Abstract
Hormones are critical for the development, maturation, and maintenance of physiological systems; therefore, understanding their involvement during maturation of the brain is important for the elucidation of mechanisms by which adults become behaviorally competent. Changes in exogenous and endogenous factors encountered during sexual maturation can have long lasting effects in mature adults. In this study, we investigated the role of the gonadotropic hormone, juvenile hormone (JH), in the modulation of adult behaviors in Drosophila. Here we utilized methoprene (a synthetic JH analog) and precocene (a JH synthesis inhibitor) to manipulate levels of JH in sexually immature male and female Drosophila with or without decreased synthesis of neuronal dopamine (DA). Locomotion and courtship behavior were assayed once the animals had grown to sexual maturity. The results demonstrate a sexually dimorphic role for JH in the modulation of these centrally controlled behaviors in mature animals that is dependent on the age of the animals assayed, and present DA as a candidate neuronal factor that differentially interacts with JH depending on the sex of the animal. The data also suggest that JH modulates these behaviors through an indirect mechanism. Since gonadotropic hormones and DA interact in mammals to affect brain development and later function, our results suggest that this mechanism for the development of adult behavioral competence may be evolutionarily conserved.
Keywords: juvenile hormone, locomotion, courtship, dopamine, female receptivity, sexual maturation
Introduction
Hormones are important throughout the lifecycle for the development, maturation, and maintenance of physiological systems (McEwen, 1992). The adolescent developmental period is particularly vulnerable to both biological and environmental factors, since perturbations in nervous system development during this time will affect mature adult behaviors, such as reproduction (Romeo et al., 2002). Work in mammalian systems has shown that the actions of steroid hormones during puberty can influence neuron survival, neurogenesis, neurite outgrowth, synaptogenesis, receptor expression, neurotransmitter synthesis and neuronal excitability (Romeo, 2003), resulting in persistent changes in behavioral outcomes.
Several similarities exist between development of the mammalian and Drosophila neuroendocrine systems (see Hartenstein, 2006 for review). The ring gland is the major endocrine gland in Drosophila and is the site of synthesis of the hormone juvenile hormone (JH). While a fly that has just emerged from the pupal case looks like an adult fly, it will not participate in reproductive behaviors until 48 hours post-eclosion, evidence that the individual is not yet fully mature (Spieth, 1974); this period can be considered comparable to the mammalian adolescent stage. During this time, JH is required for the acquisition of reproductive behaviors. For example, male Caribbean fruit flies treated with JH or its synthetic analog, methoprene, engage in sexual signaling, release pheromone, and mate at younger ages, indicating JH controls reproductive maturity and sexual signaling (Teal et al., 2000). In females, JH is necessary to trigger vitellogenesis for ovarian development (Handler and Postlethwait, 1977). Previous work has also shown that JH has a direct effect on the modeling of developing neural circuitry (Malaterre et al., 2003), and studies in honeybees have demonstrated that JH is important for the neural plasticity required for the development of mature adult behaviors (Fahrbach and Robinson, 1996). While the roles of JH in metamorphosis have been investigated, more research is required to elucidate the role of JH in the adult brain of both males and females (Strambi et al., 1999).
Studies in various insect species have established that JH interacts with dopamine (DA) (Woodring and Hoffmann, 1994; Sasaki et al., 2012). Past studies have also implied the existence of direct interactions between DA and JH to effect the organization of neural circuitry in Drosophila (Gruntenko et al., 2005 and 2007), consistent with observations that DA also functions to mediate ovarian development, sexual receptivity, and fertility (Neckameyer, 1996). Elucidation of the mechanisms underlying these neuroendocrine interactions will facilitate understanding how the actions of hormones can have lasting effects on neuronal processes.
In this study we used pharmacological manipulation of JH in sexually immature adult males and females to demonstrate that JH affects locomotion and courtship, two centrally controlled behaviors (Strauss and Heisenberg, 1993 and Ryner et al., 1996). Recently, two JH receptors have been confirmed (Charles et al., 2011; Abdou et al., 2011): methoprene tolerant and germ-cell expressed, which work together to facilitate JH signaling. Since these receptors have only recently been identified, there are not yet well-characterized genetic reagents to manipulate JH signaling. However, topical administration of methoprene (a JH analog) or precocene (a JH synthesis inhibitor) has been successfully used to mimic or inhibit actions of JH, respectively (Landers and Happ, 1980; Wilson et al., 1983; Dubrovsky et al., 2000; Dubrovsky et al., 2002). Additionally, application of methoprene has been shown to be able to reverse the effects of precocene (e.g., Gellissen and Wyatt, 1981 and Dallai et al., 1997). The long-term effects of JH observed in this study are sexually and temporally dimorphic, and dependent on the level of neuronal DA. These studies demonstrate that, in Drosophila, manipulation of gonadotropic hormones in sexually immature individuals may have persistent effects on centrally controlled mature adult behaviors, and suggest that, as in mammals, JH may function as a gonadotropic hormone with a key role in the development of mature brain circuitry.
Materials and Methods
Fly Culture
Flies were maintained in glass pint bottles containing standard agar-cornmeal-yeast food at 25°C on a 12 hour light-dark cycle. Male and female progeny were collected immediately following eclosion and maintained separately in groups of < 20 under identical conditions for 3, 5, or 8 days as described for each experiment.
Fly Strains
All stocks were obtained from the Bloomington Stock Center (Indiana University, Bloomington) unless otherwise noted. w1118 is the parental strain for the Drosophila tyrosine hydroxylase RNAi transgenic line. pP{w+mW.hs = GawB}elavC155 (elavC155) is a pan-neuronal Gal4 which drives constitutive expression in post-mitotic neural tissues. CSwu is a wild-type Canton S strain established in the laboratory of Martin Heisenberg (University of Wurzburg, Germany) in 1978 and was a gift from Dr. J. Steven de Belle (DART Neuroscience). w*;UAScytoβ-galactosidase (UAS β-galalactosidase) was a gift from Enrique Massa (Texas A&M University, Kingsville, TX, USA). The RNAi line for Drosophila tyrosine hydroxylase (THK) was generated as previously described (Neckameyer and Bhatt, 2012) and used to decrease dopamine levels, since tyrosine hydroxylase is the rate limiting step for dopamine synthesis (Neckameyer and Quinn, 1989).
Pharmacological Treatments
Methoprene (methoprene acid) (Sigma Aldrich St. Louis, MO), a synthetic JH analog, was dissolved in acetone at a concentration of 1 mg/mL. Precocene I (Sigma Aldrich St. Louis, MO), an anti-juvenoid that inhibits JH synthesis, was diluted in acetone to a concentration of 1 mg/mL. 0.2 µL of either methoprene or precocene was applied to the abdomens of newly eclosed flies using a Hamilton syringe; animals were also treated in parallel with acetone alone as a vehicle control. Comparing all methoprene- and precocene-treated animals to acetone controls helped to account for any behavioral effects of acetone or CO2, which was used to anesthetize animals during collection and application of pharmacological agents. Application was completed during collection of newly eclosed flies, which were then allowed to recover from the CO2 anesthetic, and males and females were maintained separately in food vials for 3, 5 or 8 days.
Courtship Analysis
On the day of analysis, an experimental male or female fly was aspirated into a well of a 10 position mating chamber wheel (Figure 1) with a sexually mature (5–9 day old) wild-type (CSwu) fly of the opposite sex. Successful male reproductive behavior was assessed by determining latency to copulation and number of males initiating courtship. Female receptivity was measured by the latency to copulation and whether or not copulation took place (Neckameyer, 1998). Pairs were observed in the mating chamber wheel for 30 min, unless copulation occurred before that time. If the assay went for the full 30 min without copulation occurring, the female was recorded as non-receptive. If the male did not initiate courtship within 10 minutes, observation was not continued for that pair, and the male was recorded as failing to initiate courtship. The courtship behavioral paradigm has been previously described (Gailey et al., 1986). The data was recorded in real time by 4 individuals blind to the treatment conditions. n = 50 pairs that copulated.
Figure 1.
