–91. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahimkar RM, Baricos WH, Visaya O, Pollock AS, Lovett DH. Identification, cellular distribution and potential function of the metalloprotease-disintegrin MDC9 in the kidney. J Am Soc Nephrol. 2000;11:595–603. doi: 10.1681/ASN.V114595. [DOI] [PubMed] [Google Scholar]
- 142.Marcello E, Gardoni F, Mauceri D, Romorini S, Jeromin A, et al. Synapse-associated protein-97 mediates α-secretase ADAM10 trafficking and promotes its activity. J Neurosci. 2007;27:1682–91. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335:225–28. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Deleted in proof
- 145.Deleted in proof
- 146.Deleted in proof
- 147.Deleted in proof
- 148.McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-α. J Biol Chem. 2002;277:42603–12. doi: 10.1074/jbc.M206487200. [DOI] [PubMed] [Google Scholar]
- 149.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol Cell Biol. 2004;24:5172–83. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh B, Schneider M, Knyazev P, Ullrich A. UV-induced EGFR signal transactivation is dependent on proligand shedding by activated metalloproteases in skin cancer cell lines. Int J Cancer. 2009;124:531–39. doi: 10.1002/ijc.23974. [DOI] [PubMed] [Google Scholar]
- 151.Semino CE, Kamm RD, Lauffenburger DA. Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp Cell Res. 2006;312:289–98. doi: 10.1016/j.yexcr.2005.10.029. [DOI] [PubMed] [Google Scholar]