Image of a 10 position mating chamber wheel used for courtship analysis. Each chamber of the wheel is 1cm (diameter) by 0.5cm (height).
Locomotor Analysis
Male and female flies were collected, treated, and aged as described above. On the day of analysis, individual flies were aspirated into a 60mm petri dish marked with a 1 cm2 grid on the bottom. After a 30 second recovery period, the number of grid lines crossed was determined for the first two (exploratory locomotion) and last two (basal locomotion) minutes of a 15 minute observation period as previously described (Neckameyer, 1998; Neckameyer and Weinstein, 2005; Neckameyer and Matsuo, 2008). Data were recorded in real time by 8 different individuals blind to the treatment conditions. All individuals were trained by the same person to obtain consistency. Start times were staggered allowing for approximately 4 animals to be completed over a 30 minute analysis period, which permitted time to aspirate animals into the petri dish and record the data. n = 45.
Tyrosine Hydroxylase Enzymatic Activity Assay
Males and females were collected, treated, and aged as previously described. Once animals reached the desired age, they were quick-frozen in liquid nitrogen. Enzymatic activity of tyrosine hydroxylase was determined using 150 µg of crude protein extracted from head tissue as previously described (Neckameyer et al., 2005). n = 3–5 assays with each assay consisting of an average of 2–4 samples.
Quantification of β-galactosidase
Male and female elavC155/UAS β-galactosidase animals were collected, treated, and aged as previously described, then quick-frozen in liquid nitrogen. 25µg crude protein prepared from head tissue was incubated with 50mM potassium phosphate, 1 mM MgCl2, 1mM chlorophenol red-β-D-galactoparanaside (Sigma-Aldrich St. Louis, MO, USA), pH 7.5 for 45 minutes at 37°C, and absorbance at 574nm was determined. n = 6 for males and females at each age.
Statistical Analysis
All statistical analyses were performed using SPSS for Windows from IBM Corporation (Armonk, NY, USA) and represented visually in graphs made using GraphPad Prism (GraphPad Softward, Inc. La Jolla, CA, USA). For all statistical analysis, a 95% confidence interval was used.
Results
Long-Term Neuronal Effects of JH Manipulation
To investigate the effects of JH on the maturing brain, newly eclosed elavC155/w1118 animals were treated with acetone (to serve as vehicle control), methoprene, or precocene immediately following eclosion. elavC155/w1118 animals were used for these experiments to provide an appropriate control for the transgenic lines used in the later experiments. Following pharmacological manipulation of JH, animals were then aged for 3 (cusp of sexual maturity), 5 (sexually mature), or 8 (older) days prior to behavioral analysis. Reports spanning across several species have demonstrated that methoprene can mimic the actions of JHIII (the most biologically active form of JH) (for example, Berger et al., 1992; Wilson and Fabian, 1986; Raikhel and Lea, 1990; Ahl and Brown, 1991; Noriega et al., 1997, amongst others). Additonally, methoprene has been shown to bind to the methoprene tolerant JH receptor (Miura et al. 2005; Charles et al., 2011). These data have validated the use of both methoprene and precocene to perturb JH signaling in vivo. However, the relative degree of saturation of the JH receptor in the adult stage is not known. In addition, since the pool of available JH in any tissue at a given time is unknown, the extent of reduction in JH synthesis after precocene treatment cannot be precisely measured (Brendena et al., 2011). However, both methoprene and precocene were effective in our studies in producing behavioral changes. The multiple effects of JH manipulation on behavior are summarized in Table 1.
Table 1.
Manipulation of JH alters centrally controlled behaviors. Males and females were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to behavioral analysis. For latency to copulation, animals were paired with a wild-type animal of the opposite sex. Animals were observed until copulation occurred or for 30 minutes. n = 50 animals that copulated for each population. Alternatively, animals were assayed for exploratory or basal locomotion, referring to the first and last 2 minutes of a 15 minute observation period, respectively.
| Sex | Age (days) |
Treatment | Effects on Latency to Copulation |
Effects on Exploratory Locomotion |
Effects on Basal Locomotion |
|---|---|---|---|---|---|
| Female | 3 | Methoprene | none | none | * ↓ |
| Precocene | none | none | none | ||
| 5 | Methoprene | none | *** ↓ | * ↓ | |
| Precocene | * ↑ | ** ↑ | none | ||
| 8 | Methoprene | none | none | none | |
| Precocene | *** ↑ | none | none | ||
| Male | 3 | Methoprene | none | none | none |
| Precocene | * ↓ | *** ↑ | none | ||
| 5 | Methoprene | none | none | none | |
| Precocene | none | *** ↑ | none | ||
| 8 | Methoprene | * ↑ | none | none | |
| Precocene | none | *** ↑ | none |
One-Way ANOVA,
p < 0.05,
p < 0.01
p < 0.001, with arrows indicating whether there was an increase or decrease.
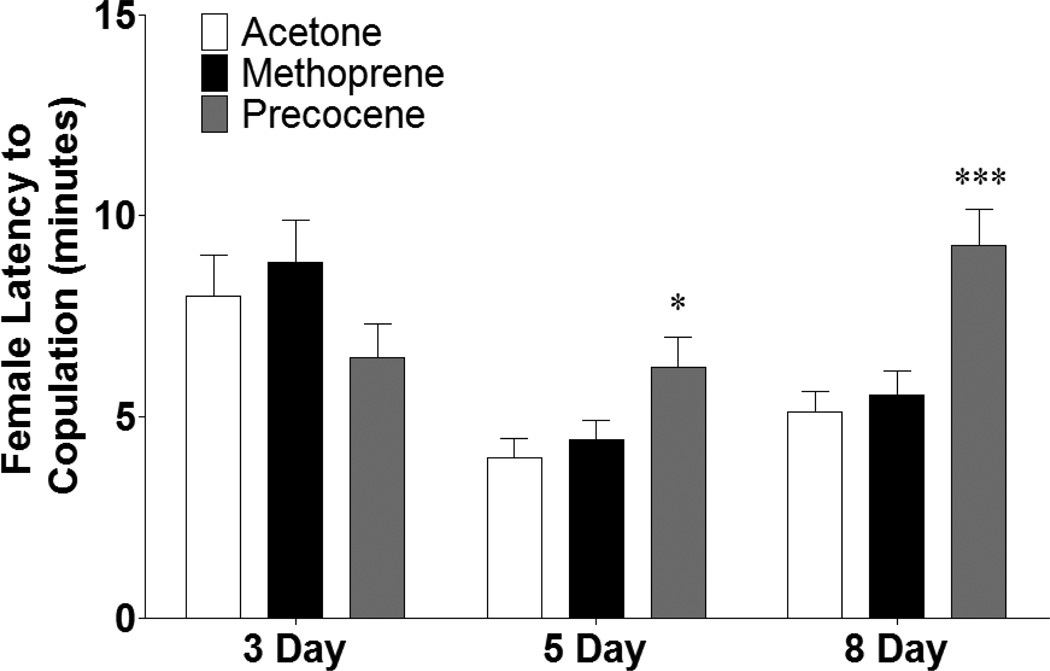
Several parameters of courtship behavior were analyzed based on known roles for JH in mature sexual signaling and reproductive behaviors. Even though the full mature pheromonal complement and array of sexual behaviors is attained by 3 days (Wicker and Jallon, 1995; Cook, 1973; Pitnick et al., 1995), the brains are still maturing and their responses (i.e. how quickly the female accepts the male or how long it takes for the male to initiate courtship) may differ from older flies, consistent with our observation that 3 day old acetone treated control females displayed an increased latency to copulation relative to older acetone treated females (Figure 2). At both 5 and 8 days, treatment with precocene increased latency to copulation (p < 0.05 and p < 0.001, respectively), while methoprene had no effect (Figure 2). No significant difference was observed in initiation of courtship by wild-type males placed with treated females, or in whether or not copulation occurred (data not shown).
Figure 2.
Early manipulation of JH alters latency to copulation in females in a temporally-dimorphic manner. Females were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to being paired with a wild-type male for courtship analysis. Animals were observed until copulation occurred or for 30 minutes. n = 50 animals that copulated for each population. One-Way ANOVA, * p < 0.05, *** p < 0.001.
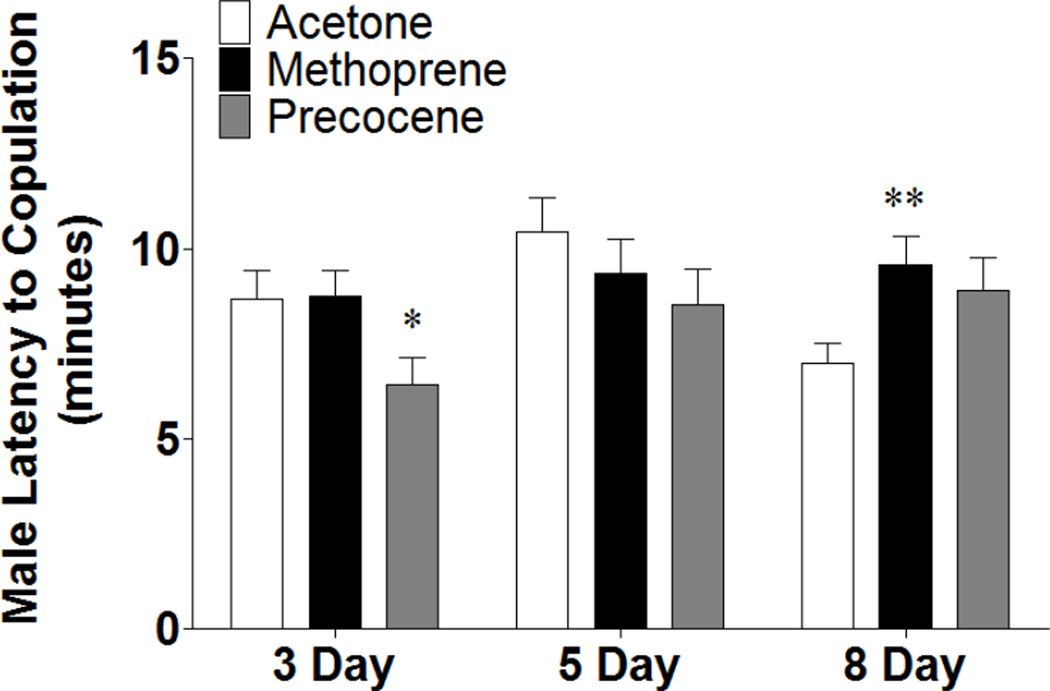
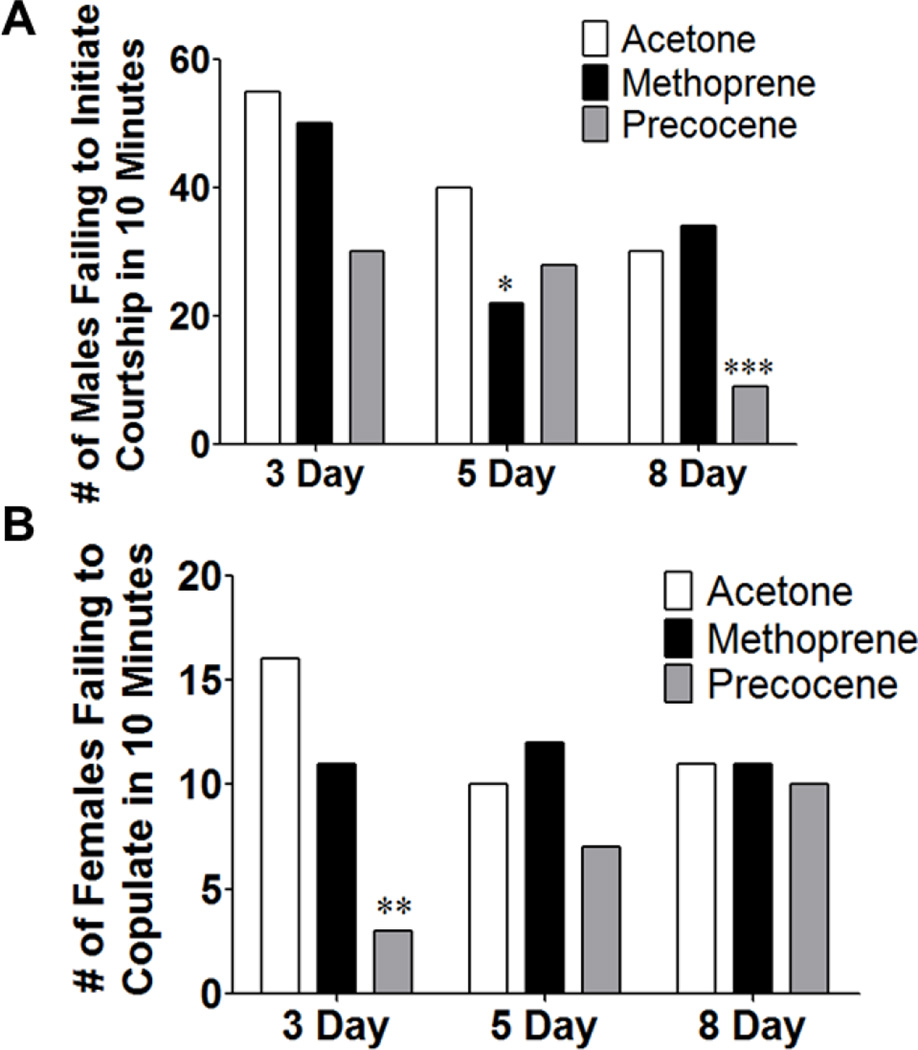
When treated males were paired with wild-type females, there was not an obvious decrease in latency to copulation with increasing age as there was with females (Figure 3). 3 day old males treated with precocene displayed a decreased latency to copulation (p < 0.05), and treatment with methoprene caused an increased latency to copulation in 8 day old males (p < 0.01) (Figure 3). The lack of an age effect on latency to copulation could suggest that males attain sexual maturity earlier than females; however, other observations were consistent with our belief that the brain of 3 day old animals is not yet fully sexually mature. For example, 3 day old males are capable of initiating courtship, but the percentage increases with increasing age and more robust sexual maturity (Figure 4A), possibly suggesting strengthening of synaptic connections with age. Early treatment with methoprene assayed at day 5 and with prococene assayed at day 8 resulted in fewer males initiating courtship (p < 0.05 and p < 0.001, respectively); which correlated with changes in latency to copulation that were observed in females, suggesting that precocene treatment delayed sexual maturation. There were also differences in whether or not copulation ultimately occurred for the treated males that did initiate courtship (Figure 4B). Additionally, wild-type females appeared to be more receptive to older males. In this case, the actions of precocene significantly increased the percentage of females successfully copulating with 3 day old males (p < 0.05), indicating that the effects of precocene on sexual maturation are complex.
Figure 3.
Early manipulation of JH alters latency to copulation in males in an age-specific manner. Males were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to being paired with a wild-type female for courtship analysis. Animals were observed until copulation occurred or for 30 minutes. n = 50 animals that copulated for each population. One-Way ANOVA, * p < 0.05, ** p < 0.01.
Figure 4.
Early manipulation of JH in males alters male initiation of courtship and female receptivity in a temporally dimorphic manner. Males were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to being paired with a wild-type female for courtship analysis. (A) The number of males that failed to initiate courtship within 10 minutes was determined. (B) The number of females that failed to copulate after 30 minutes was determined. n = 50 animals that copulated for each population, Chi-square with 50 animals copulating and the number corresponding to how many failed to either initiate (A) or copulate (B) with acetone treatment used as the expected values, * p < 0.05, ** p < 0.001.
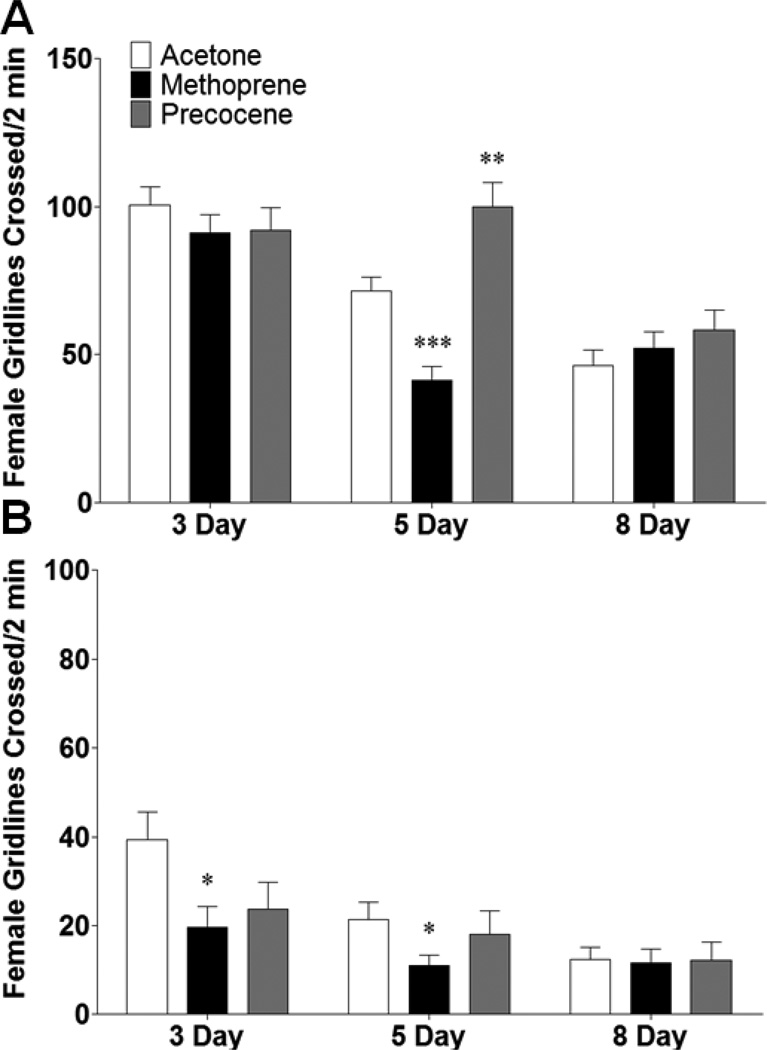
Locomotor behavior was also assessed, since this is a neuronally controlled behavior unrelated to gonadal development. Behavior was assessed during the first two (exploratory) and last two (basal) minutes of a 15 minute observation period; as for most species, there is decreased locomotion after adaptation to a novel environment (Liu et al., 2007; Neckameyer and Matsuo, 2008). There was an overall trend towards decreasing locomotion with increasing age for both exploratory and basal locomotion for acetone treated females (Figure 5). For exploratory locomotion, the only age at which there was a significant change in response to JH manipulation was at 5 days: methoprene decreased (p < 0.001) and precocene increased exploratory locomotion (p < 0.01) (Figure 5A). Methoprene caused a decrease in basal locomotion in 3 and 5 day old females (p < 0.05), but precocene had no effect (Figure 5B). The observed result that JH maniupulation had different effects on exploratory and basal locomotion suggests that these two locomotor behaviors are not regulated by identical circuits.
Figure 5.
Early manipulation of JH alters female locomotion in an age-dependent manner. Females were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to being assayed for (A) exploratory and (B) basal locomotion, which refers to the first and last 2 minutes of a 15 minute observation period, respectively. n = 45 animals for each population. One-Way ANOVA, * p < 0.05, ** p < 0.01, *** p < 0.001.
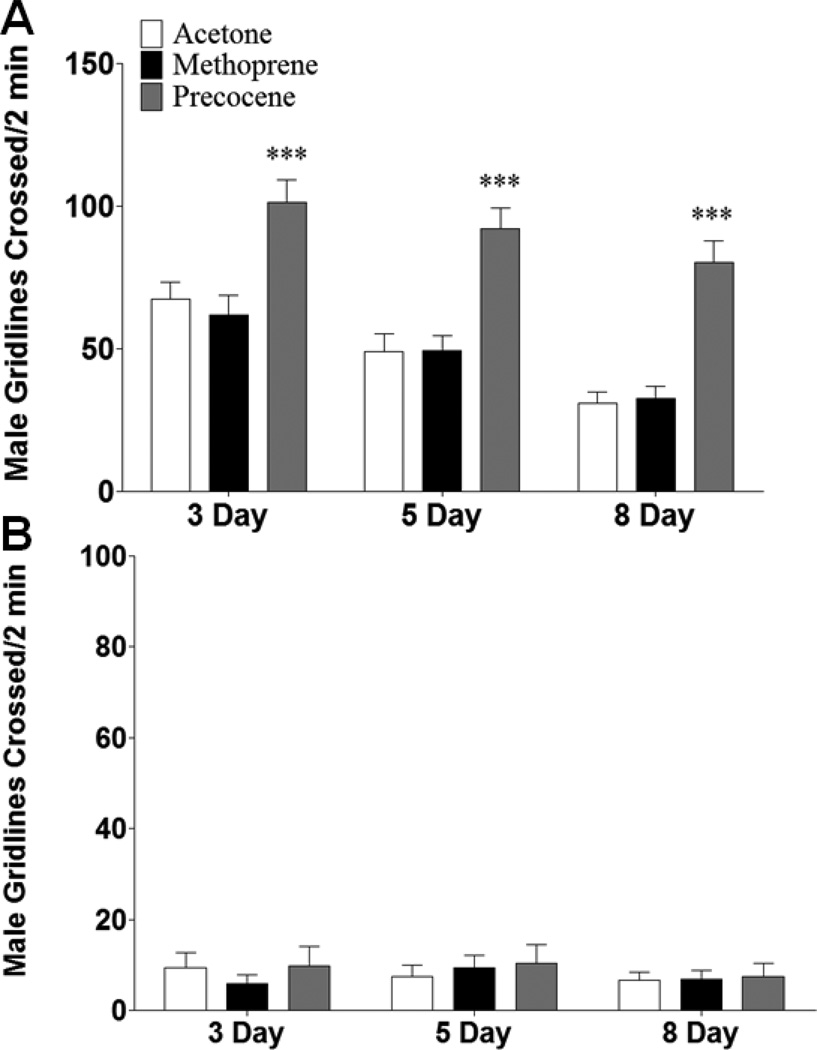
Acetone treated males assayed for exploratory locomotion displayed an overall trend of decreased locomotion with increasing age, while basal locomotion was unchanged; for both, males displayed decreased locomotion relative to females (Figure 6). At all ages, precocene treated males increased their exploratory locomotion from that of age-matched acetone treated males (p < 0.001 for all, Figure 6A).
Figure 6.
Early manipulation of JH alters male locomotion in a temporally-dimorphic manner. Males were collected and treated with acetone, methoprene, or precocene immediately following eclosion and then aged for 3, 5, or 8 days prior to being assayed for (A) exploratory and (B) basal locomotion, which refers to the first and last 2 minutes of a 15 minute observation period, respectively. n = 45 animals for each population. One-Way ANOVA, *** p < 0.001.
Effects of JH Manipulation in Animals with Altered Neuronal Dopamine Synthesis
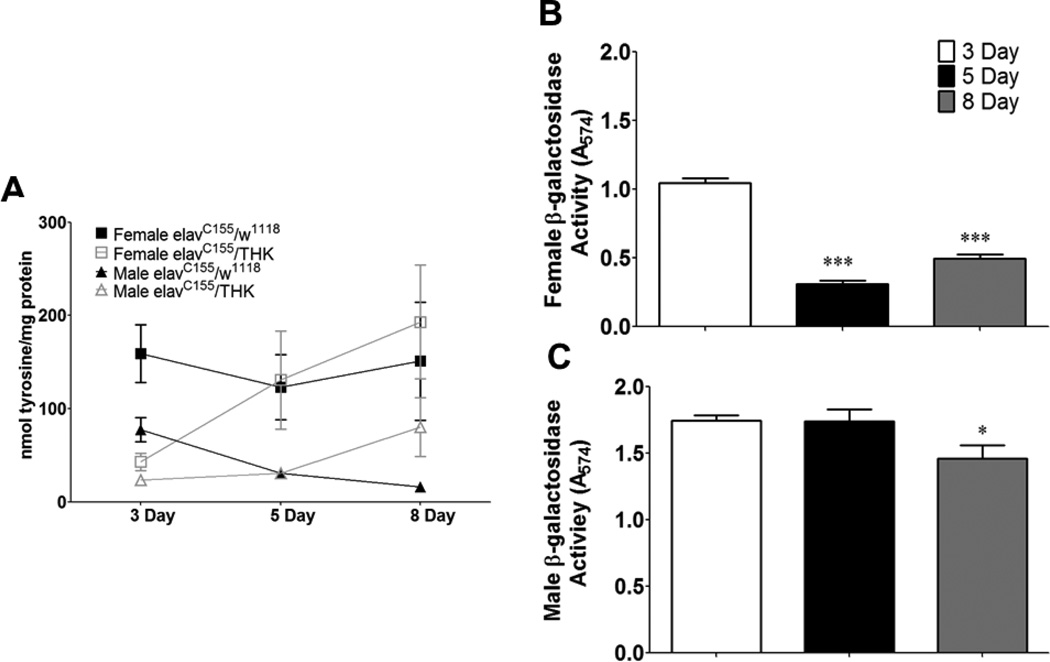
To identify whether there were interactions between JH and neuronal DA, a tyrosine hydroxylase RNAi (THK) was used to reduce tyrosine hydroxylase (TH) levels (and thus DA synthesis) within post-mitotic neurons. This transgenic line has been used successfully to decrease DA synthesis in Drosophila larvae and adults (Neckameyer and Bhatt, 2012; Argue and Neckameyer, 2013a and b). The transgene was targeted specifically to the brain using the pan-neuronal driver elavC155, and TH enzymatic activity was assayed in head tissue from 3, 5, and 8 day old control (elavC155/w1118) and elavC155/THK animals (Figure 7A). However, TH activity was only decreased in 3 day old elavC155/THK fly heads (p < 0.05). At 5 days, elavC155/THK displayed similar activity as the controls, and by 8 days, elavC155/THK actually showed slightly higher activity than the controls, but the variance was too great for this increase to reach statistical significance. Because TH activity was decreased at 3 days, and has previously been shown to decrease activity when similarly targeted to the larval brain (Neckameyer and Bhatt, 2012), we hypothesized that differences in expression of elavC155- Gal4 at ages 5 and 8 days may have been responsible for these results. This apparent decrease in expression of the elavC155 Gal4 driver with increasing age was quantified by crossing this line with one carrying a UAS-β-galactosidase reporter and assaying β-galactosidase activity (measured by absorbance at 547mm) in head tissue from 3, 5 and 8 day old males and females (Figure 7B,C). Activity was significantly decreased in 5 and 8 day old females (p < 0.001, Figure 7B) and in 8 day old males (p < 0.05, Figure 7C). All further analyses were therefore limited to 3 day old animals, since the elavC155 driver was still strongly expressed at that age in both sexes.
Figure 7.
(A) TH enzymatic activity in head tissue from control and TH-knockdown animals. Tyrosine hydroxlase activity was measured from crude protein extracted from head tissues from control (elavC155/w1118) and elavC155/THK (tyrosine hydroxylase RNAi) males and at 3, 5 and 8 days post-eclosion. elavC155/THK females and males are shown as gray boxes and triangles, respectively, and elavC155/w1118 females and males are shown as black boxes and triangles, respectively. A 2-way ANOVA comparing Genotype X Age was not significant for females or males. Bonferroni post tests did reveal a significant difference between the two genotypes at 3 days of age for both sexes (p < 0.05, n = 3–5 assays with each assay consisting of an average of 2–4 samples).
(B and C) elavC155 expression decreases with increasing age in both males and females. A574 was determined from crude protein extract from head tissue following a 45 minute incubation in 1mM chlorophenol red-β-D_galactoparanaside. B, females; C, males. n = 6, One-Way ANOVA, * p < 0.05, *** p < 0.001.
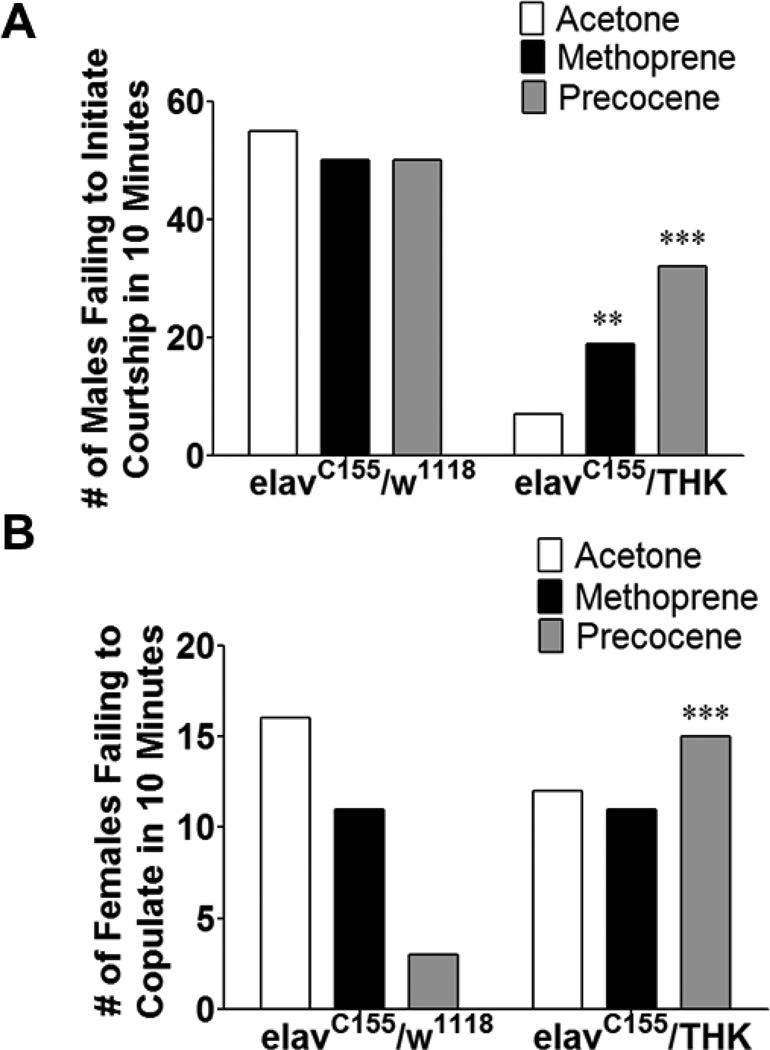
To investigate the effect of early manipulation of JH in flies with decreased neuronal DA synthesis, newly eclosed elavC155/w1118 and elavC155/THK animals were treated with methoprene or precocene, then aged for 3 days prior to behavioral analysis. Table 2 summarizes behavioral parameters in which there was a significant difference in the response to JH manipulation in animals with reduced neuronal DA synthesis compared to control animals. Specific differences are shown graphically for those behaviors in which there was a significant interaction between the level of neuronal DA synthesis and JH treatment. For courtship, acetone treated elavC155/THK males displayed a significant difference in their initiation of courtship (Figure 8A). Acetone treated elavC155/THK males were not as likely to initiate courtship compared to acetone treated elavC155/w1118 males. Both methoprene (p < 0.01) and precocene (p < 0.001) treatment resulted in increased numbers of elavC155/THK males initiating courtship; however, these increases resulted in only a partial rescue of the impaired phenotype. Methoprene and precocene treatment in elavC155/w1118 males decreased the number of wild-type females willing to copulate, but maintained (methoprene) or increased the number copulating (precocene, p < 0.001) when applied to elavC155/THK males (Figure 8B). These results suggested that both DA and JH are important for the development of the neural circuitry mediating courtship behaviors. elavC155/THK females and males assayed for latency to copulation following treatment with methoprene or precocene displayed no significant interactions between genotype and treatment when compared to sex-matched elavC155/w1118 animals (data not shown). For females, there were also no differences in the wild-type males’ initiation of courtship with the female, or in whether or not copulation occurred if courtship was initiated (data not shown).
Table 2.
Reduced neuronal DA alters behavioral responses to JH manipulation. elavC155/w1118 and elavC155/THK males and females were collected and treated with acetone, methoprene, or precocene immediately following eclosion, then aged for 3 days prior to analysis of courtship or locomotion. All animals were placed with a wild-type individual of the opposite sex for courtship analysis. Initiation of courtship refers to the number of males that failed to initiate courtship within 10 minutes. Females failing to copulate refers to the number of females that failed to copulate after 30 minutes. Exploratory and basal locomotion refer to the first and last two minutes of a 15 minute observation period, respectively. n = 50 pairs that copulated for each population for courtship, or n = 45 for locomotion.
| Sex | Treatment | Latency to Copulation |
Male Initiation of Courtship |
Females Failing to Copulate |
Exploratory Locomotion |
Basal Locomotion |
|---|---|---|---|---|---|---|
| Female | Methoprene | ns | ns | ns | ns | ns |
| Precocene | ns | ns | ns | ns | ns | |
| Male | Methoprene | ns | * | ns | ns | ** |
| Precocene | ns | *** | * | * | ns |
2-Way ANOVAs (Genotype X Treatment),
p < 0.05,
p < 0.01,
p < 0.001.
Figure 8.
Early manipulation with JH in males with reduced neuronal DA levels affects the male’s ability to initiate courtship and female receptivity. elavC155/w1118 and elavC155/THK males were collected and treated with acetone, methoprene, or precocene immediately following eclosion, then aged for 3 days prior to analysis of courtship with a wild-type female. (A) The number of males that failed to initiate courtship within 10 minutes was determined. (B) The number of females that failed to copulate after 30 minutes was determined. n = 50 pairs that copulated for each population. Chi-square using the ratio of the number of acetone to either methoprene or precocene treated elavC155/w1118 animals that failed to either initiate (A) or copulate (B) as expected values, ** p < 0.01, *** p < 0.001.
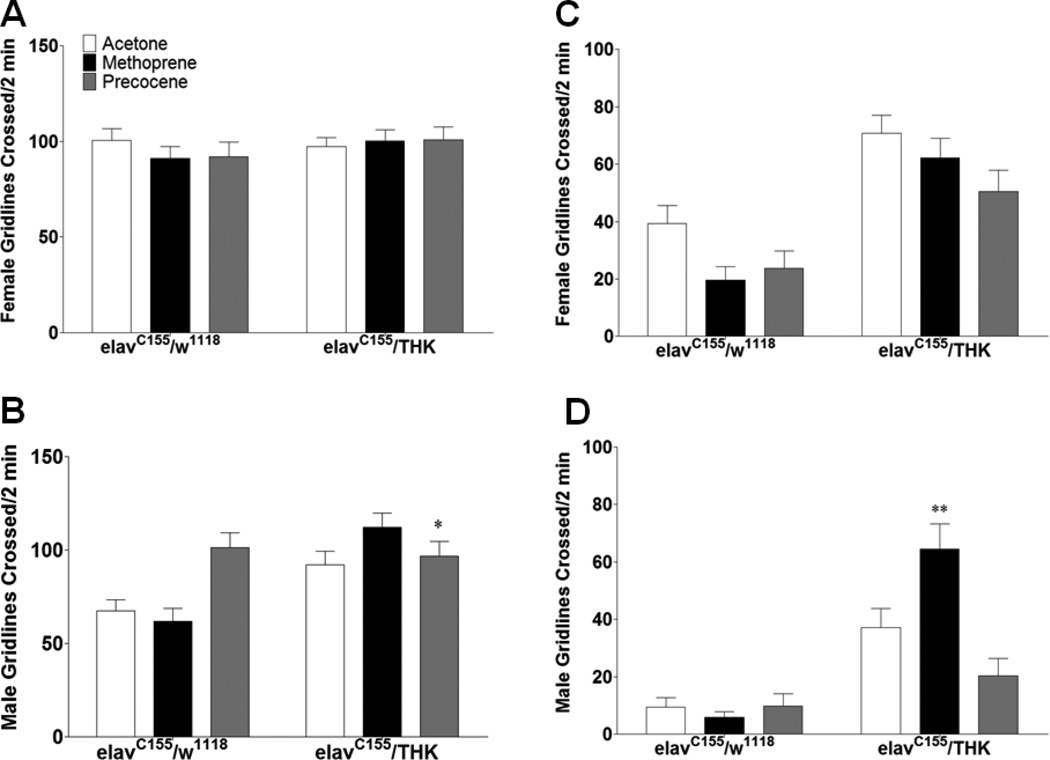
For locomotion, there was no significant difference in either exploratory (Figure 9A) or basal (Figure 9C) locomotion for females, regardless of treatment. Acetone treated elavC155/THK males assayed for exploratory locomotion displayed increased locomotion compared to acetone treated controls (Figure 9B), and increased their exploratory locomotion when treated with precocene; however, the elavC155/THK males’ already elevated locomotion was not altered by this treatment (p < 0.05). The different results observed following precocene treatment in elavC155/w1118 or elavC155/THK males are consistent with an interaction between DA and JH in development of these circuits. Acetone treated elavC155/THK males assayed for basal locomotion displayed increased locomotion compared to acetone treated controls (Figure 9D). When treated with methoprene, elavC155/THK males increased their locomotion, whereas methoprene treated control males did not significantly alter theirs (p < 0.01). These results also suggest an interaction between JH and DA, and reveal a sexual dimorphism in this interaction, since no effect was observed in females.
Figure 9.
Early manipulation with JH in females and males with reduced neuronal DA levels affects locomotion. elavC155/THK (A and C) females and (B and D) males were collected and treated with acetone, methoprene, or precocene immediately following eclosion, then aged for 3 days prior to analysis of (A and B) exploratory and basal (C and D) locomotion. n = 45, 2-way Two-Way ANOVA (Genotype X Treatment) with Bonferroni posttest, * p < 0.05, ** p < 0.01.
Discussion
Our results show that manipulation of JH immediately following eclosion affected central behaviors in the sexually mature adult, indicating that this time is critical for development and plasticity. Numerous research studies and observations of human behavior have demonstrated that adolescence, a developmental period in which mammals undergo the transition to become a sexually mature adult, is particularly vulnerable (reviewed in Somerville et al., 2010). For example, testosterone in adolescent males has been shown to regulate axon diameter, resulting in changes in neurotransmission and cell metabolism (reviewed in Paus, 2010). Similarly, estrogens have been shown to regulate myelination, neuronal survival (reviewed in Arevalo et al., 2010), and neuronal excitability (reviewed in Smith, 1994). We hypothesized that manipulation of JH during the sensitive developmental window immediately after eclosion would alter interactions with factors that mediate mature behaviors, as opposed to JH acting directly to influence a given behavior. Therefore, it was expected that the observed results would demonstrate variability rather than a persistent increase or decrease in a given behavioral measure. An indirect mechanism of action is further supported by the relatively short half-lives of both methoprene and precocene and the fact that we observed behavioral effects 8 days post-treatment (Schooley et al., 1975; McCaffery and McDowell, 2006). For example, treatment of females with precocene increased latency to copulation 5 and 8 days post-eclosion, yet displayed no effect 3 days post-eclosion. Interestingly, in males, precocene treatment decreased latency to copulation at the cusp of sexual maturity (3 days post-eclosion), yet the effect did not persist in older animals, which showed an increased latency to copulation in response to early methoprene treatment. The observation that a decrease in JH levels shortly after eclosion resulted in improved performance at 3 days, but depressed performance of the same behavior at 8 days, suggests differences in the brain milieu in 3 day old compared to 8 day old animals, implying a differential susceptibility to the changes that occurred as a result of the early JH manipulation. When male initiation of courtship was assessed, there were no observed effects until 8 days post-eclosion, in which precocene treated males displayed increased initiation of courtship. Observation of the number of females that failed to copulate with a male that had received hormonal manipulation showed an effect at 3 days post-eclosion, with females copulating more often with precocene-treated males. While seemingly small differences in latency to copulation and rates of initiation and successful copulation may have little impact in a laboratory mating chamber, they could have a much larger impact in nature where females can easily escape from an undesirable mate (Ewing and Ewing 1984, Gromko and Markow, 1993) and where changes in mate selection can have consequences on the fitness of the population. Therefore, the observed changes in courtship behavior following manipulation of JH could, in some cases, be considered failures in the acquisition of sexual competency.
It is possible that the effects on courtship behavior could be related to effects of JH manipulation on ovarian development or pheromone synthesis. The role for JH in ovarian development has been well established (Handler and Postlethwait, 1977), and while it is feasible that our manipulations of JH altered ovarian development, we do not believe that feedback between the ovaries and brain regions necessary for normal courtship behavior were primarily responsible for the observed changes in latency to copulation. Decreased synthesis of neuronal DA resulted in changes in courtship behaviors; however, this manipulation does not affect ovarian development (unpublished observations), suggesting that these effects must be occurring centrally. JH is also known to be involved in pheromone synthesis (Liu et al., 2008). Immature flies contain long-chain hydrocarbons that disappear and are replaced with shorter-chain hydrocarbons by 3 days post-eclosion (Wicker and Jallon, 1995). When JH levels are reduced, males have reduced levels of aggregation pheromone and females have reduced levels of courtship pheromone (Liu et al., 2008). We would have expected that changes in pheromone synthesis would either be consistent amongst the different age groups, or, if there were an immediate effect, it would have disappeared 8 days after the treatment. The observed variations in courtship behaviors amongst the different age groups suggests that while pheromone synthesis could contribute to the alterations in behavior, it was not the only factor responsible for these changes.
While effects on latency to copulation could be attributed to changes locomotion, the summary of the behavioral effects (Table I) clearly demonstrates that there was no correlation between courtship behaviors and locomotion. The only apparent instance of a correlation was observed in females treated with precocene and assayed for exploratory locomotion. These females displayed an increase in locomotion, which could correlate with increased latency to copulation. However, we observed increased latency to copulation at 8 days without a corresponding change in locomotion, and the decreases seen for exploratory locomotion at 5 days and for basal locomotion at 3 and 5 days in response to treatment with methoprene did not correspond to changes in copulation. Similarly, males treated with precocene increased their exploratory locomotion at all three ages, yet there were no corresponding changes in copulation. Therefore, the changes we observed for locomotor behaviors were distinct from those for reproduction, and lend further support to our hypothesis that JH has diverse actions on multiple behavioral circuits. In support of this, numerous studies have demonstrated that gonadotropic hormones in mammals can alter neuronal synaptic transmission. One of the first events during the onset of puberty in mammals is an increase in gonadotropic releasing hormone (GnRH), resulting in increases in gonadotropin and leutinizing hormone, followed by increased gonadal hormones (Styne, 1994). Treating hippocampal slices with luteinizing hormone-releasing hormone resulted in changes in electrophysiological properties, suggesting that gonadotropic hormones can act as neurotransmitters or neuromodulators (Wong et al., 1990). Other studies demonstrated that treatment of hippocampal slices with GnRH resulted in dose-dependent changes in estradiol synthesis and spine synapse density, demonstrating synaptic changes in response to gonadotropic hormones (Prange-Kiel et al., 2008).
Previous studies have suggested DA can interact with JH (Gruntenko et al., 2005 and 2007), but these studies did not specifically compare changes occurring with age in both males and females. Our data suggests the interaction between DA and JH is sexually dimorphic. For male initiation of courtship, a decrease in neuronal DA synthesis resulted in an increase in male initiation of courtship. Both decreased and increased JH levels in these males resulted in fewer males initiating courtship. One potential explanation could be that inappropriate JH levels (whether too much or too little) activate a compensatory mechanism to normalize DA levels or DA signaling. It is important to note that in this scenario JH does not necessarily interact directly with DA, but rather is involved in the modulation of DA levels. It is also possible that JH regulates another signaling molecule that can compensate in the regulation of specific behaviors when there are insufficient levels of DA. We also saw that a decrease in neuronal DA synthesis in males increased success with females in terms of the number of pairs that successfully copulated. While treatment with methoprene had no effect on this aspect of courtship behavior, treatment with precocene increased the number of females that failed to copulate with the treated males. For male exploratory locomotion the decrease in neuronal dopamine ablated the increase in locomotion that was seen in control animals following early treatment with precocene. For basal locomotion treatment with methoprene further increased the already elevated locomotion in the males with decreased neuronal DA synthesis.
While none of the behaviors assayed at 3 days post-eclosion revealed an interaction between JH and DA in females, we feel confident that evidence of an interaction would have been observed at other ages. However, this and the initial data showing the effects of early manipulation of JH in sexually mature individuals, do illustrate sex differences in its actions. Although males and females were not compared directly in order to focus more specifically on the effects of JH, it is clear from the data summary in Table 1 that there is actually only one instance (exploratory locomotion observed 5 days post-eclosion in response to early treatment with precocene) in which both sexes had a similar response to the treatment. In every other case, males and females had distinct responses to the manipulations of JH. It will be important, as we move forward in elucidating factors that interact with JH and its targets, to investigate how these components differ across different ages and the sexes to ultimately produce appropriate mature adult behavior.
Conclusions
Our studies have revealed a sexually and temporally dimorphic role for JH in the modulation of sexually mature, centrally controlled behaviors in Drosophila. We have presented DA as a neuronal factor that can interact with JH in a complex mechanism in which the two potentially modulate levels of one another; this interaction is also sexually dimorphic. Similar mechanisms have been demonstrated in mammalian species, suggesting that this role for gonadotropic hormones and their neuronal interactions in the modulation of centrally controlled behaviors may be evolutionarily conserved across species. Further research elucidating the specific nature of the interaction between DA and JH and identification of other factors that interact with JH will be useful for understanding the acquisition of sexually mature behavior and the role of gonadotropic hormones in this process.
Highlights.
early life manipulation of juvenile hormone alters adult behavior
effects of early life juvenile hormone manipulation are sexually dimorphic
effects of juvenile hormone manipulation depend on neuronal dopamine levels
elavC155 Gal4 expression decreases with increasing age
some actions of juvenile hormone are similar to mammalian gonadotropic hormones
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdou MA, He Q, Wen D, Zyaan O, Wang J, Xu J, Baumann AA, Joseph J, Wilson TG, Li S, Wang J. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011;41:938–945. doi: 10.1016/j.ibmb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Ahl JS, Brown JJ. The effect of juvenile hormone III, methyl farnesoate, and methoprene on Na/K-ATPase activity in the larvae of the brine shrimp, Artemia. Comp Biochem Physiol A Comp Physiol. 1991;100:155–158. doi: 10.1016/0300-9629(91)90199-m. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Argue KJ, Neckameyer WS. Sexually dimorphic recruitment of dopamine neurons into the stress response circuitry in Drosophila melanogaster. Behav. Neurosci. 2013(a) doi: 10.1037/a0033807. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, Neckameyer WS. Temporally dimorphic recruitment of dopamine neurons into stress response circuitry in Drosophila. Behav. Neurosci. 2013(b) doi: 10.1037/a0033602. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendena WG, Zhang J, Burtenshaw SM, Tobe SS. Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen. Comp. Endocrinol. 2011;172:56–61. doi: 10.1016/j.ygcen.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Berger EM, Goudie K, Klieger L, Berger M, DeCato R. The juvenile hormone analogue, methoprene, inhibits ecdysterone inductions of small heat shock protein gene expression. Dev. Biol. 1992;151:410–418. doi: 10.1016/0012-1606(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. USA. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RM. Physiological factors in the courtship processing of Drosophila melanogaster. J. Insect Physiol. 1973;19:397–406. doi: 10.1016/0022-1910(73)90114-5. [DOI] [PubMed] [Google Scholar]
- Dallai R, Baldari C, Marchini D, de Filippis T, Rosetto M, Manetti AG. Juvenile hormone regulates the expression of the gene encoding ceratotoxin a, an antibacterial peptide from the female reproductive accessory glands of the medfly Ceratitis capitata. J. Insect Physiol. 1997;43:1161–1167. doi: 10.1016/s0022-1910(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Drobrovsky EB, Dubrovskaya VA, Berger EM. Juvenile hormone signaling during oogenesis in Drosophila melanogaster. Insect. Biochem. Mol. Biol. 2002;32:1555–1565. doi: 10.1016/s0965-1748(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Dubrovsky EB, Dubrovskaya VA, Bilderback AL, Berger EM. The isolation of two juvenile hormone-inducible genes in Drosophila melanogaster. Dev. Biol. 2000;224:486–495. doi: 10.1006/dbio.2000.9800. [DOI] [PubMed] [Google Scholar]
- Ewing LS, Ewing AW. Courtship in Drosophila melanogaster: behavior of mixed-sex groups in large observation chambers. Behaviour. 1984;90:184–202. [Google Scholar]
- Fahrbach SE, Robinson GE. Juvenile hormone, behavioral maturation, and brain structure in the honey bee. 1996;18:102–114. doi: 10.1159/000111474. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Lacaillade RC, Hall JC. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav. Genet. 1986;16:375–405. doi: 10.1007/BF01071319. [DOI] [PubMed] [Google Scholar]
- Gellissen G, Wyatt GR. Production of lipophorin in the fat body of adult Locusta migratoria: comparison with vitellogenin. Can. J. Biochem. 1981;59:648–654. doi: 10.1139/o81-090. [DOI] [PubMed] [Google Scholar]
- Gromko MH, Markow TA. Courtship and remating in field populations of Drosophila. Anim. Behav. 1993;45:253–262. [Google Scholar]
- Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Bogomolova EV, Bownes M, Rauschenbach IY. Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine, and 20-hydroxyecdysone contents in Drosophila. Arch. Insect Biochem. Physiol. 2007;65:85–94. doi: 10.1002/arch.20187. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Saprykina ZV, Bownes N, Rauschenbach IY. Effects of dopamine on juvenile hormone metabolism and fitness in Drosophila virilis. J. Insect Physiol. 2005;51:959–968. doi: 10.1016/j.jinsphys.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Handler AM, Postlethwait JH. Endocrine control of vitellogenesis in Drosophila melanogaster: effects of the brain and corpus allatum. J. Exp. Zool. 1977;202:389–402. doi: 10.1002/jez.1402020309. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. The neuroendocrine system of invertebrates: a developmental and evolutionary perspective. J. Endocrinol. 2006;190:555–570. doi: 10.1677/joe.1.06964. [DOI] [PubMed] [Google Scholar]
- Landers MH, Happ GM. Precocene inhibition of vitellogenesis in Drosophila melanogaster. Experientia. 1980;36:619–620. [Google Scholar]
- Liu L, Davis RL, Roman G. Exploratory activity in Drosophila requires the Kurtz nonvisual arrestin. Genetics. 2007;175:1197–1212. doi: 10.1534/genetics.106.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li X, Prasifka JR, Jurenka R, Bonning BC. Overexpression of Drosophila juvenile hormone esterase binding protein results in anti-JH effects and reduced pheromone abundance. Gen. Comp. Endocrinol. 2008;156:164–172. doi: 10.1016/j.ygcen.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Malaterre J, Strambi C, Aouane A, Strambi A, Rougon G, Cyre M. Effect of hormones and growth factors in the proliferation of adult cricket neural progenitor cells in vitro. J. Neurobiol. 2003;56:387–397. doi: 10.1002/neu.10244. [DOI] [PubMed] [Google Scholar]
- McCaffery AR, McDowell PG. Titres of precocene ii (6,7-dimethoxy-2,2-dimethylchromene) and its metabolites in the haemolymph of larva of the African armyworm Spodoptera exempta (walker) following topical treatment with the compound. Pesticide Science. 1987;19:185–196. [Google Scholar]
- McEwen BS. Steroid hormones: effect on brain development and function. Horm. Res. 1992;37:1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Multiple roles for dopamine in Drosophila development. Dev. Biol. 1996;176:209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Dopamine modulates female sexual receptivity in Drosophila melanogaster. J. Neurogenet. 1998;12:101–114. doi: 10.3109/01677069809167259. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Bhatt P. Neurotrophic actions of dopamine on the development of a sertonergic feeding circuit in Drosophila melanogaster. BMC Neurosci. 2012;13:26. doi: 10.1186/1471-2202-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Holt B, Paradowski TJ. Biochemical conservation of recombinant Drosophila tyrosine hydroxylase with its mammalian cognates. Biochem. Genet. 2005;43:425–443. doi: 10.1007/s10528-005-6781-3. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience. 2008;156:841–856. doi: 10.1016/j.neuroscience.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Quinn WG. Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron. 1989;2:1167–1175. doi: 10.1016/0896-6273(89)90183-9. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Weinstein JS. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress. 2005;8:117–132. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Shah DK, Wells MA. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect Mol. Biol. 1997;6:63–66. doi: 10.1046/j.1365-2583.1997.00154.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Markow TA, Spicer GS. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl. Acad. Sci. U S A. 1995;92:10614–10618. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol. 2008;180:417–426. doi: 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. Gen. Comp. Endocrinol. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones and neurobehavioural development. J. Neuroendocrinol. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci. Beiobehav. Rev. 2002;26:381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Akasaka S, Mezawa R, Shimada K, Maekawa K. Regulation of the brain dopaminergic system by juvenile hormone in honey bee males (Apis Mellifera L.) Insect Mol. Biol. 2012;21:502–509. doi: 10.1111/j.1365-2583.2012.01153.x. [DOI] [PubMed] [Google Scholar]
- Schooley DA, Creswell KM, Staiger LE, Quistad GB. Environmental degradation of the insect growth regulator isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate (methoprene). IV. Soil metabolism. J. Agric. Food Chem. 1975;23:369–373. doi: 10.1021/jf60199a067. [DOI] [PubMed] [Google Scholar]
- Smith SS. Female sex steroid hormones: from receptors to networds to performance—actions on the sensorimotor system. Prog. Neurobiol. 1994;44:55–86. doi: 10.1016/0301-0082(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth HT. Courtship behavior in Drosophila. Annu. Rev. Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- Strambi C, Cayre M, Strambi A. Neural plasticity in the adult insect brain and its hormonal control. Int. Rev. Cytology. 1999;190:137–174. [Google Scholar]
- Strauss R, Heisenberg M. A higher center of locomotor behavior in the Drosophila brain. J. Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne DM. Physiology of puberty. Horm. Res. 1994;41:3–6. doi: 10.1159/000183949. [DOI] [PubMed] [Google Scholar]
- Teal PE, Gomez-Simuta Y, Proveaux AT. Mating experience and juvenile hormone enhance sexual signaling and mating in male Caribbean fruit flies. Proc. Natl. Acad. Sci. U S A. 2000;97:3708–3712. doi: 10.1073/pnas.060034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker C, Jallon JM. Hormonal control of sex pheromone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 1995;41:65–70. [Google Scholar]
- Wilson TG, Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Landers MH, Happ GM. Precocene I and II inhibition of vitellogenic oocyte development in Drosophila melanogaster. J. Insect Physiol. 1983;29:249–254. [Google Scholar]
- Wong M, Eaton MJ, Moss RL. Electrophysiological actions of luteinizing hormone-releasing hormone: intracellular studies in the rat hippocampal slide preparation. Synapse. 1990;5:65–70. doi: 10.1002/syn.890050106. [DOI] [PubMed] [Google Scholar]
- Woodring J, Hoffmann KH. The effects of octopamine, dopamine and serotonin on juvenile hormone synthesis, in vitro, in the cricket, Cryllus bimac. J. Insect Physiol. 1994;40:797–802. [Google Scholar]